我国蛋鸡存栏量、鸡蛋产量、禽料产量屡创新高,饲料安全直接影响了蛋鸡的安全生产和鸡蛋安全。镉(Cd)、铅(Pb)、汞(Hg)、铬(Cr)等重金属(heavy metal,HEM)在动物生存环境、饲料和动物可食性组织中同时存在,且含量较高。畜禽饲料中重金属主要来源于环境及矿物质添加剂。已有大量调研数据显示,我国各省市的畜禽饲料存在严重重金属超标现象[1-2]。Cd、Pb、Hg为畜禽非必需金属元素,可在动物各组织沉积,危害生殖器官,引起离子紊乱。Cr为畜禽必需金属元素,但过量添加同样会导致动物中毒。研究发现,蛋鸡饲粮途径单一重金属暴露[Cr、Pb、Cd、钒(V)及钼(Mo)]可引起重金属沉积和必需金属离子紊乱,降低蛋鸡产蛋率和鸡蛋中必需金属元素含量[3-8]。Kim等[9]研究发现,蛋鸡饲粮途径两两联合暴露不同含量的重金属(Hg+Pb、Pb+Cd、Hg+Cd),可引起血浆、肾脏、胫骨、肝脏和肌胃等组织重金属沉积。Hallak等[10]和Jordan等[11]研究发现,鸭饲粮途径3种重金属联合暴露(Cd+Pb+Hg)对肝脏的毒性大于两两联合暴露(Hg+Pb、Pb+Cd、Hg+Cd)。研究发现,饲粮中添加亚硒酸钠可降低部分饲粮重金属在肉鸡肌肉、肝脏、肾脏等组织中的沉积量[12-15]。但Jihen等[16]研究却发现,亚硒酸钠对雄性大鼠Cd中毒无明显缓解作用。Xing等[17]研究结果显示,酵母硒可缓解Cd导致的氧化应激,恢复硒蛋白表达水平,减少肝细胞焦亡。
虽然我国卫生标准规定了相应重金属的限量,但是由于利益驱使、管控力度不够以及矿物质添加剂的劣质,仍然会有饲料及生产环节中重金属超标的现象,尤其是Pb和Cr。目前,国内外有关重金属及硒对重金属毒性的缓解研究主要集中在单一或两两重金属暴露,但实际生产中各种重金属同时存在,而有关多种重金属联合暴露对蛋鸡的金属离子稳态的研究较少。因此,本试验旨在探索多种重金属联合暴露对蛋鸡肝脏、肾脏、胫骨及血清中金属离子稳态的毒性及Se的缓解作用,为进一步保障蛋鸡饲料安全和人类食品安全提供科学依据。
1 材料与方法 1.1 试验动物试验动物选用160只处于产蛋后期(63周龄)的健康粉壳罗曼蛋鸡。
1.2 试验设备及试剂 1.2.1 试验设备高分辨率连续光源原子吸收光谱仪(ContrAA 700,德国Analytik Jena公司)、智能微波消解仪(CEM-MARS6,上海博通化学科技有限公司)、双道原子荧光光度计(AES-230E,北京海光仪器公司)、测汞仪(DMA 80 evo,意大利Milestone公司)。
1.2.2 试验试剂氯化镉(CdCl2,分析纯)、氯化铬(CrCl3,分析纯)、硝酸铅[(Pb(NO3)2,分析纯]、氯化汞(HgCl2,分析纯)、浓硝酸、高氯酸(成都市科龙化工试剂厂),酵母硒(0.2%,吉隆达生物科技有限公司),氧化镧(La2O3,成都市科龙化工试剂厂)、氯化铯(CsCl,上海生工生物技术)、超纯水。Hg、Cr、Se标准溶液(1 000 μg/mL)和Cd、Pb、Cu、Fe、Mn、Zn、Na、K、Mg、Ca标准溶液(100 μg/mL)(国家标准物质中心)。
1.3 试验设计选择160只63周龄的健康粉壳罗曼蛋鸡,随机分为4个组,每组10个重复,每个重复4只鸡。对照组(CON组)饲喂玉米-豆粕型基础饲粮,加硒组(Se组)在基础饲粮中添加0.4 mg/kg Se(酵母硒),重金属联合暴露组(HEM组)基础饲粮中添加5 mg/kg Cd(CdCl2)、5 mg/kg Cr(CrCl3)、50 mg/kg Pb[Pb(NO3)2]和3 mg/kg Hg(HgCl2),重金属联合暴露加硒组(HEM+Se组)在HEM组饲粮中添加0.4 mg/kg Se(酵母硒)。基础饲粮组成及营养水平见表 1,基础饲粮金属离子含量见表 2。预试期1周,正试期12周。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
|
|
表 2 基础饲粮金属离子含量(风干基础) Table 2 Metal ion contents of the basal diet (air-dry basis) |
试验在四川农业大学动物营养所试验场进行。采用3层全阶梯笼养,保证每天16 h光照,舍内温度控制在25 ℃左右,试验全期自由采食和饮水。
1.5 样品采集和测定指标饲粮配制完成后,按照四分法采样,采集基础饲粮和试验饲粮各250 g于自封袋中,4 ℃保存。试验第12周末,从每个重复中随机选取2只鸡(每组20只,共80只),翅静脉采血并提取血清,采集胫骨、肝脏、肾脏样品,-20 ℃保存。参照罗成等[18]的方法测定Cd、Cr、Pb含量,参照GB 5009.17—2014[19]的方法测定Hg含量,参照王宏慧等[20]的方法测定Cu、Fe、Mn、Zn、Na、K、Mg、Ca含量。
