2. 河南农业职业学院牧业工程学院, 郑州 451450
2. College of Animal Husbandry Engineering, Henan Vocational College of Agriculture, Zhengzhou 451450, China
开食料的物理形态及其中谷物饲料的加工方式是影响幼龄反刍动物瘤胃发育的重要因素,受到人们的广泛关注。在犊牛上的相关研究表明,将开食料中的玉米进行蒸汽压片处理能够影响开食料的适口性和犊牛的采食量[1],进而影响犊牛瘤胃的发酵和发育[2];与粉碎[3-4]、压碎[1]和颗粒化[5]开食料相比,将开食料中的玉米进行蒸汽压片处理更有利于犊牛生长性能的提高和瘤胃发酵[6];同时,饲粮的物理形态和化学组成能够显著影响犊牛的瘤胃微生物组成[7]。在羔羊上也有类似研究,但相对较少。Karimizadeh等[8]研究表明,相对于磨碎或颗粒化开食料,采食块状饲料的育肥绵羊表现出较好的养分消化、瘤胃发酵和生长性能。而Mirmohammadi等[9]的研究发现,与块状饲料相比,磨碎饲料组生长肥育羊的生长效率更高。Abouheif等[10]的研究表明,与较短(9.5 mm)苜蓿干草相比,在饲粮中添加较长(14 mm)的苜蓿干草可提高生长肥育羊的中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)消化率,延长反刍时间。但有关开食料中玉米加工方式对羔羊早期断奶前和断奶后瘤胃发酵和微生物区系影响的研究报道较少。因此,本试验设计2种玉米加工方式的开食料,从瘤胃发酵和微生物区系2个方面研究开食料中玉米加工方式对羔羊瘤胃早期发育的影响,为肉羊生产中开食料的研发和应用提供理论依据。
1 材料与方法 1.1 试验设计与试验动物选择初生重相近的24只8日龄湖羊公羔[平均体重(5.04±0.75) kg,购于甘肃省白银市康瑞种羊养殖公司],随机分为2组,每组12只,分别饲喂颗粒化开食料(PS组,玉米及其他原料全部粉碎后制粒)和蒸汽压片玉米开食料(SFS组,玉米进行蒸汽压片处理后破碎,其他原料粉碎后制粒,然后将破碎的蒸汽压片玉米与其他原料制成的颗粒料混合后饲喂)。
1.2 开食料的制备参照NRC(2007)标准,设计羔羊开食料配方(表 1),由甘肃傲农饲料科技有限公司(武威)生产加工。其中,颗粒化开食料是将各种饲料原料粉碎后环模制粒(温度84~86 ℃,4.5 mm,压缩比1∶5,直径6 mm);蒸汽压片玉米开食料是将玉米蒸汽压片、破碎(直径4~5 mm),其他原料粉碎后制粒(工艺及参数同颗粒化开食料),然后将二者按比例混合而成。
|
|
表 1 开食料组成及营养水平(饲喂基础) Table 1 Composition and nutrient levels of starter feeds (as-fed basis) |
羔羊1~7日龄随母羊哺乳。8日龄与母羊分开,单栏(0.8 m×1.3 m)饲养,按体重的2%在08:00、14:00、20:00分3次等量饲喂代乳粉(购于北京精准动物营养研究中心,其营养成分含量:粗蛋白质23%,粗脂肪12%,粗纤维3%,钙0.6%~1.5%,食盐0.1%~1.2%,赖氨酸2.2%,蛋氨酸1%,苏氨酸1%,维生素E 80 IU,维生素A 13 000~50 000 IU,维生素D 3 000~10 000 IU),同时开始饲喂开食料,自由采食和饮水。35日龄断代乳粉,42日龄试验结束。试验期间,每天早晨清理羊栏内卫生,每2周消毒1次,保证羊舍干燥、清洁。
1.4 样品采集与制备分别于21日龄(断奶前)、42日龄(断奶后),每组随机选择6只羔羊,屠宰,采集瘤胃内容物,混匀,1份用4层纱布过滤,将滤液分装于10 mL无菌离心管中,-20 ℃保存,用于测定瘤胃发酵参数;1份装入EP管,投入液氮速冻,-80 ℃保存,用于测定瘤胃微生物。
1.5 测定指标及方法 1.5.1 瘤胃液挥发性脂肪酸含量采用气相色谱法[11]测定瘤胃液挥发性脂肪酸含量。取约10 mL瘤胃液,在4 ℃下10 000~120 00×g离心10 min,取上清液,加入25%偏磷酸(200 μL/mL挥发性脂肪酸样品),如前法再次离心,取上清液,采用气相色谱仪(AI 3000, Thermo公司,德国)测瘤胃液挥发性脂肪酸含量。进样量:0.6 μL,色谱柱:AE-FFAP毛细管色谱柱(30 m×0.25 mm×0.33 μm,兰州中科安泰分析科技有限公司)。色谱条件:进样口温度200 ℃,氮气(N2)流量2.0 mL/min,分流比40∶1,程序升温模式(120 ℃ 3 min,10 ℃/min升至180 ℃,保持1 min),检测器250 ℃,氢火焰离子化检测器(FID)空气、氢气(H2)和N2流量分别为450、40和45 mL/min,柱箱升温程序为以20 ℃/min由45 ℃升至150 ℃,保持5 min。
1.5.2 瘤胃液氨态氮含量瘤胃液氨态氮含量采用比色法[12]测定。
1.5.3 瘤胃微生物区系瘤胃微生物区系测定在北京诺禾致源科技股份有限公司(中国)完成。因贮存过程中有1个样品损坏,每组均取5个样品进行测定。
