在我国粗饲料资源短缺的现状下,通过提高饲粮中精料的比例来满足反刍动物高增长的营养需要是一种极其普遍的生产现象。但精料比例过高极易引起亚急性瘤胃酸中毒(SARA)[1],造成细菌内毒素(LPS)、组胺和D-乳酸等异常代谢产物在瘤胃中大量积累[2],这些异常代谢产物破坏瘤胃内环境的同时使瘤胃上皮屏障功能受损,严重影响动物机体健康及生产性能。前人研究表明,高精料会造成瘤胃紧密连接蛋白表达下调,细胞间隙变大,瘤胃屏障功能受损[3];同时,LPS通过旁细胞通路和跨细胞转运等方式易位进入血液或者瘤胃上皮被免疫细胞和上皮细胞识别[4],通过LPS/Toll样受体4(TLR4)信号传导途径触发炎症反应,产生大量的促炎细胞因子和炎症介质[5-6],炎症因子和炎症介质进一步破坏瘤胃上皮屏障[7]。苦豆子(Sophora alopecuroides L.)是豆科槐属多年生药用植物,其中含有生物碱类、黄酮类、有机酸类等多种活性成分[8],具有抑菌、抗炎、抗癌、降血压、降血脂等作用[9-10]。目前,关于苦豆子的研究较多是生物碱分离鉴定[11]。本课题组前期研究发现,低剂量的苦豆子即呈现出了提高肉羊日增重和机体健康的效果[12-13],尤其在高精料饲粮条件下,但机制尚不明确。其是否通过影响瘤胃屏障功能发挥了潜在作用?这值得深入研究。因此,本试验通过在高精料饲粮中添加不同剂量的苦豆子,研究其对绵羊瘤胃上皮c-Jun氨基末端蛋白激酶(JNK)/p38丝裂原活化蛋白激酶(p38MAPK)信号通路及紧密连接蛋白表达的影响,以期为苦豆子作为中草药饲料添加剂调控瘤胃上皮屏障生理功能提供新思路。
1 材料与方法 1.1 试验动物及试验设计选用32只体重为(25.73±2.17) kg的杜蒙羔羊,随机分为4组,每组8只。对照组饲喂高精料基础饲粮(精粗比为7 ∶ 3),3个试验组分别饲喂在基础饲粮基础上添加0.1%(T1组)、0.3%(T2组)、0.5%(T3组)苦豆子的试验饲粮。苦豆子为市售产品,将苦豆子加热炒至变色,研磨粉碎过60目筛后和预混料一起混在精料中饲喂。试验预试期15 d,正试期60 d。试验期羔羊单笼饲养,自由采食,自由饮水,统一免疫、驱虫。每天08:00和18:00各饲喂1次。
1.2 基础饲粮试验用基础饲粮参考《肉羊饲养标准》(NY/T 816—2004)配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the basal diet (DM basis) |
在60 d的饲养试验结束时,每组选择5只试验羔羊进行屠宰,采集新鲜瘤胃腹囊处组织,将组织切成约5 cm×5 cm小块,4%多聚甲醛固定,用于石蜡切片的制作。另外选取瘤胃腹囊处的上皮组织,分离黏膜,经0.9%的生理盐水漂洗后分装至冻存管中,置于-80 ℃冰箱冻存待测。
1.4 测定指标与方法 1.4.1 瘤胃液pH测定使用口腔采样法于正试期第30天晨饲后0(晨饲前)、6 h,第31天晨饲后2、8 h以及第32天晨饲后4、10 h采集瘤胃液各10 mL,用4层纱布过滤,使用pH计(奥豪斯,美国)测定其pH。
1.4.2 瘤胃上皮组织形态结构检测取4%多聚甲醛固定的瘤胃组织,常规苏木精-伊红(HE)染色法制作组织学切片。每只羔羊取5张切片,每张切片选5个视野,挑选视野下最长乳头,采用ImageView图像分析软件测定瘤胃乳头高度、宽度、面积以及肌层厚度。
