2. 江苏海洋大学, 江苏省海洋生物产业技术协同创新中心, 连云港 222005;
3. 江苏省海洋资源开发研究院, 连云港 222005
2. Co-Innovation Center of Jiangsu Marine Bio-industry Technology, Jiangsu Ocean University, Lianyungang 222005, China;
3. Jiangsu Marine Resources Development Research Institute, Lianyungang 222005, China
β-羟基-β-甲基丁酸(β-hydroxy-β-methylbutyrate,HMB)是必需氨基酸亮氨酸的中间代谢产物之一,广泛存在于自然界的各种动植物体内,目前主要通过人工化学合成的方法获得。HMB因具有促进动物体肌肉生长、延缓肌肉疲劳、防止肌肉萎缩、增加免疫力等作用而被作为营养补充剂广泛应用。2011年,我国卫生部批准β-羟基-β-甲基丁酸钙(β-hydroxy-β-methylbutyrate calcium,HMB-Ca)作为食品添加剂进入新资源食品目录。随着高密度集约化养殖业的发展,我国水产养殖产量不断攀升,但水产养殖动物表现出生长缓慢、肌肉品质和免疫力下降等问题[1-3],严重制约了水产养殖业的健康可持续发展。目前,越来越多的绿色添加剂被应用于水产动物饲料中以改善动物的生长性能和免疫功能等[4-6]。HMB作为饲料添加剂在提高水产动物生长性能和抗病力等方面也表现出了较好的效果[7-10]。本文综述了HMB的作用机制及其对水产动物生长性能、蛋白质代谢、脂质代谢、肌肉品质和免疫功能的影响,并与在畜禽等其他动物中的研究做比较,分析了目前HMB在水产动物中的应用进展与不足。
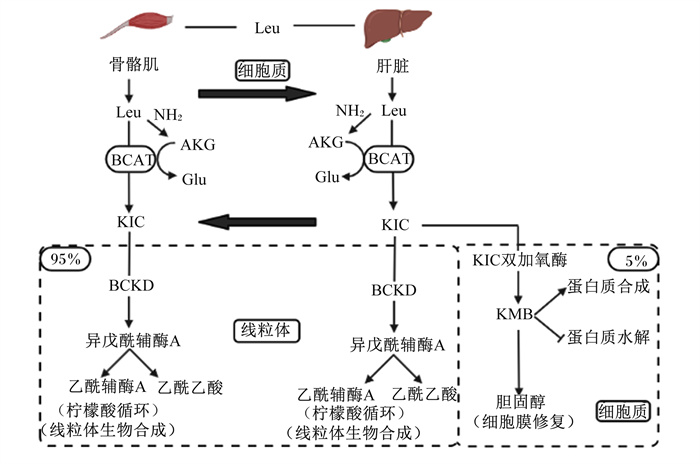
1 HMB的代谢及其作用机制亮氨酸是动物的必需氨基酸之一,绝大部分的亮氨酸会通过周转用于合成自身的蛋白质,只有少量的亮氨酸(大约5%)会在体内经过代谢后最终转化为HMB[11]。因为HMB有许多与亮氨酸相似的作用,故HMB被认为是亮氨酸发挥作用的关键中间代谢产物。HMB合成的第1步是亮氨酸在支链氨基酸转氨酶的作用下被转氨为α-酮异己酸(α-ketoisocaproate acid,KIC),这一步主要发生在肝脏外组织中(如骨骼肌等)。大部分KIC被转运至肝脏线粒体后通过支链酮酸脱氢酶的作用产生异戊酰辅酶A再生成三羧酸循环的中间代谢物乙酰辅酶A和乙酰乙酸[12]。只有极少部分KIC通过KIC双加氧酶代谢产生HMB[11-12]。除随尿液排出以外,体内的部分HMB生成β-羟基-β-甲基丁酸-CoA后经酯化转化为β-羟基-β-甲基戊二酸单酰辅酶A(β-hydroxy-β-methylglutaryl coenzyme A,HMG-CoA),最终合成胆固醇[12]。HMB的代谢过程如图 1所示。
|
AKG:α-酮戊二酸α-ketoglutarate;BCAT:支链氨基酸转氨酶branched chain amino acid transferase;BCKD:支链酮酸脱氢酶branched chain ketoacid dehydrogenase;Glu:谷氨酸glutamic acid;HMB:β-羟基-β-甲基丁酸β-hydroxy-β-methylbutyrate;KIC:α-酮异己酸α-ketoisocaproate acid;Leu:亮氨酸leucine。 图 1 β-羟基-β-甲基丁酸的代谢 Fig. 1 β-hydroxy-β-methylbutyrate metabolism[13] |
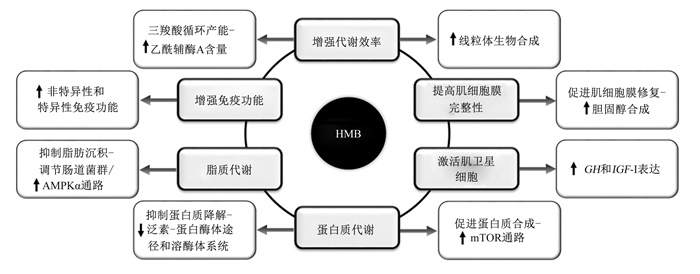
HMB具有促进动物体肌肉生长、延缓肌肉疲劳、防止肌肉萎缩、增强免疫功能等作用,因而被广泛应用于运动补剂、健身产品以及高端保健食品等。HMB发挥作用的可能机制(图 2)包括:1)直接或间接增加肌肉细胞中能量产生,增强代谢效率。摄食HMB产生的HMG-CoA会在HMG-CoA合成酶的作用下转化为乙酰乙酰辅酶A进而增加三羧酸循环的中间代谢物乙酰辅酶A含量,直接增加ATP的产生[12];HMB还通过增加线粒体生物合成提高能量代谢,Stancliffe等[14]发现HMB可使C2C12细胞的线粒体生物合成增加50%。2)提高肌肉细胞膜的完整性。