1.5.3.1 基因组DNA提取和PCR扩增采用CTAB方法提取样本中基因组DNA,用琼脂糖凝胶电泳检测DNA的纯度和浓度,然后取适量的样本DNA于离心管中,用无菌水稀释样本至1 ng/μL。基于稀释后的基因组DNA模板,根据测序区域(16S V4区),选择使用带Barcode的特异引物(515F:5′-GTGCCAGCMGCCGCGGTAA-3′;806R:5′-GGACTACHVGGGTWTCTAAT-3′),采用Phusion® High-Fidelity PCR Master Mix with GC Buffer(New England Biolabs公司)及高效高保真酶进行PCR扩增。PCR总反应体系为37.5 μL:2×Taq PCR mix 25 μL,上、下游引物(10 μmol/L)各1 μL,gDNA 2.5 μL,dH2O 8.0 μL。PCR反应程序:95 ℃预变性5 min;94 ℃变性1 min,57 ℃退火45 s,72 ℃延伸1 min,共34个循环;72 ℃延伸10 min。PCR扩增产物采用2%的琼脂糖凝胶进行电泳检测。回收产物,使用TruSeq® DNA PCR-Free Sample Preparation Kit建库试剂盒进行文库构建,构建好的文库经过Qubit和Q-PCR定量,文库合格后,用于构建测序文库。
1.5.3.2 16S rDNA PCR产物高通量测序及分析建库后使用HiSeq2500 PE250进行上机测序。原始数据进行过滤和去除嵌合体等处理后,对优质序列按相似度97%进行操作分类单元(OTUs)聚类分析。对OTUs列表中的各样品微生物进行α多样性和β多样性分析,对微生物的种类和结构进行注释。采用LEfSe分析2组羔羊瘤胃中相对丰度存在显著差异的物种。
1.5.3.3 微生物功能预测分析采用Tax4Fun方法[13],基于16S Silva数据库对瘤胃微生物区系的功能进行预测,具体如下:首先提取KEGG数据库中原核生物全部基因组16S rRNA序列,然后利用BLASTN算法,在SILVA SSU Ref NR数据库中(BLAST bitscore>1 500)与其比对,建立相关矩阵,再通过2种方法(UProC和PAUDA),将注释到KEGG数据库中的原核生物全部基因组功能信息与SILVA数据库中基因组功能信息进行比对,从而达到SILVA数据库功能注释的目的。
1.6 统计分析采用SPSS 20.0统计软件对数据进行t检验,以P<0.05作为差异显著标准。
2 结果与分析 2.1 瘤胃发酵参数由表 2可知,与PS组相比,SFS组羔羊断奶前和断奶后瘤胃液中总挥发性脂肪酸、丙酸、丁酸含量均显著提高(P<0.05),断奶前分别提高了23.06%、35.58%、56.44%,断奶后分别提高了7.82%,28.87%,32.47%;其他指标不受开食料中玉米加工方式的影响(P>0.05)。
|
|
表 2 羔羊瘤胃发酵参数 Table 2 Rumen fermentation parameters of lambs |
由表 3可知,开食料中玉米加工方式对羔羊断奶前和断奶后瘤胃微生物α多样性各项指标均无显著影响(P>0.05)。
|
|
表 3 瘤胃微生物α多样性 Table 3 α diversity of rumen microbes |
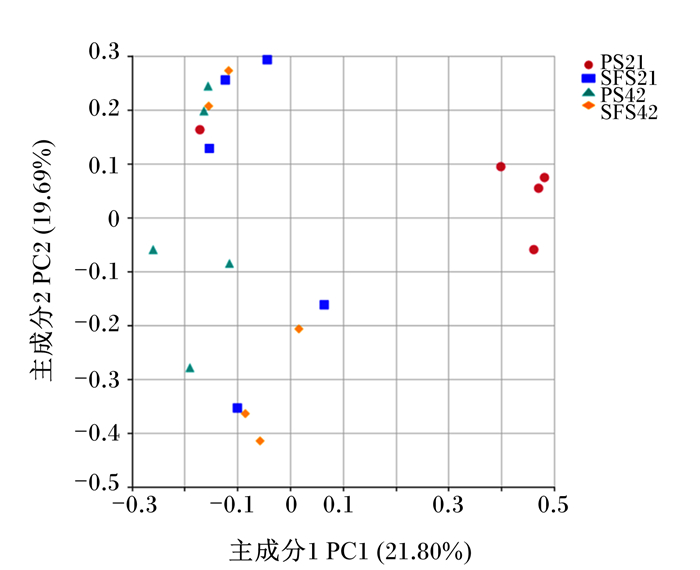
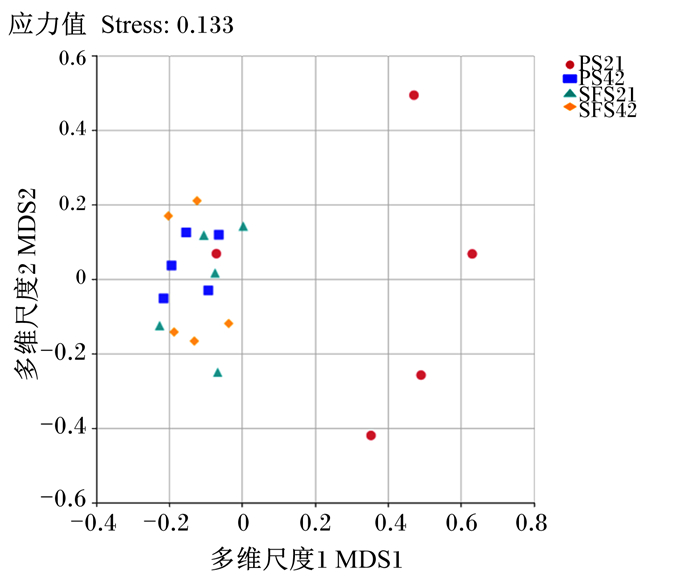
由微生物OTUs的主坐标分析(PCoA,图 1)可以看出,主成分1(PC1)和主成分2(PC2)对样本间变异的贡献率分别为21.80%和19.69%,可以充分反映样本间的差异;PCoA结果显示,组内样品距离较近。非度量多维尺度(NMDS,图 2)结果显示,Stress值(0.133)小于0.2,说明能够准确表示这些数据;PS组断奶前羔羊多数样本与其他3组样本远远分开,反映出组间菌群结构和多样性存在差异。
|
PS21:PS组断奶前;SFS21:SFS组断奶前;PS42:PS组断奶后;SFS42:SFS组断奶后。下图同。 PS21: pre-weaned for PS group; SFS21: pre-weaned for SFS group; PS42: pre-weaned for PS group; SFS42: pre-weaned for SFS group. The same as below. 图 1 瘤胃微生物OTUs主坐标分析 Fig. 1 PCoA of rumen microbial OTUs |
|
图 2 瘤胃微生物OTUs非度量多维尺度分析 Fig. 2 Non-metric multi-dimensional scaling analysis of rumen microbial OTUs |
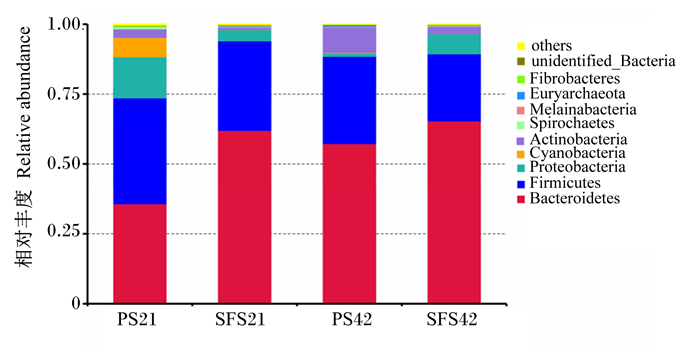
由图 3可知,2组羔羊断奶前和断奶后瘤胃中的优势菌门均为拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes),相对丰度均大于24%。SFS组拟杆菌门的相对丰度(61.96%)在断奶前就已超过厚壁菌门(32.08%)和变形菌门(Proteobacteria,3.99%),成为第一优势菌门,且接近断奶后水平(65.36%);而PS组拟杆菌门的相对丰度(57.28%)在断奶后才超过厚壁菌门(31.21%)和变形菌门(1.15%),且仍低于断奶前和断奶后SFS组拟杆菌门的相对丰度(61.96%和65.36%)。