1.6 数据统计分析采用SAS 9.4软件的PROC MIXED模型对肝脏、肾脏、胫骨及血清中金属离子含量的数据进行分析,组间采用Tukey法进行多重比较,结果用“平均值±标准误”表示,P < 0.05表示差异显著。采用SPSS 26.0软件的Pearson相关检验分析肝脏、肾脏、胫骨及血清中各金属离子之间的相关性,r > 0.50和P < 0.05认为相关关系显著。
2 结果 2.1 重金属对蛋鸡肝脏中金属离子平衡的毒性及Se的缓解效果肝脏中金属离子含量变化如图 1所示。与CON组相比,HEM组肝脏中Cd、Pb、Hg、Fe、Zn、K含量显著升高(P < 0.05),Se组和HEM+Se组肝脏中Se含量显著升高(P < 0.05)。与HEM组相比,HEM+Se组肝脏中Cd、Pb、Fe、Zn、K含量无显著差异(P > 0.05)。
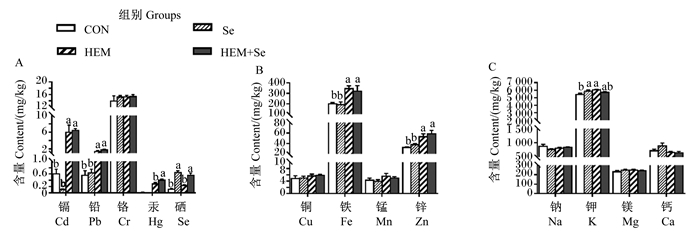
|
数据柱标相同小写字母表示差异不显著(P > 0.05),不同小写字母表示差异显著(P < 0.05)。下图同。 Value columns with the same small letter mean no significant difference (P > 0.05), while with different small letters mean significant difference (P < 0.05). The same as below. 图 1 重金属对肝脏中金属离子含量的影响及Se的缓解效果 Fig. 1 Effects of HEM on metal ion content in liver and ameliorative effect of Se |
肝脏中金属离子含量的相关性如表 3所示。肝脏中13种金属离子含量之间共存在19种显著相关关系(P < 0.05)。其中,Cd含量与Pb、Hg、Fe含量呈显著正相关(P < 0.05);Pb含量与Cd、Hg、Fe、Zn含量呈显著正相关(P < 0.05);Cr含量与Na含量呈显著正相关(P < 0.05);Hg含量与Cd、Pb、Fe、Zn、Mn含量呈显著正相关(P < 0.05);Cu含量与Mn、Zn、Mg含量呈显著正相关(P < 0.05);Fe含量与Cd、Pb、Hg、Zn含量呈显著正相关(P < 0.05);Mn含量与Mg、Hg、Cu含量呈显著正相关(P < 0.05);Zn含量与Pb、Hg、Cu、Fe含量呈显著正相关(P < 0.05);Na含量与Cr含量呈显著正相关(P < 0.05),与K和Mg含量呈显著负相关(P < 0.05);K含量与Na含量呈显著负相关(P < 0.05),与Mg含量呈显著正相关(P < 0.05)。
|
|
表 3 肝脏中金属离子含量的相关性 Table 3 Correlation of metal ion contents in liver |
肾脏中金属离子含量变化如图 2所示。与CON组相比,HEM组肾脏中Cd、Hg、Pb含量显著升高(P < 0.05)。与HEM组相比,HEM+Se组肾脏中Cd、Pb、Hg含量无显著差异(P > 0.05)。
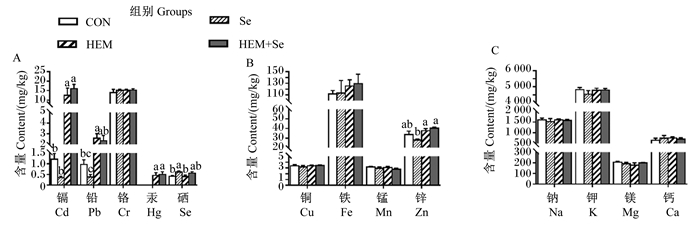
|
图 2 重金属对肾脏中金属离子含量的影响及Se的缓解效果 Fig. 2 Effects of HEM on metal ion content in kidney and ameliorative effect of Se |
肾脏中金属离子含量的相关性如表 4所示。肾脏中13种金属离子含量之间共存在12种显著相关关系(P < 0.05)。其中,Cd含量与Pb、Hg、Fe、Zn含量呈显著正相关(P < 0.05);Pb含量与Cd、Hg、Zn含量呈显著正相关(P < 0.05);Hg含量与Cd、Pb、Zn含量呈显著正相关(P < 0.05);Cu含量与Zn、K含量呈显著正相关(P < 0.05);Fe含量与Cd、Ca含量呈显著正相关(P < 0.05);Zn含量和Cd、Pb、Hg、K含量呈显著正相关(P < 0.05);K含量和Cu、Zn、Mg含量呈显著正相关(P < 0.05)。
|
|
表 4 肾脏中金属离子含量的相关性 Table 4 Correlation of metal ion contents in kidney |