受损伤的肌肉细胞可能不能够产生足够的HMG-CoA以维持正常的细胞功能[15],而HMB可通过转化为HMG-CoA合成胆固醇进而促进肌肉细胞膜的修复[13]。3)上调生长激素(growth hormone,GH)和胰岛素样生长因子-Ⅰ(insulin-like growth factor-Ⅰ,IGF-Ⅰ)的表达[16],诱导肌卫星细胞和成肌因子的激活,促进肌肉生长和再生[17-18]。4)通过激活哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路增加蛋白质合成。HMB可诱导小鼠C2C12细胞mTOR、p70核糖体蛋白S6激酶1(p70 ribosomal protein S6 kinase 1,p70S6K1)和真核起始因子4E结合蛋白-1(eukaryotic translation initiation factor 4E binding protein-1,4EBP-1)表达上调以及蛋白质合成增加[19]。5)通过调控蛋白质水解系统抑制蛋白质降解。离体和在体试验均证明HMB可抑制泛素-蛋白酶体途径进而减少蛋白质降解[20-21];此外,HMB还会抑制含半胱氨酸的天冬氨酸蛋白水解酶(caspase)和溶酶体系统诱导的自噬过程,减弱骨骼肌蛋白质降解[22]。6)调控脂质代谢。通过调节肠道菌群和激活腺苷酸活化蛋白激酶α(AMP-activated protein kinase α,AMPKα)通路,抑制脂肪酸合成,促进脂肪分解和脂肪酸氧化,从而抑制动物脂肪沉积和肥胖[23-24]。7)增强机体免疫功能。畜禽动物和鱼类中均证明HMB通过提高吞噬细胞的吞噬活性、溶酶体活性以及免疫球蛋白含量等增强动物免疫力[8, 25]。
|
AMPKα:腺苷酸活化蛋白激酶α AMP-activated protein kinase α;GH:生长激素growth hormone;HMB:β-羟基-β-甲基丁酸β-hydroxy-β-methylbutyrate;IGF-Ⅰ:胰岛素样生长因子-Ⅰ insulin-like growth factor-Ⅰ;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin。 图 2 β-羟基-β-甲基丁酸发挥作用的可能机制 Fig. 2 β-hydroxy-β-methylbutyrate possible mechanisms of action |
HMB在水产动物中的应用和研究主要集中在探究其对鱼类生长性能、蛋白质代谢和免疫功能的影响,而对脂质代谢和肌肉品质影响的研究较少(表 1)。
|
|
表 1 β-羟基-β-甲基丁酸在水产动物中应用的研究 Table 1 Studies of application of β-hydroxy-β-methylbutyrate in aquatic animals |
饲料中添加0.1% HMB-Ca使大菱鲆(Scophthalmus maximus L.)幼鱼的终末体重增加了13.3%,特定生长率增加了5.88%,对其存活率、摄食率和饲料效率均没有显著影响[7]。同时离体试验也发现,培养基中添加3种不同浓度(25、50和100 μg/mL)的HMB-Ca均可显著提高大菱鲆肌肉细胞的增殖率,即促进了大菱鲆肌肉细胞的生长[7]。相似地,饲料中添加0.1% HMB也显著增加了大黄鱼(Larimichthys crocea)的增重率和特定生长率[8]。然而,饲料中添加HMB未能显著提高尼罗罗非鱼(Oreochromis niloticus)[30]和杂交条纹鲈(Morone chrysops × M. saxatilis)[31]的生长性能。王磊[7]推测不同的试验结果可能是由于不同的试验对象和试验条件造成的。以尼罗罗非鱼为研究对象的养殖试验仅进行了2周,以杂交条纹鲈为研究对象的养殖试验结束后,鱼的终末体重仅比初始体重增加了1倍。这可能是由于养殖时间太短和体重增加太少的原因,没有足够的空间表现出HMB的有益作用,轻微的有益趋势在生物统计学上并未显现出显著差异。大量研究已证明,饲料中适宜水平的HMB可提高畜禽动物的生长性能。在巴马香猪饲粮中添加0.13% HMB显著提高了其生长性能[33]。Qiao等[34]研究表明,饲粮中添加0.1% HMB-Ca可显著改善肉鸡的生长性能,促进胸肌发育,且在生长初期效果较为明显,这可能与血清中甲状腺激素水平的提高有关。然而,HMB对虾蟹类等其他水产动物生长性能的影响还未见报道。