|
Bacteroidetes:拟杆菌门;Firmicutes:厚壁菌门;Proteobacteria:变形菌门;Cyanobacteria:蓝藻菌门;Actinobacteria:放线菌门;Spirochaetes: 螺旋体门;Melainabacteria:黑水仙菌门;Euryarchaeota:广古菌门;Fibrobacteres: 纤维杆菌门;unidentified_Bacteria: 未鉴定细菌;others: 其他。 图 3 羔羊瘤胃内容物中前10种微生物门水平组成 Fig. 3 Phylum-level composition of top 10 microbes in rumen content of lambs |
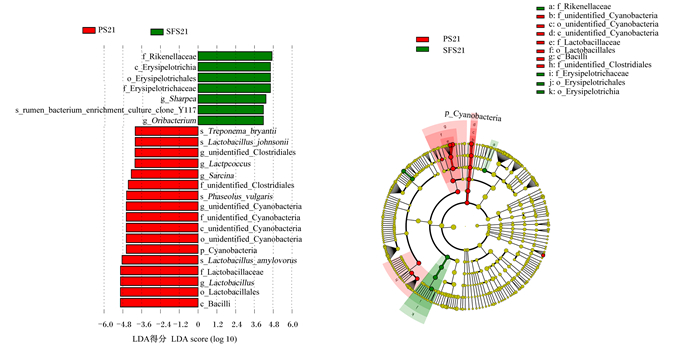
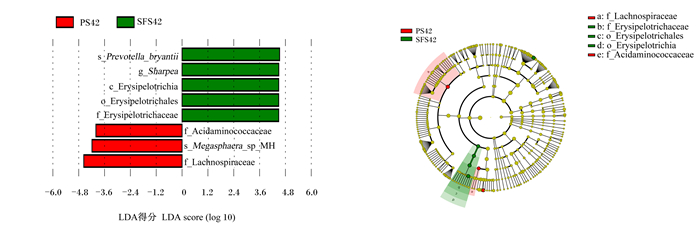
以LDA>4为判定标准,筛选断奶前、后相对丰度存在显著差异的物种。在属水平上,断奶前(图 4),SFS组梭菌目未鉴定属(unidentified_Clostridiales)、乳球菌属(Lactpcoccus)、乳杆菌属(Lactobacillus)、八叠球菌属(Sarcina)和蓝藻菌门未鉴定属(unidentified_Cyanobacteria)的相对丰度较PS组显著降低(P<0.05),夏普氏菌属(Sharpea)和原细菌属(Oribacterium)的相对丰度则较PS组显著增加(P<0.05);断奶后(图 5),SFS组夏普氏菌属的相对丰度较PS组显著增加(P<0.05)。
|
f_Rikenellaceae:理研菌科;c_Erysipelotrichia:丹毒杆菌纲;o_Erysipelotrichales:丹毒菌目;f_Erysipelotrichaceae:丹毒菌科;g_Sharpea:夏普氏菌属;s_rumen_bacterium_enrichment_culture_clone_Y117:瘤胃细菌富集培养克隆Y117;g_Oribacterium:原细菌属;s_Treponema_bryantii:布氏密螺旋体;s_Lactobacillus_johnsonii:约翰逊乳杆菌种;g_unidentified_Clostridiales:梭菌目未鉴定属;g_Lactpcoccus:乳球菌属:g_Sarcina:八叠球菌属;f_unidentified_Clostridiales:梭菌目未鉴定科;s_Phaseolus_vulgaris:菜豆;g_unidentified_Cyanobacteria:蓝藻菌门未鉴定属;f_unidentified_Cyanobacteria:蓝藻菌门未鉴定科;c_unidentified_Cyanobacteria:蓝藻菌门未鉴定纲;o_unidentified_Cyanobacteria:蓝藻菌门未鉴定目;p_Cyanobacteria:蓝藻菌门;s_Lactobacillus_amylovorus:淀粉乳杆菌种;f_Lactobacillaceae:乳酸杆菌科;g_Lactobacillus:乳酸杆菌属;o_Lactobacillales:乳酸杆菌目;c_Bacilli:杆菌纲。 图 4 断奶前PS组与SFS组羔羊瘤胃微生物区系线性判别分析及效应值 Fig. 4 Linear discriminant analysis and effect size on rumen microflora of pre-weaned lambs in PS and SFS groups |
|
s_Prevotella_bryantii:布氏普氏菌种;g_Sharpea:夏普氏菌属;c_Erysipelotrichia:丹毒杆菌纲;o_Erysipelotrichales:丹毒菌目;f_Erysipelotrichaceae丹毒菌科;f_Acidaminococcaceae:酸氨基球菌科;s_Megasphaera_sp_MH:巨球型菌种;f_Lachnospiraceae:毛螺菌科。 图 5 断奶后PS组与SFS组羔羊瘤胃微生物区系线性判别分析及效应值 Fig. 5 Linear discriminant analysis and effect size on rumen microflora of post-weaned lambs in PS and SFS groups |
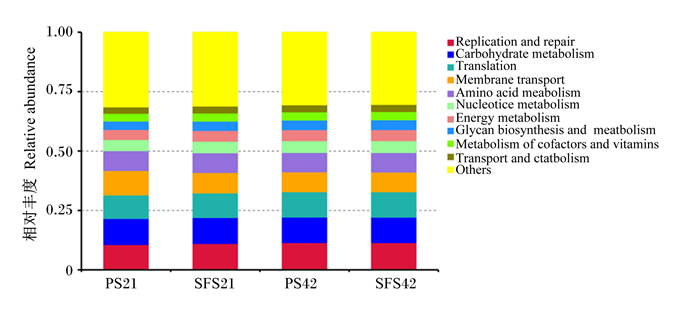
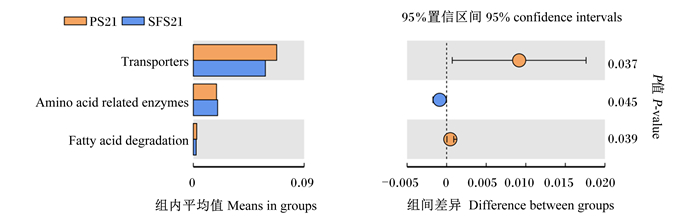
在KEGG水平2中,与PS组相比,羔羊断奶前和断奶后SFS组均无显著富集的功能基因(P>0.05,图 6)。仅在KEGG水平3中,羔羊断奶前SFS组与酶相关的氨基酸代谢的功能基因表达量显著高于PS组(P<0.05),转运因子、脂肪酸降解代谢的功能基因表达量显著低于PS组(P<0.05,图 7)。
|