血清中金属离子含量变化如图 3所示。与CON组相比,HEM组血清中Se、Mn、Mg、Ca含量显著下降(P < 0.05);Se组血清中Se含量显著升高(P < 0.05),血清中Mn、Mg、Ca含量显著下降(P < 0.05)。与HEM组相比,HEM+Se组血清中Hg含量显著升高(P < 0.05),血清中Mn、Mg、Ca含量无显著差异(P > 0.05)。
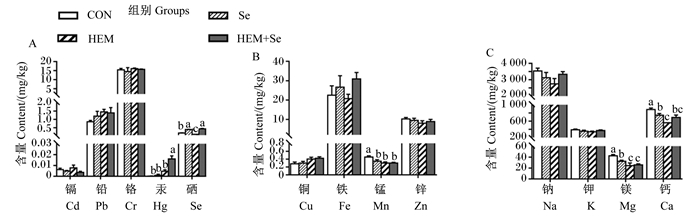
|
图 3 重金属对血清中金属离子含量的影响及Se的缓解效果 Fig. 3 Effects of HEM on metal ion contents in serum and ameliorative effect of Se |
血清中金属离子含量的相关性如表 5所示。血清中13种金属离子含量之间共存在12种显著相关关系(P < 0.05)。其中,Hg含量与Mn、Mg含量呈显著负相关(P < 0.05);Mn含量与Hg含量呈显著负相关,与Zn、Mg、Ca含量呈显著正相关(P < 0.05);Zn含量与Mn、Mg、Ca含量呈显著正相关(P < 0.05);Na含量与K、Mg、Ca含量呈显著正相关(P < 0.05);K含量与Mg含量呈显著正相关(P < 0.05);Mg含量与Mn、Zn、Na、K、Ca含量呈显著正相关(P < 0.05),与Hg含量呈显著负相关(P < 0.05)。
|
|
表 5 血清中金属离子含量的相关性 Table 5 Correlation of metal ion contents in serum |
胫骨中金属离子含量变化如图 4所示。与CON组相比,HEM组胫骨中Cd、Pb、Hg、Se、Na、K含量显著升高(P < 0.05),胫骨中Mn含量显著降低(P < 0.05)。与HEM组相比,HEM+Se组胫骨中Pb、Se含量显著升高(P < 0.05),胫骨中Na、K含量显著降低(P < 0.05),胫骨中Cd、Hg、Mn含量无显著差异(P > 0.05)。
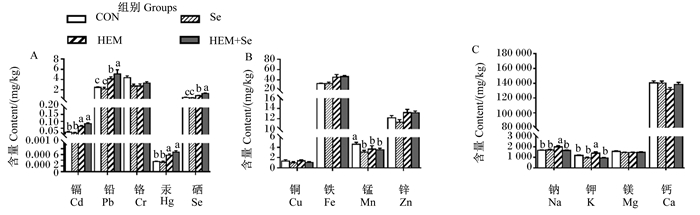
|
图 4 重金属对胫骨中金属离子含量的影响及Se的缓解效果 Fig. 4 Effects of HEM on metal ion contents in tibia and ameliorative effect of Se |
胫骨中金属离子含量的相关性如表 6所示。胫骨中13种金属离子含量之间共存在7种显著相关关系(P < 0.05)。其中,Cd含量与Pb、Hg、Se含量呈显著正相关(P < 0.05);Pb含量与Cd、Hg、Se含量呈显著正相关(P < 0.05);Hg含量与Cd、Pb含量呈显著正相关(P < 0.05);Zn含量与Mg含量呈显著正相关(P < 0.05);Se含量与Cd、Pb含量呈显著正相关(P < 0.05);Na含量与K含量显著正相关(P < 0.05)。
|
|
表 6 胫骨中金属离子含量的相关性 Table 6 Correlation of metal ion contents in tibia |
饲料及动物性食品中重金属污染通过食物链威胁动物和人类的健康[21],鸡蛋中的重金属含量与摄入饲料受重金属污染程度呈正相关[22]。我国各省市饲料原料均有重金属超标现象,各饲料原料中Cr超标率可达100%,Pb超标率高达30%以上[2]。我国饲料原料中Cd、Cr、Pb、Hg、Se的国家标准限量分别为0.5、5.0、5.0、0.1和0.5 mg/kg[23-24]。本试验结合国家限量标准和重金属的污染情况设计了联合重金属暴露的剂量。酵母硒因其高生物利用度在畜禽中被广泛使用,本试验结合国家限量设计了其添加剂量。
3.1 联合重金属暴露增加了相应重金属的沉积本试验结果显示,联合重金属暴露引起蛋鸡肝脏、肾脏、胫骨中Cd、Pb、Hg的含量显著升高。饲粮中重金属在肠道吸收,经尿液或粪便排出,无法随粪尿排出的重金属沉积在体内的各个部位,以肝脏、肾脏为主[25]。本研究中,肾脏中Cd含量最高,与Tsuruoka等[26]的研究结果一致,其沉积原理可能是:Cd随血液循环进入肝脏后,首先与金属硫蛋白(MT)结合形成络合物Cd-MT,Cd-MT一般会先到达肾脏,通过肾小管膜进入管状体后几乎在近曲小管被完全吸收,之后再通过胞饮作用进入肾小管溶酶体降解,分离并释放出游离的Cd发挥毒性作用。胫骨中Pb含量最高,这与杨晓刚等[27]的研究结果吻合,其原因可能与胫骨中Pb的存在形式有关,蛋鸡体内95%的Pb以不溶性磷酸Pb的形式与骨骼中的一些化合物结合形成稳定有机盐,长期蓄积在骨骼中。Hg化合物主要经动物肠道消化,其中有7%~15%被进一步吸收,主要沉积到肾脏中[22]。