1.4.3 实时荧光PCR(RT-PCR)法检测瘤胃上皮炎症相关因子及紧密连接蛋白基因mRNA相对表达量 1.4.3.1 总RNA的提取与反转录使用RNA提取试剂盒RNAiso Plus(TaKaRa)提取羔羊瘤胃上皮组织中总RNA,并使用反转录试剂盒Prime Script RT reagent Kit with gDNA Eras(TaKaRa)将其反转录成cDNA,于-20 ℃冰箱保存待测。
1.4.3.2 引物设计和RT-PCR检测采用RT-PCR检测羔羊瘤胃上皮组织中白细胞介素-1β(IL-1β)、白细胞介素-(IL-6)、白细胞介素-10(IL-10)、γ-干扰素(IFN-γ)、肿瘤坏死因子-α(TNF-α)、封闭蛋白-1(claudin-1)、闭锁蛋白(occludin)和闭锁小带蛋白-1(ZO-1)基因表达情况,β-肌动蛋白(β-actin)、甘油醛-3-磷酸脱氢酶(GAPDH)为内参基因,引物序列见表 2。使用LightCycler 96实时荧光定量PCR仪(罗氏,瑞士)进行PCR扩增,扩增程序为:95 ℃预变性30 s;95 ℃变性10 s,退火温度(Tm)下退火30 s,72 ℃延伸10 s,45个循环。PCR反应体系为10 μL:0.5 μL上游引物、0.5 μL下游引物、5 μL SYBRTM Premix Ex TaqTM Ⅱ(Tli RNaseH Plus)(TaKaRa)、0.5 μL模板cDNA、3.5 μL ddH2O。双内参基因方法校正基因表达量,使用2-△△Ct法计算目的基因mRNA相对表达量,计算公式如下:
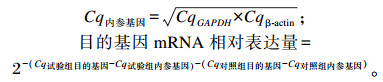
|
|
|
表 2 引物序列 Table 2 Primer sequences |
每个瘤胃上皮组织样本称取0.1 g,加入0.9 mL磷酸盐缓冲液(PBS,pH=7.4),用匀浆器匀充分浆,3 000 r/min离心20 min收集上清液,根据IFN-γ、IL-1β、IL-6、IL-10和TNF-α ELISA试剂盒(武汉基因美生物科技有限公司)说明书测定相应指标含量。
1.4.5 Western blotting法检测瘤胃上皮JNK/p38MAPK信号通路及紧密连接蛋白的蛋白相对表达量使用Western及IP细胞裂解液(上海碧云天生物技术有限公司)提取瘤胃上皮总蛋白,BCA蛋白浓度试剂盒(增强型,上海碧云天生物技术有限公司)对其进行蛋白定量,蛋白上样缓冲液制备上样样品。将分装好的蛋白样品置于100 ℃水浴变性5 min,每条泳道中蛋白上样量为40 μg,经十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)后,半干转膜仪转印至聚偏二氟乙烯(PVDF)膜上,WB无蛋白封闭液室温振荡封闭1 h,加入一抗[1 ∶ 1 000,磷酸化JNK(p-JNK)、JNK、磷酸化p38MAPK(p-p38MAPK)、p38MAPK、claudin-1、β-actin(上海碧云天生物技术有限公司),ZO-1、occludin(武汉三鹰生物技术有限公司)],37 ℃恒温培养箱孵育1 