Replication and repair:复制与修复;Carbohydrate metabolism:碳水化合物代谢;Translation:翻译;Membrane transport:膜转运;Amino acid metabolism:氨基酸代谢;Nucleotide metabolism:核苷酸代谢:Energy metabolism:能量代谢;Glycan biosynthesis and metabolism:聚糖生物合成和代谢;Metabolism of cofactors and vitamins:辅助因子和维生素的代谢;Transport and catabolism:运输和分解代谢;Others:其他。 图 6 断奶前和断奶后PS组与SFS组羔羊瘤胃微生物KEGG水平2功能预测 Fig. 6 Functional prediction of rumen microflora in level 2 of KEGG for pre-weaning and post-weaned lambs in PS and SFS groups |
|
Transporters:转运因子;Amino acid related enzymes:与酶相关的氨基酸代谢;Fatty acid degradation:脂肪酸降解代谢。 图 7 断奶前PS组与SFS组羔羊瘤胃微生物KEGG水平3功能预测 Fig. 7 Functional prediction of rumen microflora in level 3 of KEGG for pre-weaning lambs in PS and SFS groups |
瘤胃液中挥发性脂肪酸是碳水化合物的主要发酵产物,包括乙酸、丙酸和丁酸等,是反刍动物的主要能源。另外,挥发性脂肪酸也是瘤胃上皮生长发育的主要促进因子,且丁酸的刺激作用最强[14]。本试验中,SFS组羔羊瘤胃液中总挥发性脂肪酸含量在断奶前和断奶后均显著高于PS组,表明蒸汽压片玉米开食料更有利于羔羊的瘤胃发酵,原因可能是此开食料中玉米的蒸汽压片处理导致玉米的物理性状和化学成分均发生了改变。蒸汽压片玉米是对玉米进行高温和高湿处理,其胚芽中淀粉颗粒周围的胚层和蛋白基质均遭到破坏,导致淀粉颗粒中的半结晶结构破坏[15],淀粉糊化度提高(本实验室测定发现玉米蒸汽压片处理后的淀粉糊化度提高了556%),淀粉分子的结晶结构扩展,表面积增加,与酶的接触面积增大[16],从而更易被消化,产生更多的挥发性脂肪酸。SFS组羔羊瘤胃液中丙酸含量高于PS组的结果同时也说明,该开食料更有利于饲料的能量利用,从而有利于肉用反刍动物的育肥。本实验室同期试验中SFS羔羊的采食量、生长速度、体尺和体重比PS组显著提高的结果[17-18]证明了这一结论。而SFS组羔羊丁酸含量显著升高的结果也从物理加工方式不同所导致的瘤胃中发酵产物变化的角度解释了蒸汽压片玉米开食料为什么更有利于瘤胃的发育,这与本实验室同期试验结果中瘤胃重量和组织形态学的结果(SFS组的瘤胃相对重量、乳头高度、乳头宽度和肌层厚度等均显著优于PS组)[17]一致,从而促进了羔羊对养分的消化和生长性能的发挥[17-18]。犊牛的相关研究也得到了类似结果。Lesmeister等[19]报道,与整粒、干压碎和烘烤压碎玉米相比,饲喂包含蒸汽压片玉米的开食料的犊牛有较高的血液总挥发性脂肪酸和瘤胃液丙酸含量。Pavlata等[20]研究发现,与颗粒化开食料相比,饲喂蒸汽压片玉米开食料犊牛有较高的瘤胃液总挥发性脂肪酸、乙酸和丙酸含量。这些结果表明,包含蒸汽压片玉米的开食料含有较多易发酵碳水化合物,有利于幼龄反刍动物的瘤胃发育。
瘤胃液氨氮含量可在一定程度上反映瘤胃微生物分解含氮物质产生氨氮的速度以及其对氨的摄取利用情况,同时反映出在特定饲粮组成下蛋白质降解与合成之间的平衡关系。瘤胃微生物生长所需的最佳氨氮含量为6.3~27.5 mg/dL[21]。本试验中,羔羊瘤胃液氨氮含量未受开食料中玉米加工方式的显著影响,且均处于瘤胃微生物生长所需要的最佳氨氮含量范围之内,表明开食料中玉米加工方式不影响早期断奶前和断奶后羔羊瘤胃微生物对含氮化合物的降解,其可能原因是2组开食料的配方和营养成分相同,区别仅在玉米的加工方式,而玉米的蒸汽压片处理主要造成了碳水化合物中淀粉的变化,对含氮物质的影响不明显。其他相关研究也得出了类似结果,例如,Beharka等[22]和Pazoki等[6]研究表明,采食颗粒化和蒸汽压片玉米开食料的犊牛的瘤胃液氨氮含量无显著差异;嘎尔迪等[23]研究也发现,玉米通过制粒、烘烤和蒸汽加工处理后,不会显著影响绵羊瘤胃内容物中的氨氮含量。
微生物α多样性指标中,OTUs反映了聚类多少,Chao1指数、ACE指数、Shannon指数和Simpson指数用于评估微生物群落的丰度和多样性,后四者中,Chao1指数和Ace指数反映微生物群落的相对丰度,二者的数值越大,群落丰富度越高;而Simpson指数和Shannon指数则反映了微生物群落的多样性,Shannon指数越大,群落多样性越高;Simpson指数越小,群落多样性越高[24]。本试验中,2组羔羊瘤胃微生物α多样性指标在断奶前和断奶后均无显著差异,该结果表明开食料中的玉米加工方式不影响羔羊瘤胃微生物的种类、丰度和多样性。
在反刍动物瘤胃微生物区系中,拟杆菌门和厚壁菌门是两大优势菌群[25]。拟杆菌门在饲粮淀粉和蛋白质的降解、微生物蛋白的合成以及肽和氨基酸的吸收中发挥重要作用[26]。而厚壁菌门包含很多可分解纤维的菌属,如瘤胃球菌属(Ruminococciis)、真细菌属(Eubacterium)、假丁酸弧菌属(Pseudobutyrivibrio)、丁酸弧菌属(Butyvibro)和颤螺旋菌属(Oscillibacter)等,其主要功能是分解纤维素[27]。Jiang等[28]比较研究了发酵与未发酵玉米蛋白粉对断奶前犊牛瘤胃微生物组成的影响,发现发酵玉米蛋白粉组的拟杆菌门相对丰度显著高于未发酵组,而厚壁菌门相对丰度则刚好相反。