3.2 联合重金属暴露引起金属离子失衡的可能原因研究表明,饲粮中联合重金属暴露能引起组织中金属离子紊乱,重金属离子之间可能存在协同关系[7-10]。本研究结果与以上研究结果一致。相关性分析结果表明,机体内的离子失衡与重金属的蓄积有关。肝脏中13种金属离子含量之间存在19种显著的相关关系,其中Cd含量与Fe含量,Hg含量与Zn含量,Pb含量与Zn含量以及Fe含量与Zn含量之间都存在显著的正相关关系,这与Mouro等[28]发现的肝脏中Cd含量与Fe含量呈负相关关系不同,原因可能与Cd的毒性作用有关。Cu与Zn、Mn一起作为超氧化物歧化酶(SOD)的辅助因子,在对超氧化物[29]引起的氧化损伤的第一反应中具有活性,而Cd由于与Cu、Fe、Mn等离子存在竞争性结合,可以增加活性氧(ROS)的产生,从而导致蛋鸡体内抗氧化系统损伤;同时,Cd的竞争性结合使SOD活性中心的二价离子解离,导致组织中金属离子含量升高,引起蛋鸡组织中金属离子紊乱。肾脏中13种金属离子含量之间存在12种显著的相关关系,Armbrecht等[30]研究显示,肾脏中Cd含量与Ca含量呈负相关,其原因是高含量的Cd会影响肾小球的形态和正常的生理功能,而肾小球是1, 25-二羟基维生素D32-D3[1, 25-(OH)]转化为活性形式的场所,肾小球的结构和功能受损会降低血清中1, 25-(OH)2-D3含量,进而导致Ca含量的降低。但在本试验中,肾脏中Cd含量与Ca含量无显著相关性,而Fe含量与Ca含量呈显著正相关,这与前人的研究结果不一致,可能是因为Fe与Cd竞争肠道中的二价金属转运体1(DMT1),减少了Cd的吸收[31],使Cd含量无法对Ca含量产生显著影响。血清中13种金属离子含量之间存在12种显著的相关关系,Mn含量与Zn、Mg、Ca含量均存在显著正相关,Mg含量与Ca含量也存在显著正相关,这与Liu等[32]的研究结果一致。机体组织和体液中必需金属的含量反映动物的病理和营养状况[33]。Cd、Pb、Hg降解性差,易在动物心脏、肝脏、肾脏、输卵管和骨骼等组织中蓄积,竞争性地与其他必需营养素争夺相同的跨膜载体,扰乱体内微量元素的平衡,造成神经、生殖等系统毒性,威胁鸡蛋安全[34-37]。胫骨中13种金属离子含量之间存在7种显著的相关关系,且主要是在重金属元素之间,说明重金属元素在胫骨中的关系可能是协同作用。
多个因素间的相互作用明确分为累加、协同、拮抗和独立[38]。秦卫红等[39]研究发现,饲粮途径重金属联合暴露(Pb+Cd、Pb+Hg和Hg+Cd)导致重金属在蛋鸡组织中的沉积加剧,可能具有协同效应。Kim等[9]研究发现,饲粮途径Pb+Hg联合暴露8周,蛋鸡血浆中Pb、Hg含量显著增加。相关性分析结果表明,肝脏、肾脏和胫骨中Cd、Pb、Hg之间可能为协同作用;在肾脏中,Fe与Cd可能为拮抗作用,与Ca、Zn可能为协同作用。
3.3 Se对金属离子稳态的影响组织中Se含量反映机体对Se的吸收利用率,Se可部分缓解有害重金属在肝脏、肾脏等组织中的沉积[40-41]。本研究中,HEM+Se组血清Hg含量显著高于HEM组,可能是因为Hg暴露影响血清中Se含量和硒蛋白的分布,硒蛋白P在较高的Hg暴露浓度下会结合更多的Hg[42]。在本研究中,与CON组相比,Se组胫骨中Se含量无显著差异,但HEM组和HEM+Se组胫骨中Se含量显著升高,且HEM+Se组胫骨中Se含量显著高于HEM组,与鞠耿越[43]研究结果不同,可能是因为在联合重金属暴露过程中重金属的存在使Se向胫骨转移,促进了Se的排出,但具体的机制需要进一步探索。
4 结论在本试验条件下,Cd、Pb、Hg、Cr联合暴露增加了相应重金属元素的沉积,扰乱了蛋鸡肝脏、肾脏、血清、胫骨中金属离子稳态,饲粮中添加0.4 mg/kg酵母硒未能缓解重金属暴露引起的金属离子紊乱毒性。
| [1] |
陈甫, 朱风华, 徐丹, 等. 2015年山东省肉鸡饲料原料中重金属污染情况调查及风险评估[J]. 动物营养学报, 2016, 28(10): 3175-3182. CHEN F, ZHU F H, XU D, et al. Investigation and risk assessment of heavy metal pollution of feedstuffs in broiler chickens in Shandong province in 2015[J]. Chinese Journal of Animal Nutrition, 2016, 28(10): 3175-3182 (in Chinese). DOI:10.3969/j.issn.1006-267x.2016.10.020 |