2.2 HMB对水产动物蛋白质代谢的影响研究表明,HMB可通过调控mTOR信号通路以及泛素-蛋白酶体途径等蛋白质水解系统进而影响机体蛋白质代谢。在大黄鱼中的研究发现,饲料中添加0.1%和0.8% HMB可提高肌肉中TOR和IGF-Ⅰ的mRNA表达水平,同时降低肌肉中泛素连接酶肌环指蛋白-1(muscle ring finger-1,Murf-1)的mRNA表达水平,即促进了肌肉蛋白质合成而抑制了其分解[8]。相似地,饲料中添加0.1% HMB-Ca显著降低了大菱鲆幼鱼肌肉中泛素连接酶Murf-1和肌肉萎缩盒F基因(muscle atrophy F-box,MAFbx/Atrogin-1)的mRNA表达水平[7],即抑制了蛋白质泛素化过程,降低了肌肉蛋白质分解。在哺乳动物中也得到了类似的结果,HMB诱导小鼠成肌细胞(C2C12)mTOR、p70S6K1和4EBP-1表达上调以及蛋白质合成增加,而且这一过程可被雷帕霉素(mTOR抑制剂)抑制[19]。Aversa等[35]研究发现,以HMB处理体外培养的小鼠肌管细胞能够降低Murf-1和MAFbx/Atrogin-1的mRNA及蛋白表达水平。HMB和亮氨酸对机体蛋白质代谢的调控途径存在许多重叠或相似之处,在C2C12细胞中的研究证明,HMB抑制饥饿诱导的蛋白质降解的效果优于亮氨酸[36]。最近的研究甚至发现,亮氨酸在C2C12细胞中发挥抑制蛋白质降解作用需要转化为HMB实现[37]。然而在水产动物中,HMB和亮氨酸调控蛋白质代谢的效果是否存在差异以及亮氨酸是否也需要转化为HMB才能发挥该调控作用尚未可知,仍需进一步探究。
2.3 HMB对水产动物脂质代谢的影响除了调控机体蛋白质代谢外,HMB还影响动物脂质代谢。然而,目前有关HMB对水产动物脂质代谢影响的报道较少。在大黄鱼中发现,饲料中添加0.1% HMB会显著增加鱼体血清甘油三酯和肌肉粗脂肪含量,而对血清中总胆固醇、高密度脂蛋白和低密度脂蛋白含量无显著影响[8]。相似地,在杂交条纹鲈饲料中添加1 000 mg/kg HMB显著增加了鱼体脂肪含量[31]。然而,饲料中添加0.1% HMB-Ca对大菱鲆幼鱼的鱼体粗脂肪含量无显著影响[7]。与水产动物中的结果不同,在畜禽动物中发现HMB可抑制脂肪沉积,提高瘦肉率。如饲粮中添加HMB减少了肉鸡[34]、猪[33]等动物的脂肪沉积。此外,饲粮HMB还会增加猪肌肉中n-3多不饱和脂肪酸(n-3 PUFA)含量[38-39],即改善肌肉脂肪酸组成。最近在小鼠和巴马香猪中的研究证明,HMB通过调节肠道菌群和激活AMPKα通路,抑制脂肪酸合成,促进脂肪分解和脂肪酸氧化,从而抑制动物脂肪沉积和肥胖[23-24]。此类研究在水产动物中均未见报道,因此HMB对水产动物脂质代谢的影响及其机制有待深入挖掘。
2.4 HMB对水产动物肌肉品质的影响研究发现,HMB可以改善动物肌肉品质。饲料中添加0.1%和0.2% HMB显著提高了大黄鱼肌肉硬度,0.05%和0.10% HMB显著增加了肌肉持水力[8]。同样,饲喂0.1% HMB-Ca显著增加了大菱鲆幼鱼的硬度、弹性和咀嚼性[7]。动物肌肉的质构参数(硬度、弹性和咀嚼性等)可以反映肌肉质地特性,而质地的好坏是评价鱼肉品质的一个关键指标,也是消费者关注的重要感官指标,同时在水产品加工流通领域会显著影响鱼肉的加工性能。研究表明,肌纤维作为构成肌肉组织的基本单位,其直径、数目和类型是肌肉生长和品质形成的生物学基础,和肉质性状密切相关,被认为是肌肉质地的重要决定因素[40-41]。研究发现,肌纤维的密度与肌肉硬度、弹性和咀嚼性等存在正相关关系[42-43]。饲料中0.1% HMB-Ca显著降低了大菱鲆幼鱼的肌纤维横截面积,意味着单位面积内存在更多的肌纤维,肌纤维更加细密,这可能是HMB-Ca改善大菱鲆质地特性的原因[7]。
根据动物骨骼肌肌纤维的形态、收缩功能和代谢特征可将其分为两大类,即氧化型(Ⅰ型)纤维和酵解型(Ⅱ型)纤维[44]。不同类型的肌纤维因具有不同的收缩速率和代谢能力而直接影响肌肉品质,当肌肉中酵解型肌纤维比例较高时,其较高的糖酵解能力和ATP酶活性会增加肌肉pH下降的速度和程度,进而导致肌肉持水力和质地较差[41]。哺乳动物中的研究发现,饲粮中补充KIC会导致生长猪肌肉支链氨基酸失衡,促进肌纤维向酵解型转变,而饲粮添加HMB会促进更多氧化型肌纤维的生成,并促进肌肉生长,尤其是氧化型骨骼肌,这些作用可能与HMB激活AMPKα-沉默信息调节因子1-过氧化物酶体增殖物激活受体γ共激活因子-1α(AMPKα-silent information regulator 1-peroxisome proliferator-activated receptor-γ coactivator-1α,AMPKα-Sirt1-PGC-1α)轴和线粒体的生物合成有关[45]。然而,水产动物中缺乏HMB对骨骼肌肌纤维类型影响的报道。