本试验中,SFS组羔羊断奶前和断奶后瘤胃微生物中拟杆菌门的相对丰度显著高于PS组、且SFS组拟杆菌门在断奶前就已成为第一优势菌门,该结果说明开食料中的玉米加工方式影响了羔羊早期断奶前和断奶后门水平上的瘤胃微生物群落结构,蒸汽压片玉米开食料更有利于羔羊尽早建立优势的拟杆菌门。其可能原因是蒸汽压片玉米开食料中的玉米经过蒸汽压片处理提高了淀粉的糊化度,含有较多易发酵碳水化合物,从而促进了瘤胃拟杆菌门的增殖和发酵。这与瘤胃发酵参数中总挥发性脂肪酸和丙酸含量在SFS组显著高于PS组的结果一致。瘤胃中夏普氏菌属的主要发酵产物是乳酸,乳酸的形成可进一步促进夏普氏菌属的发酵,且促使乳酸向丁酸转变[29]。本试验中,SFS组显著增加了断奶前和断奶后瘤胃微生物中夏普氏菌属的相对丰度,与瘤胃液丁酸含量在SFS组显著高于PS组的试验结果一致。这也从微生物学角度揭示了蒸汽压片玉米开食料为什么比颗粒化开食料更有助于瘤胃的发育、瘤胃中酶的产生和活性的提高、养分的消化和羔羊生长性能的发挥[17-18]。
瘤胃微生物区系是微生物共生联合体,它既是反刍动物中重要的蛋白质来源,也是反刍动物通过发酵产生挥发性脂肪酸的主要能量来源。因此,利用瘤胃微生物区系这个庞大的生物资源库,积极挖掘一些与重要营养生理功能密切相关的瘤胃菌群功能是非常有必要的,如碳水化合物转运及代谢、氨基酸运输和代谢、挥发性脂肪酸生成等。Abhauer等[13]证实蛋白质和碳水化合物代谢是微生物生存必需的一项基本功能。本试验中,SFS组比PS组在断奶前KEGG水平3中显著增加了与酶相关的氨基酸代谢的功能基因表达量,同时降低了转运因子、脂肪酸降解代谢的功能基因表达量的结果说明,SFS组羔羊断奶前瘤胃氨基酸和脂肪酸代谢功能较好,从而促进瘤胃发酵,产生更多挥发性脂肪酸,这与SFS组瘤胃液总挥发性脂肪酸含量提高、拟杆菌门和夏普氏菌属的相对丰度增加的结果一致。
4 结论开食料中玉米加工方式影响了羔羊早期断奶前和断奶后的瘤胃发酵和微生物区系。相对于颗粒化开食料,蒸汽压片玉米开食料通过提高羔羊断奶前和断奶后瘤胃液总挥发性脂肪酸、丙酸和丁酸含量,促进瘤胃中优势菌群拟杆菌门的及早建立和增加夏普氏菌属、原细菌属的相对丰度等促进早期断奶羔羊的瘤胃发育。
| [1] |
NEJAD J G, TORBATINEJAD N, NASERIAN A A, et al. Effects of processing of starter diets on performance, nutrient digestibility, rumen biochemical parameters and body measurements of brown Swiss dairy calves[J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(7): 980-987. DOI:10.5713/ajas.2011.11457 |
| [2] |
MIRZAEI M, KHORVASH M, GHORBANI G R, et al. Interactions between the physical form of starter (mashed versus textured) and corn silage provision on performance, rumen fermentation, and structural growth of Holstein calves[J]. Journal of Animal Science, 2016, 94(2): 678-686. DOI:10.2527/jas.2015-9670 |
| [3] |
FRANKLIN S T, AMARAL-PHILLIPS D M, JACKSON J A, et al. Health and performance of Holstein calves that suckled or were hand-fed colostrum and were fed one of three physical forms of starter[J]. Journal of Dairy Science, 2003, 86(6): 2145-2153. DOI:10.3168/jds.S0022-0302(03)73804-1 |
| [4] |
HILL T M, BATEMAN Ⅱ H G, ALDRICH P J M, et al. High-starch, coarse-grain, low-fiber diets maximize growth of weaned dairy calves less than 4 months of age[J]. The Professional Animal Scientist, 2012, 28: 325-331. DOI:10.15232/S1080-7446(15)30363-6 |
| [5] |