h后于4 ℃过夜,TBST洗涤3次,每次5 min,加入荧光二抗[山羊抗兔IgG 1 ∶ 10 000、山羊抗鼠IgG 1 ∶ 5 000(LI-COR公司,美国)],室温孵育1 h,TBST洗涤3次,每次10 min,Licor Odyssey双色近红外荧光成像系统(LI-COR公司,美国)扫描PVDF膜(n=3),利用Image StudioVer 5.2软件分析成像结果,根据灰度值分析计算目的蛋白相对表达量。
1.5 数据分析试验数据采用Excel 2016进行初步整理,SAS 9.2软件进行单因素方差分析(one-way ANOVA),Duncan氏法进行多重比较,GraphPad Prism8绘图,以P<0.05表示差异显著,P<0.01表示差异极显著,结果用平均值±标准差表示。
2 结果 2.1 苦豆子对羔羊瘤胃液pH的影响由表 3可知,在采食后2 h时,T3组瘤胃液pH显著高于对照组和T2组(P < 0.05);在采食后6 h时,T1组瘤胃液pH显著高于对照组(P < 0.05);采食后0、4、8、10 h时,瘤胃液pH各组间差异不显著(P>0.05)。饲喂高精料条件下,羔羊瘤胃液pH发生较大变化,并且随着进食时间的延长,各组羔羊瘤胃液pH均先降低后升高至正常水平。
|
|
表 3 苦豆子对羔羊瘤胃液pH的影响 Table 3 Effects of Sophora japonica L. on rumen fluid pH of lambs |
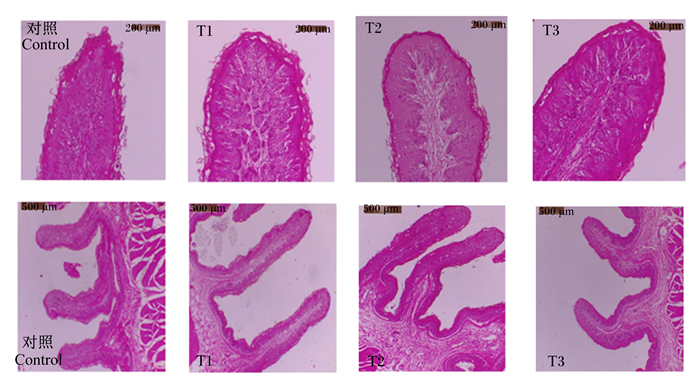
由HE染色结果(图 1)可知,对照组部分瘤胃乳头出现断裂、角质化严重、乳头顶部肿大等异常形态,各试验组瘤胃上皮组织角质化、异常脱落、乳头断裂等异常形态有所改善,乳头表面圆润、角质化及异常脱落减少。
|
图 1 苦豆子对羔羊瘤胃上皮组织形态的影响 Fig. 1 Effects of Sophora alopecuroides L. on morphology of rumen epithelium of lambs |
由表 4可知,各试验组瘤胃乳头长度均显著高于对照组(P<0.05);T1和T2组瘤胃肌层厚度均极显著高于对照组(P<0.01)。瘤胃乳头面积、宽度各组间差异不显著(P>0.05)。
|
|
表 4 苦豆子对羔羊瘤胃乳头长度、宽度、面积及肌层厚度的影响 Table 4 Effects of Sophora japonica L. on rumen papillae length, width, area and muscle thickness of lambs |
由表 5可知,T1、T2组羔羊瘤胃上皮IFN-γ mRNA相对表达量分别极显著(P<0.01)和显著(P<0.05)低于对照组;T1组羔羊瘤胃上皮IL-6 mRNA相对表达量显著低于对照组和T3组(P<0.05);T2组羔羊瘤胃上皮IL-1β mRNA相对表达量显著低于对照组(P<0.05),而T1组极显著高于T2组(P<0.01),且显著高于T3组(P<0.05);T1、T2组羔羊瘤胃上皮TNF-α mRNA相对表达量极显著低于对照组和T3组(P<0.01);T1、T2组羔羊瘤胃上皮IL-10 mRNA相对表达量极显著高于对照组(P<0.01),且显著高于T3组(P<0.05)。