2.5 HMB对水产动物免疫功能的影响大量研究表明,HMB能改善畜禽动物的免疫功能。Peterson等[25]在体外培养鸡巨噬细胞系时添加HMB能够显著增强巨噬细胞的吞噬功能,促进细胞增殖,进而改善仔鸡的免疫功能。在母猪妊娠后期饲粮中添加15 mg/kg HMB能显著提高母猪初乳中免疫球蛋白G和免疫球蛋白M的含量[46]。Zheng等[47]研究发现,饲粮中添加0.60% HMB改善了脂多糖胁迫下仔猪的肠道完整性、功能和微生物区系,表明HMB可以缓解免疫应激引起的肠道功能紊乱。
虽然水生动物的免疫系统与陆生动物不同,但研究表明HMB也能提高水产动物的免疫功能。Estévez等[27]通过细胞培养和养殖试验表明,HMB能诱导大鳞大马哈鱼(Oncorhynchus tshawytscha)细胞系多种非特异性免疫基因的表达,而摄食HMB能显著增加大西洋鲑(Salmo salar)幼鱼鳃中抗菌肽的表达,但不影响皮肤中抗菌肽的表达。大黄鱼中的研究发现,饲料中添加HMB可显著提高鱼体血清溶菌酶活性以及补体和免疫球蛋白的含量,同时显著上调肠道抑炎症因子白细胞介素-8(interleukin-8,IL-8)和白细胞介素-10(interleukin-10,IL-10)的基因表达,而下调促炎症因子肿瘤坏死因子α(tumour necrosis factor α,TNFα)和白细胞介素-1β(interleukin-1β,IL-1β)的表达[8]。Siwicki等[23]通过离体试验发现,培养基中添加10~100 μg/mL HMB显著增加了虹鳟(Oncorhynchus mykiss)和鲤鱼(Cyprinus carpio)吞噬细胞的活性,同时显著提高了伴刀豆球蛋白A和脂多糖刺激的T淋巴细胞和B淋巴细胞的增殖能力。饲料中添加HMB显著增加了虹鳟多形核白细胞和单个核细胞的呼吸爆发活性、血浆溶菌酶活性以及血清免疫球蛋白含量,同时提高了虹鳟对杀鲑气单胞菌(Aeromonas salmonicida)感染的抵抗力[9]。在白梭吻鲈(Sander lucioperca)中也发现,饲料中添加50 mg/kg HMB可显著提高其吞噬细胞的吞噬活性和鱼体对鲁氏耶尔森氏菌(Yersinia ruckeri)感染的抵抗力[10]以及血浆溶菌酶活性和鱼体对杀鲑气单胞菌感染的抵抗力[26]。相似地,体内外试验均证明HMB可显著提高欧洲巨鲶(Silurius glanis)的免疫功能[28-29]。然而,也有报道不支持此类结论,如饲料中添加HMB并未显著提高尼罗罗非鱼感染海豚链球菌(Streptococcus iniae)后抗体的产生以及存活率[30];HMB不能提高杂交条纹鲈幼鱼头肾细胞内外超氧阴离子产生以及血清溶菌酶活性[31]。这些不同结果可能与不同的物种、生长阶段以及养殖周期等有关。
3 小结与展望HMB是一种非常有应用前景的营养补充剂,已经在临床和动物中有了大量的试验及应用,且已证明了其安全性。HMB具有多种有益作用,包括促进肌肉蛋白质合成、抑制肌肉蛋白质降解、提高肌肉品质和免疫力等。同时,HMB是一种无氮补剂,应用于水产动物养殖中对水体的污染小,不会造成水体中氨氮和亚硝酸含量的升高。因此,HMB作为饲料添加剂,在提高水产养殖动物生长性能、肌肉品质和免疫功能方面具有非常广阔的应用前景。HMB的作用机制非常复杂,目前还在探索阶段,需要大量的试验验证。HMB在水产动物中的应用和研究非常有限,许多在畜禽动物中常见的研究在水产动物尤其是甲壳动物中却鲜有报道,未来需要大量的研究来验证HMB在水产动物中的作用及其机制。
| [1] |
GAO X L, PANG G W, LUO X, et al. Effects of stocking density on the survival and growth of Haliotis discus hannai ♀×H. fulgens ♂ hybrids[J]. Aquaculture, 2020, 529: 735693. DOI:10.1016/j.aquaculture.2020.735693 |
| [2] |