| [2] |
杨柳, 邢冠中, 张丽阳, 等. 河北省鸡主要饲料原料重金属污染分析[J]. 中国家禽, 2019, 41(20): 23-27. YANG L, XING G Z, ZHANG L Y, et al. Analysis on heavy metal pollution in main feedstuffs for chicken in Hebei province[J]. China Poultry, 2019, 41(20): 23-27 (in Chinese). |
| [3] |
LI J H, XING L, ZHANG R X. Effects of Se and Cd co-treatment on the morphology, oxidative stress, and ion concentrations in the ovaries of laying hens[J]. Biological Trace Element Research, 2018, 183(1): 156-163. DOI:10.1007/s12011-017-1125-9 |
| [4] |
QU K C, LI H Q, TANG K K, et al. Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles[J]. Biological Trace Element Research, 2020, 193(1): 234-240. DOI:10.1007/s12011-019-01682-x |
| [5] |
XU T, GAO X J, LIU G W. The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver[J]. Biological Trace Element Research, 2016, 169(2): 365-373. DOI:10.1007/s12011-015-0422-4 |
| [6] |
CAO H B, GAO F Y, XIA B, et al. The co-induced effects of molybdenum and cadmium on the mRNA expression of inflammatory cytokines and trace element contents in duck kidneys[J]. Ecotoxicology and Environmental Safety, 2016, 133: 157-163. DOI:10.1016/j.ecoenv.2016.07.007 |
| [7] |
ŞAHIN K, ŞAHIN N, KÜÇÜK O. Effects of dietary chromium picolinate supplementation on serum and tissue mineral contents of laying Japanese quails[J]. The Journal of Trace Elements in Experimental Medicine, 2002, 15(3): 163-169. DOI:10.1002/jtra.10013 |
| [8] |
TAO C, ZHANG B Y, WEI X T, et al. Effects of dietary cadmium supplementation on production performance, cadmium residue in eggs, and hepatic damage in laying hens[J]. Environmental Science and Pollution Research, 2020, 27(26): 33103-33111. DOI:10.1007/s11356-020-09496-4 |
| [9] |
KIM E, WICKRAMASURIYA S S, SHIN T K, et al. Bioaccumulation and toxicity studies of lead and mercury in laying hens: effects on laying performance, blood metabolites, egg quality and organ parameters[J]. The Journal of Poultry Science, 2019, 56(4): 277-284. DOI:10.2141/jpsa.0180118 |