|
|
表 5 苦豆子对羔羊瘤胃上皮炎症因子基因mRNA相对表达量的影响 Table 5 Effects of Sophora alopecuroides L. on mRNA relative expression levels of inflammatory factor genes in rumen epithelium of lambs |
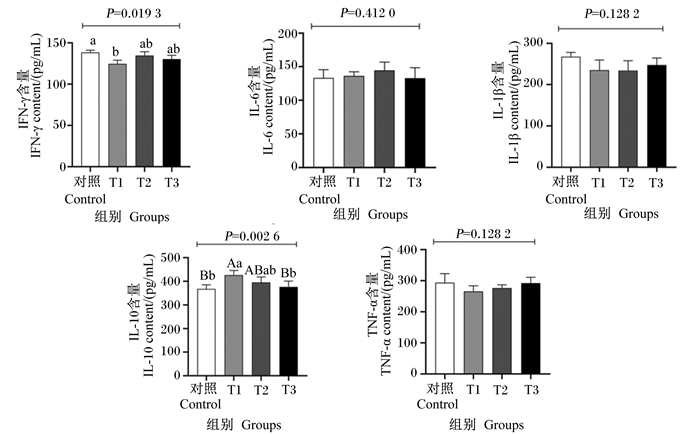
由图 2可知,T1组羔羊瘤胃上皮IFN-γ含量显著低于对照组(P<0.05);T1组羔羊瘤胃上皮IL-10含量极显著高于对照组和T3组(P<0.01);瘤胃上皮其余炎症因子含量各组间差异均不显著(P>0.05)。
|
数据柱标注不同小写字母表示差异显著(P<0.05),不同大写字母表示差异极显著(P<0.01)。下图同。 Data columns with different small letters mean significant difference (P < 0.05), and with different capital letters mean extremely significant difference (P < 0.01). The same as below. 图 2 苦豆子对羔羊瘤胃上皮炎症因子含量的影响 Fig. 2 Effects of Sophora alopecuroides L. on inflammatory factor contents in rumen epithelium of lambs |
由表 6可知,T1组羔羊瘤胃上皮组织ZO-1 mRNA相对表达量极显著高于T3组(P<0.01),显著高于对照组(P<0.05),同时T2组显著高于T3组(P<0.05);T1组羔羊瘤胃上皮组织occludin mRNA相对表达量极显著高于对照组和T3组(P<0.01),且T2组显著高于T3组(P<0.05);T1组羔羊瘤胃上皮组织claudin-1 mRNA相对表达量极显著高于对照组(P<0.01),显著高于T2、T3组(P < 0.05)。
|
|
表 6 苦豆子对羔羊瘤胃上皮紧密连接蛋白基因mRNA相对表达量的影响 Table 6 Effects of Sophora alopecuroides L. on mRNA relative expression levels of tight junction protein genes in rumen epithelium of lambs |
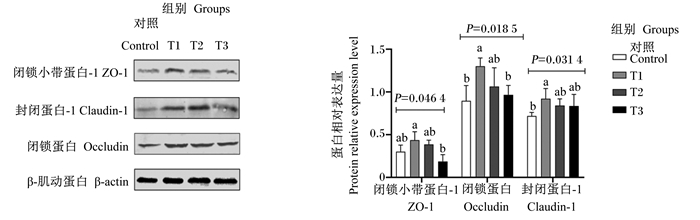
由图 3可知,T1组羔羊瘤胃上皮ZO-1蛋白相对表达量显著高于T3组(P<0.05);T1组羔羊瘤胃上皮occludin蛋白相对表达量显著高于对照组和T3组(P<0.05);T1组羔羊瘤胃上皮claudin-1蛋白相对表达量显著高于对照组(P<0.05)。
|
图 3 苦豆子对羔羊瘤胃上皮紧密连接蛋白蛋白相对表达量的影响 Fig. 3 Effects of Sophora alopecuroides L. on protein relative expression levels of tight junction proteins in rumen epithelium of lambs |