XU H G, ZHANG X, WEI Y L, et al. Effects of dietary phosphorus level and stocking density on tiger puffer Takifugu rubripes: growth performance, body composition, lipid metabolism, deposition of phosphorus and calcium, serum biochemical parameters, and phosphorus excretion[J]. Aquaculture, 2020, 529: 735709. DOI:10.1016/j.aquaculture.2020.735709 |
| [3] |
刘慧玲, 郭文俊, 王成桂, 等. 养殖密度对墨吉明对虾生长、代谢和免疫的影响[J]. 水产科学, 2021, 40(5): 679-685. LIU H L, GUO W J, WANG C G, et al. Effects of stocking density on growth, metabolism and immune functions of banana prawn Fenneropenaeus meiguiensis[J]. Fisheries Science, 2021, 40(5): 679-685 (in Chinese). |
| [4] |
ĪNANAN B E, ACAR Ü, ĪNANAN T. Effects of dietary Ferula elaeochytris root powder concentrations on haematology, serum biochemical parameters, spermatozoa parameters, and oxidative status in tissues of males goldfish (Carassius auratus)[J]. Aquaculture, 2021, 544: 737087. DOI:10.1016/j.aquaculture.2021.737087 |
| [5] |
符振强, 董扬帆, 汤上上, 等. 低盐胁迫下饲料中添加α-硫辛酸对凡纳滨对虾生长、抗氧化能力及肠道健康的影响[J]. 动物营养学报, 2021, 33(9): 5203-5218. FU Z Q, DONG Y F, TANG S S, et al. Effects of dietary α-lipoic acid on growth, antioxidant capacity and intestinal health of Litopenaeus vannamei under low salinity stress[J]. Chinese Journal of Animal Nutrition, 2021, 33(9): 5203-5218 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.09.040 |
| [6] |
DENG G W, DUAN J C, MU H, et al. Effects of dietary short-chain fatty acid salts on the growth performance, digestive, antioxidant and immune enzyme activities, immune-related gene expression and resistance to Vibro parahaemolytics infection in juvenile ridgetail white prawn (Exopalaemon carinicauda)[J]. Aquaculture Research, 2021, 52(12): 6716-6725. DOI:10.1111/are.15542 |
| [7] |
王磊. 植物乳杆菌p8发酵豆粕及β-羟基-β-甲基丁酸钙(HMB-Ca)在大菱鲆饲料中应用的研究[D]. 博士学位论文. 青岛: 中国海洋大学, 2016. WANG L. The effect of dietary supplementation of Lactobacillus plantarum P8 fermented soybean meal or β-hydroxy-β-methylbutyrate calcium (HMB-Ca) for juvenile turbot (Scophthalmus maximus L. )[D]. Ph. D. Thesis. Qingdao: Ocean University of China, 2016. (in Chinese) |