| [10] |
HALLAK A K, BAYKOV B, KIROV K. Influence of lead and cadmium on productivity of laying-hens[C]//Proceedings of 13th International Congress in Animal Hygiene. Tartu: Estonia, 2007: 17-21.
|
| [11] |
JORDAN S A, BHATNAGAR M K. Hepatic enzyme activity after combined administration of methylmercury, lead and cadmium in the pekin duck[J]. Bulletin of Environmental Contamination and Toxicology, 1990, 44(4): 623-628. DOI:10.1007/BF01700886 |
| [12] |
ZHANG C, WANG L L, CAO C Y, et al. Selenium mitigates cadmium-induced crosstalk between autophagy and endoplasmic reticulum stress via regulating calcium homeostasis in avian leghorn male hepatoma (LMH) cells[J]. Environmental Pollution, 2020, 265(Pt A): 114613. |
| [13] |
JORDAN S A, BHATNAGAR M K, BETTGER W J. Combined effects of methylmercury, lead, and cadmium on hepatic metallothionein and metal concentrations in the pekin duck[J]. Archives of Environmental Contamination and Toxicology, 1990, 19(6): 886-891. DOI:10.1007/BF01055055 |
| [14] |
万惠愚. 不同剂量的硒对鸡铬中毒肾脏毒性影响的研究[D]. 硕士学位论文. 泰安: 山东农业大学, 2017. WAN H Y. Effect of various selenium doses on chromium-induced nephrotoxicity in chickens[D]. Master's Thesis. Tai'an: Shandong Agricultural University, 2017. (in Chinese) |
| [15] |
JIAO X Y, YANG K, AN Y, et al. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius[J]. Environmental Science and Pollution Research International, 2017, 24(8): 7555-7564. DOI:10.1007/s11356-016-8329-y |
| [16] |
JIHEN E H, IMED M, FATIMA H, et al. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation[J]. Food and Chemical Toxicology, 2008, 46(11): 3522-3527. DOI:10.1016/j.fct.2008.08.037 |
| [17] |
XING P C, XU S, WEI C, et al. Yeast selenium exerts an antioxidant effect by regulating the level of selenoprotein to antagonize Cd-induced pyroptosis of chicken liver[J/OL]. Biological Trace Element Research. (2020-10-30)[2021-11-10]. https://doi.org/10.1007/s12011-021-02984-9. DOI: 10.1007/s12011-021-02984-9.