MOEINI H, MAHDAVI A H, RIASI A, et al. Effects of physical form of starter and forage provision to young calves on blood metabolites, liver composition and intestinal morphology[J]. Journal of Animal Physiology and Animal Nutrition, 2017, 101(4): 755-766. DOI:10.1111/jpn.12485 |
| [6] |
PAZOKI A, GHORBANI G R, KARGAR S, et al. Growth performance, nutrient digestibility, ruminal fermentation, and rumen development of calves during transition from liquid to solid feed: effects of physical form of starter feed and forage provision[J]. Animal Feed Science and Technology, 2017, 234: 173-185. DOI:10.1016/j.anifeedsci.2017.06.004 |
| [7] |
DE MENEZES A B, LEWIS E, O'DONOVAN M, et al. Microbiome analysis of dairy cows fed pasture or total mixed ration diets[J]. FEMS Microbiology Ecology, 2011, 78(2): 256-265. DOI:10.1111/j.1574-6941.2011.01151.x |
| [8] |
KARIMIZADEH E, CHAJI M, MOHAMMADABADI T. Effects of physical form of diet on nutrient digestibility, rumen fermentation, rumination, growth performance and protozoa population of finishing lambs[J]. Animal Nutrition, 2017, 3(2): 139-144. DOI:10.1016/j.aninu.2017.01.004 |
| [9] |
MIRMOHAMMADI D, ROUZBEHAN Y, FAZAELI H. The effect of the inclusion of recycled poultry bedding and the physical form of diet on the performance, ruminal fermentation, and plasma metabolites of fattening lambs[J]. Journal of Animal Science, 2015, 93(8): 3843-3853. DOI:10.2527/jas.2014-8789 |
| [10] |
ABOUHEIF M A, AL-SAIADY M Y, AL-MUFARREJ S I, et al. Effect of physical form of diet and frequency of feeding on digesta retention time and digestion in Najdi lambs[J]. Journal of Animal and Veterinary Advances, 2012, 11(11): 1774-1779. DOI:10.3923/javaa.2012.1774.1779 |
| [11] |
刘婷, 李发弟, 王维民, 等. 不同日龄补饲开食料对湖羊羔羊瘤胃形态及表皮生长相关基因表达的影响[J]. 畜牧兽医学报, 2016, 47(12): 2441-2449. LIU T, LI F D, WANG W M, et al. Effects of starter feeding on rumen papilla genes expression involved in cellular growth and morphology in Hu lamb at different ages[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(12): 2441-2449 (in Chinese). DOI:10.11843/j.issn.0366-6964.2016.12.014 |
| [12] |
冯宗慈, 高民. 通过比色测定瘤胃液氨氮含量方法的改进[J]. 畜牧与饲料科学, 2010(6): 37. FENG Z C, GAO M. An improved method for determination of ammonia nitrogen in rumen liquid by colorimetry[J]. Animal Husbandry and Feed Science, 2010(6): 37 (in Chinese). DOI:10.3969/j.issn.1672-5190.2010.06.015 |