| [8] |
唐梦曦. β-羟基-β-甲基丁酸盐(HMB)对大黄鱼生长效应、肌肉品质和肠道健康影响的研究[D]. 硕士学位论文. 青岛: 中国海洋大学, 2018. TANG M X. Effects of β-hydroxy-β-methylbutyrate (HMB) on growth, muscle quality and intestinal health of large yellow croaker Larimichthys crocea[D]. Master's Thesis. Qingdao: Ocean University of China, 2018. (in Chinese) |
| [9] |
SIWICKI A K, MORAND M, FULLER J, J r, et al. Influence of feeding the leucine metabolite β-hydroxy-β-methylbutyrate (HMB) on the non-specific cellular and humoral defence mechanisms of rainbow trout (Oncorhynchus mykiss)[J]. Journal of Applied Ichthyology, 2003, 19(1): 44-48. DOI:10.1046/j.1439-0426.2003.00348.x |
| [10] |
SIWICKI A K, ZAKE S ' Z, FULLER J C, J r, et al. The effect of feeding the leucine metabolite β-hydroxy-β-methylbutyrate (HMB) on cell-mediated immunity and protection against Yersinia ruckeri in pikeperch (Sander lucioperca)[J]. Aquaculture Research, 2005, 36(1): 16-21. DOI:10.1111/j.1365-2109.2004.01176.x |
| [11] |
VAN KOEVERING M, NISSEN S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo[J]. The American Journal of Physiology, 1992, 262(1 Pt.1): E27-E31. |
| [12] |
NISSEN S L, ABUMRAD N N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB)[J]. The Journal of Nutritional Biochemistry, 1997, 8(6): 300-311. DOI:10.1016/S0955-2863(97)00048-X |
| [13] |
LIMA-SOARES F, CABIDO C E T, PESSÔA K A, et al. 23-HMB supplementation: clinical and performance-related effects and mechanisms of action[M]//BAGCHI D. Sustained energy for enhanced human functions and activity. London: Academic Press, 2017: 363-381.
|
| [14] |
STANCLIFFE R A. Role of beta-hydroxy-beta-methylbutyrate (HMB) in leucine stimulation of mitochondrial biogenesis and fatty acid oxidation[D]. Master's Thesis. Knoxville: The University of Tennessee, 2012.