|
| [18] |
罗成. 鸡蛋中镉、铬、铅快速检测技术研究及风险监测[D]. 硕士学位论文. 雅安: 四川农业大学, 2017. LUO C. Study on rapid detection and risk monitoring of cadmium, chromium, lead in the egg[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2017. (in Chinese) |
| [19] |
中华人民共和国国家卫生和计划生育委员会. 食品安全国家标准食品安全国家标准食品中总汞及有机汞的测定: GB 5009.17—2014[S]. 北京: 中国标准出版社, 2015. National Health and Family Planning Commission of the People's Republic of China. National food safety standard-determination of total mercury and organic mercury in food: GB 5009.17—2014[S]. Beijing: Standards Press of China, 2015. (in Chinese) |
| [20] |
王宏慧, 吴云芳, 杨喆, 等. 利用微波消解-火焰原子吸收法测定不同类型饲料中常量元素含量的可行性分析[J]. 中国饲料, 2019(19): 92-95. WANG H H, WU Y F, YANG Z, et al. Feasibility analysis report of microwave digestion-flame atomic absorption spectrometry for the determination of major elements in different types of feed stuffs[J]. China Feed, 2019(19): 92-95 (in Chinese). |
| [21] |
TAO C, WEI X T, ZHANG B Y, et al. Heavy metal content in feedstuffs and feeds in Hubei province, China[J]. Journal of Food Protection, 2020, 83(5): 762-766. DOI:10.4315/0362-028X.JFP-18-539 |
| [22] |
KABEER M S, HAMEED I, KASHIF S U R, et al. Contamination of heavy metals in poultry eggs: a study presenting relation between heavy metals in feed intake and eggs[J]. Archives of Environmental & Occupational Health, 2021, 76(4): 220-232. |
| [23] |
佚名. GB 13078—2017饲料卫生标准[J]. 饲料与畜牧, 2018(1): 16-24. Anon. GB 13078—2017 hygienic standard for feed[J]. Feed and Husbandry, 2018(1): 16-24 (in Chinese). |
| [24] |
中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 饲料中硒的允许量: GB 26418—2010[S]. 北京: 中国标准出版社, 2011. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. Limited content of selenium in feeds: GB 26418—2010[S]. Beijing: Standards Press of China, 2011. (in Chinese) |
| [25] |
ARUN K B, MADHAVAN A, SINDHU R, et al. Probiotics and gut microbiome-prospects and challenges in remediating heavy metal toxicity[J]. Journal of Hazardous Materials, 2021, 420: 126676. DOI:10.1016/j.jhazmat.2021.126676 |
| [26] |
TSURUOKA S, SUGIMOTO K, MUTO S, et al. Acute effect of cadmium-metallothionein on glucose and amino acid transport across the apical membrane of the rabbit proximal tubule perfused in vitro[J]. The Journal of Pharmacology and Experimental Therapeutics, 2000, 292(2): 769-777. |
| [27] |
杨晓刚, 余东游, 许梓荣. 动物铅毒性研究进展[J]. 中国畜牧杂志, 2006(19): 57-59. YANG X G, YU D Y, XU Z R. Advances in lead toxicity in animals[J]. Chinese Journal of Animal Science, 2006(19): 57-59 (in Chinese). DOI:10.3969/j.issn.0258-7033.2006.19.020 |
| [28] |
MOURO V G S, LADEIRA L C M, LOZI A A, et al. Different routes of administration lead to different oxidative damage and tissue disorganization levels on the subacute cadmium toxicity in the liver[J]. Biological Trace Element Research, 2021, 199(12): 4624-4634. DOI:10.1007/s12011-020-02570-5 |
| [29] |
DJURIC A, BEGIC A, GOBELJIC B, et al. Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium[J]. Food and Chemical Toxicology, 2015, 86: 25-33. DOI:10.1016/j.fct.2015.09.004 |
| [30] |
ARMBRECHT H J, WONGSURAWAT N, ZENSER T V, et al. In vitro modulation of renal 25-hydroxyvitamin D3 metabolism by vitamin D3 metabolites and calcium[J]. Archives of Biochemistry and Biophysics, 1983, 220(1): 52-59. DOI:10.1016/0003-9861(83)90386-7 |