| [13] |
AΒHAUER K P, WEMHEUER B, DANIEL R, et al. Tax4fun: predicting functional profiles from metagenomic 16S rRNA data[J]. Bioinformatics, 2015, 31(17): 2882-2884. DOI:10.1093/bioinformatics/btv287 |
| [14] |
TAMATE H, MCGILLIARD A D, JACOBSON N L, et al. Effect of various dietaries on the anatomical development of the stomach in the calf[J]. Journal of Dairy Science, 1962, 45(3): 408-420. DOI:10.3168/jds.S0022-0302(62)89406-5 |
| [15] |
BATEMAN H G 2ND, HILL T M, ALDRICH J M, et al. Effects of corn processing, particle size, and diet form on performance of calves in bedded pens[J]. Journal of Dairy Science, 2009, 92(2): 782-789. DOI:10.3168/jds.2008-1242 |
| [16] |
阿米娜木·司马义. 口感化和颗粒化开食料对荷斯坦公犊牛生长性能及胃肠道发育的影响[D]. 硕士学位论文. 乌鲁木齐: 新疆农业大学, 2014. SIMAYI A M N M. Effects of texturized and pelleted starter on growth characteristics and gastrointestinal development in Holstein male calves[D]. Master's Thesis. Urumqi: Xinjiang Agricultural University, 2014. (in Chinese) |
| [17] |
LI Y, GUO Y L, ZHANG C X, et al. Effects of physical forms of starter feed on growth, nutrient digestibility, gastrointestinal enzyme activity, and morphology of pre- and post-weaning lambs[J]. Animal, 2021, 15(1): 100044. DOI:10.1016/j.animal.2020.100044 |
| [18] |
蔡小芳, 张成新, 李勇, 等. 口感化及颗粒化开食料对早期断奶羔羊生长和胃肠道发育的影响[J]. 草业科学, 2021, 38(8): 1596-1604. CAI X F, ZHANG C X, LI Y, et al. Effect of texturized and pelleted starter on growth and gastrointestinal development of early weaning lambs[J]. Pratacultural Science, 2021, 38(8): 1596-1604 (in Chinese). |
| [19] |
LESMEISTER K E, HEINRICHS A J. Effects of corn processing on growth characteristics, rumen development, and rumen parameters in neonatal dairy calves[J]. Journal of Dairy Science, 2004, 87(10): 3439-3450. DOI:10.3168/jds.S0022-0302(04)73479-7 |
| [20] |
PAVLATA L, ŠT'ASTNÍK O, KŘIVOVÁ Š, et al. The effect of different physical forms of starter feed on rumen fermentation indicators and weight gain in calves after weaning[J]. Acta Veterinaria Brno, 2017, 86: 285-291. DOI:10.2754/avb201786030285 |
| [21] |
GRUMMER R R, CLARK J H, DAVIS C L, et al. Effect of ruminal ammonia-nitrogen concentration on protein degradation in situ[J]. Journal of Dairy Science, 1984, 67(10): 2294-2301. DOI:10.3168/jds.S0022-0302(84)81577-5 |
| [22] |