|
| [15] |
PIERNO S, DE LUCA A, TRICARICO D, et al. Potential risk of myopathy by HMG-CoA reductase inhibitors: a comparison of pravastatin and simvastatin effects on membrane electrical properties of rat skeletal muscle fibers[J]. Journal of Pharmacology and Experimental Therapeutics, 1995, 275(3): 1490-1496. |
| [16] |
GERLINGER-ROMERO F, GUIMARÃES-FERREIRA L, GIANNOCCO G, et al. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-Ⅰ axis and induces hyperinsulinemia in rats[J]. Growth Hormone & IGF Research, 2011, 21(2): 57-62. |
| [17] |
SZCZEŚNIAK K A, CIECIERSKA A, OSTASZEWSKI P, et al. Characterisation of equine satellite cell transcriptomic profile response to β-hydroxy-β-methylbutyrate (HMB)[J]. British Journal of Nutrition, 2016, 116(8): 1315-1325. DOI:10.1017/S000711451600324X |
| [18] |
WAN H F, ZHU J T, SHEN Y, et al. Effects of dietary supplementation of β-hydroxy-β-methylbutyrate on sow performance and mRNA expression of myogenic markers in skeletal muscle of neonatal piglets[J]. Reproduction in Domestic Animals, 2016, 51(1): 135-142. DOI:10.1111/rda.12657 |
| [19] |
ELEY H L, RUSSELL S T, BAXTER J H, et al. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli[J]. American Journal of Physiology.Endocrinology and Metabolism, 2007, 293(4): E923-E931. DOI:10.1152/ajpendo.00314.2007 |
| [20] |
SMITH H J, WYKE S M, TISDALE M J. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate[J]. Cancer Research, 2004, 64(23): 8731-8735. DOI:10.1158/0008-5472.CAN-04-1760 |
| [21] |
HOLECEK M, MUTHNY T, KOVARIK M, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues[J]. Food and Chemical Toxicology, 2009, 47(1): 255-259. DOI:10.1016/j.fct.2008.11.021 |
| [22] |
GIRÓN M D, VÍLCHEZ J D, SHREERAM S, et al. β-hydroxy-β-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle[J]. PLoS One, 2015, 10(2): e0117520. DOI:10.1371/journal.pone.0117520 |
| [23] |
DUAN Y H, ZHONG Y Z, XIAO H, et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets[J]. The FASEB Journal, 2019, 33(9): 10019-10033. DOI:10.1096/fj.201900665RR |
| [24] |
ZHENG J, ZHENG C B, SONG B, et al. HMB improves lipid metabolism of Bama Xiang mini-pigs via modulating the Bacteroidetes-acetic acid-AMPKα axis[J]. Frontiers in Microbiology, 2021, 12: 736997. DOI:10.3389/fmicb.2021.736997 |
| [25] |
PETERSON A L, QURESHI M A, FERKET P R, et al. In vitro exposure with beta-hydroxy-beta-methylbutyrate enhances chicken macrophage growth and function[J]. Veterinary Immunology and Immunopathology, 1999, 67(1): 67-78. DOI:10.1016/S0165-2427(98)00211-6 |
| [26] |
SIWICKI A K, ZAKE S ' Z, FULLER J C, J r, et al. Influence of β-hydroxy-β-methylbutyrate on nonspecific humoral defense mechanisms and protection against furunculosis in pikeperch (Sander lucioperca)[J]. Aquaculture Research, 2006, 37(2): 127-131. DOI:10.1111/j.1365-2109.2005.01407.x |
| [27] |
SIWICKI A K, FULLER J C, J r, NISSEN S, et al. In vitro effects of beta-hydroxy-beta-methylbutyrate (HMB) on cell-mediated immunity in fish[J]. Veterinary Immunology and Immunopathology, 2000, 76(3/4): 191-197. |
| [28] |
SIWICKI A K, FULLER J C, J r, NISSEN S, et al. Effect of HMB (β-hydroxy-β-methylbutyrate) on in vitro proliferative responses of sheatfish (Silurus glanis) and catfish (Ictalurus melas) lymphocytes stimulated by mitogens[J]. Acta Veterinaria Brno, 2004, 73: 119-222. DOI:10.2754/avb200473010119 |
| [29] |
SIWICKI A K, SZCZEPKOWSKI M, TERECH-MAJEWSKA E, et al. Effect of dietary administration of the β-hydroxy-β-methylbutyrate (HMB) on the innate immunity and protection against Aeromonas septicaemia in European catfish (Silurus glanis) fingerlings[J]. Annals of Aquaculture and Research, 2016, 3(3): 1022. |
| [30] |
WHITTINGTON R, SHOEMAKER C A, LIM C, et al. Effects of dietary β-hydroxy-β-methylbutyrate on growth and survival of Nile tilapia, Oreochromis niloticus, vaccinated against Streptococcus iniae[J]. Journal of Applied Aquaculture, 2004, 14(3/4): 25-36. |