| [31] |
ZHAO D, JUHASZ A L, LUO J, et al. Mineral dietary supplement to decrease cadmium relative bioavailability in rice based on a mouse bioassay[J]. Environmental Science & Technology, 2017, 51(21): 12123-12130. |
| [32] |
LIU Y H, HAO P, ZHANG X, et al. Effects of excess Cr3+ on trace element contents in the brain and serum in chicken[J]. Biological Trace Element Research, 2017, 177(1): 180-186. DOI:10.1007/s12011-016-0875-0 |
| [33] |
ABDULLA M, CHMIELNICKA J. New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals[J]. Biological Trace Element Research, 1989, 23(1): 25-53. DOI:10.1007/BF02917176 |
| [34] |
SUGANYA T, SENTHILKUMAR S, DEEPA K, et al. Metal toxicosis in poultry-a review[J]. International Journal of Science, Environment and Technology, 2016, 5(2): 515-524. |
| [35] |
ABBAS M, CHAND N, KHAN R U, et al. Public health risk of heavy metal residues in meat and edible organs of broiler in an intensive production system of a region in Pakistan[J]. Environmental Science and Pollution Research, 2019, 26(22): 23002-23009. DOI:10.1007/s11356-019-05639-4 |
| [36] |
MARTELLI A, ROUSSELET E, DYCKE C, et al. Cadmium toxicity in animal cells by interference with essential metals[J]. Biochimie, 2006, 88(11): 1807-1814. DOI:10.1016/j.biochi.2006.05.013 |
| [37] |
MARETTOVÁ E, MARETTA M, LEGÁTH J, et al. The retention of cadmium and selenium influence in fowl and chickens of F1 generation[J]. Biological Trace Element Research, 2012, 147(1/2/3): 130-134. |
| [38] |
WHO Expert Committee on Health Effects of Combined Exposures in the Work Environment & World Health Organization. Health effects of combined exposures in the work environment: report of a WHO expert committee [meeting held in Geneva from 9 to 15 December 1980]: World Health Organization technical report series 662[R]. Geneva: World Health Organization, 1981: 1-76.
|
| [39] |
秦卫红, 王倩倩, 陈大伟. 砷镉联合暴露对蛋鸡肝脏功能的影响及硫辛酸保护效应研究[J]. 安徽农业科学, 2018, 46(33): 58-60. QIN W H, WANG Q Q, CHEN D W. Effects of combined exposure of arsenic and cadmium on the liver functions of laying hens and the protective effect of α-lipoic acid[J]. Journal of Anhui Agricultural Sciences, 2018, 46(33): 58-60 (in Chinese). DOI:10.3969/j.issn.0517-6611.2018.33.019 |
| [40] |
ZHANG R X, XING L, BAO J, et al. Selenium supplementation can protect from enhanced risk of keel bone damage in laying hens exposed to cadmium[J]. RSC Advances, 2017, 7(12): 7170-7178. DOI:10.1039/C6RA26614B |
| [41] |
XIONG X Y, ZHANG Y, XING H J, et al. Ameliorative effect of selenomethionine on cadmium-induced hepatocyte apoptosis via regulating PI3K/AKT pathway in chickens[J]. Biological Trace Element Research, 2020, 195(2): 559-568. DOI:10.1007/s12011-019-01858-5 |
| [42] |
CHEN C Y, YU H W, ZHAO J J, et al. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure[J]. Environmental Health Perspectives, 2006, 114(2): 297-301. DOI:10.1289/ehp.7861 |
| [43] |
鞠耿越. 不同硒源和硒水平对仔鹅生产性能、抗氧化性能和组织微量元素含量的影响[D]. 硕士学位论文. 扬州: 扬州大学, 2019. JU G Y. Effects of different selenium sources and levels on production performance, antioxidant capacity and tissue trace elements content in goose[D]. Master's Thesis. Yangzhou: Yangzhou University, 2019. (in Chinese) |