BEHARKA A A, NAGARAJA T G, MORRILL J L, et al. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves[J]. Journal of Dairy Science, 1998, 81(7): 1946-1955. DOI:10.3168/jds.S0022-0302(98)75768-6 |
| [23] |
嘎尔迪, 齐智利, 张润厚, 等. 玉米的不同加工处理对绵羊瘤胃内pH值、NH3-N和VFA浓度的影响[J]. 黑龙江畜牧兽医, 2002(9): 18-20. GA E D, QI Z L, ZHANG R H, et al. Effects of processed corns on rumen pH, NH3-N and VFA concentrations in rumen[J]. Heilongjiang Journal of Animal Science and Veterinary Medicine, 2002(9): 18-20 (in Chinese). DOI:10.3969/j.issn.1004-7034.2002.09.012 |
| [24] |
曾钰, 高彦华, 彭忠利, 等. 饲粮中添加酵母培养物对舍饲牦牛瘤胃发酵参数及微生物区系的影响[J]. 动物营养学报, 2020, 32(4): 1721-1733. ZENG Y, GAO Y H, PENG Z L, et al. Effects of yeast culture supplementation in diets on rumen fermentation parameters and microflora of house-feeding yak[J]. Chinese Journal of Animal Nutrition, 2020, 32(4): 1721-1733 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.04.031 |
| [25] |
唐鹏. 日粮能蛋水平对陕北白绒山羊生产性能、瘤胃微生物区系和代谢组学的影响[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2018. TANG P. Effects of dietary energy and protein levels on growth performance, rumen microbial flora and metabolomics of Shanbei white cashmere goats[D]. Master's Thesis. Yangling: Northwest A & F University, 2018. (in Chinese) |
| [26] |
CASTILLO-LOPEZ E, MOATS J, ALUTHGE N D, et al. Effect of partially replacing a barley-based concentrate with flaxseed-based products on the rumen bacterial population of lactating Holstein dairy cows[J]. Journal of Applied Microbiology, 2018, 124(1): 42-57. DOI:10.1111/jam.13630 |
| [27] |
EVANS N J, BROWN J M, MURRAY R D, et al. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes[J]. Applied and Environmental Microbiology, 2011, 77(1): 138-147. DOI:10.1128/AEM.00993-10 |
| [28] |
JIANG X, MA G M, CUI Z Q, et al. Effects of fermented corn gluten meal on growth performance, plasma metabolites, rumen fermentation and bacterial community of Holstein calves during the pre-weaning period[J]. Livestock Science, 2020, 231: 103866. DOI:10.1016/j.livsci.2019.103866 |
| [29] |
KUMAR S, TRELOAR B P, TEH K H, et al. Sharpea and Kandleria are lactic acid producing rumen bacteria that do not change their fermentation products when co-cultured with a methanogen[J]. Anaerobe, 2018, 54: 31-38. DOI:10.1016/j.anaerobe.2018.07.008 |