| [31] |
LI P, GATLIN Ⅲ D M. Evaluation of dietary supplementation of graded levels of β-hydroxy-β-methylbutyrate for hybrid striped bass Morone chrysops×M. saxatilis[J]. Journal of Applied Aquaculture, 2007, 19(3): 77-88. DOI:10.1300/J028v19n03_05 |
| [32] |
ESTÉVEZ R A, MOSTAZO M G C, RODRIGUEZ E, et al. Inducers of salmon innate immunity: an in vitro and in vivo approach[J]. Fish & Shellfish Immunology, 2018, 72: 247-258. |
| [33] |
郑昌炳, 宋博, 郑界, 等. 饲粮中添加β-羟基-β-甲基丁酸对巴马香猪生长性能和肝脏脂肪代谢的影响[J]. 动物营养学报, 2021, 33(6): 3176-3184. ZHENG C B, SONG B, ZHENG J, et al. Effects of dietary supplementation of beta-hydroxy-beta-methylbutyrate on growth performance and liver lipid metabolism of Bama Xiang pigs[J]. Chinese Journal of Animal Nutrition, 2021, 33(6): 3176-3184 (in Chinese). |
| [34] |
QIAO X, ZHANG H J, WU S G, et al. Effect of β-hydroxy-β-methylbutyrate calcium on growth, blood parameters, and carcass qualities of broiler chickens[J]. Poultry Science, 2013, 92(3): 753-759. DOI:10.3382/ps.2012-02341 |
| [35] |
AVERSA Z, ALAMDARI N, CASTILLERO E, et al. β-hydroxy-β-methylbutyrate (HMB) prevents dexamethasone-induced myotube atrophy[J]. Biochemical and Biophysical Research Communications, 2012, 423(4): 739-743. DOI:10.1016/j.bbrc.2012.06.029 |
| [36] |
DUAN Y H, LI F N, GUO Q P, et al. β-hydroxy-β-methyl butyrate is more potent than leucine in inhibiting starvation-induced protein degradation in C2C12 myotubes[J]. Journal of Agricultural and Food Chemistry, 2018, 66(1): 170-176. DOI:10.1021/acs.jafc.7b04841 |
| [37] |
DUAN Y H, ZHONG Y Z, SONG B, et al. Suppression of protein degradation by leucine requires its conversion to β-hydroxy-β-methyl butyrate in C2C12 myotubes[J]. Aging, 2019, 11(24): 11922-11936. DOI:10.18632/aging.102509 |
| [38] |
ZHONG Y Z, SONG B, ZHENG C B, et al. α-Ketoisocaproate and β-hydroxy-β-methyl butyrate regulate fatty acid composition and lipid metabolism in skeletal muscle of growing pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2019, 103(3): 846-857. DOI:10.1111/jpn.13077 |
| [39] |
ZHENG C B, SONG B, GUO Q P, et al. Alterations of the muscular fatty acid composition and serum metabolome in Bama Xiang mini-pigs exposed to dietary beta-hydroxy beta-methyl butyrate[J]. Animals, 2021, 11(5): 1190. DOI:10.3390/ani11051190 |
| [40] |
RINCÓN L, CASTRO P L, ÁLVAREZ B, et al. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo)[J]. Aquaculture, 2016, 451: 195-204. DOI:10.1016/j.aquaculture.2015.09.016 |
| [41] |
LEFAUCHEUR L. A second look into fibre typing-relation to meat quality[J]. Meat Science, 2010, 84(2): 257-270. DOI:10.1016/j.meatsci.2009.05.004 |
| [42] |
JOHNSTON I A, ALDERSON R, SANDHAM C, et al. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.)[J]. Aquaculture, 2000, 189(3/4): 335-349. |
| [43] |
PERIAGO M J, AYALA M D, LÓPEZ-ALBORS O, et al. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L.[J]. Aquaculture, 2005, 249(1/2/3/4): 175-188. |
| [44] |
MIZUNOYA W, IWAMOTO Y, SHIROUCHI B, et al. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle[J]. PLoS One, 2013, 8(11): e80152. DOI:10.1371/journal.pone.0080152 |
| [45] |
DUAN Y H, ZENG L M, LI F N, et al. Beta-hydroxy-beta-methyl butyrate promotes leucine metabolism and improves muscle fibre composition in growing pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(5): 1328-1339. DOI:10.1111/jpn.12957 |
| [46] |
DAVIS H E, JAGGER S, TOPLIS P, et al. Feeding β-hydroxy β-methyl butyrate to sows in late gestation improves litter and piglet performance to weaning and colostrum immunoglobulin concentrations[J]. Animal Feed Science and Technology, 2021, 275: 114889. DOI:10.1016/j.anifeedsci.2021.114889 |
| [47] |
ZHENG C B, SONG B, DUAN Y H, et al. Dietary β-hydroxy-β-methylbutyrate improves intestinal function in weaned piglets after lipopolysaccharide challenge[J]. Nutrition, 2020, 78: 110839. DOI:10.1016/j.nut.2020.110839 |