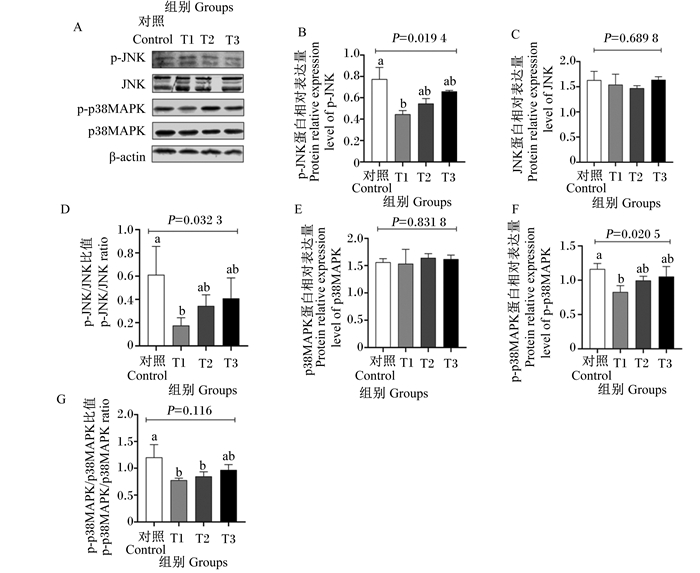
与对照组相比,T1组羔羊瘤胃上皮p-JNK、p38MAPK蛋白相对表达量显著下调(P<0.05)(图 4-B和图 4-F);苦豆子对羔羊瘤胃上皮JNK、p38MAPK蛋白相对表达量没有显著影响(P>0.05)(图 4-C和图 4-E);与对照组相比,T1组羔羊瘤胃上皮p-JNK/JNK比值(即JNK蛋白磷酸化水平)显著降低(P<0.05)(图 4-D);与对照组相比,T1、T2组羔羊瘤胃上皮p-p38MAPK/p38MAPK比值(即p38MAPK蛋白磷酸化水平)显著降低(P<0.05)(图 4-G)。
|
JNK:c-Jun氨基末端蛋白激酶c-Jun N-terminal kinase;p-JNK:磷酸化c-Jun氨基末端蛋白激酶phosphorylated c-Jun N-terminal kinase;p38MAPK:p38丝裂原活化蛋白激酶p38 mitogen-activated protein kinase;p-p38MAPK:磷酸化p38丝裂原活化蛋白激酶phosphorylated p38 mitogen-activated protein kinase;β-actin:β-肌动蛋白。 图 4 苦豆子对羔羊瘤胃上皮JNK/p38MAPK信号通路的影响 Fig. 4 Effects of Sophora alopecuroides L. on JNK/p38MAPK signaling pathway in rumen epithelium of lambs |
瘤胃是反刍动物体内容积最大的消化器官,同时也是免疫系统重要的组成成分,高精料饲粮会引起羔羊瘤胃上皮损伤。Steele等[14]发现,饲喂高精料饲粮的羔羊瘤胃上皮过度角质化,瘤胃角质层厚度、乳头高度及宽度均呈下降趋势;瘤胃乳头上皮细胞表面有脱落,乳头表面形态坚硬,角质化细胞松散。邬宇航[15]用不同非纤维性碳水化合物/中性洗涤纤维(NFC/NDF)饲粮饲喂奶山羊后发现,高NFC/NDF组瘤胃乳头高度、宽度及角质层厚度均减小,瘤胃上皮角质化严重,出现明显损伤和脱落。本试验结果显示,饲粮中添加苦豆子的各试验组瘤胃上皮角质化、异常脱落、乳头断裂等异常形态较对照组有所改善,乳头表面圆润、角质化及异常脱落减少;并且,各试验组瘤胃乳头长度均显著高于对照组,且T1和T2组瘤胃肌层厚度极显著高于对照组同时乳头面积较对照组有升高的趋势,说明适量苦豆子能够促进羔羊瘤胃乳头及肌层的发育,缓解高精料饲粮对瘤胃上皮造成的损伤。
瘤胃上皮屏障位于瘤胃的最内层,是机体阻止瘤胃内细菌入侵和LPS移位的重要屏障。前人研究表明,高精料饲粮可损害瘤胃上皮屏障,导致瘤胃上皮细胞出现空洞,紧密连接结构松散或被破坏,细胞间隙增大,导致瘤胃内的LPS发生移位进入血液循环[3]。本课题组前期研究发现,试验第60天时,高精料组绵羊血清中脂多糖结合蛋白(LBP)浓度增加,说明LPS发生了移位[16]。TLR4是LPS受体,LPS通过LPS-TLR4途径激活核转录因子-κB(NF-κB)、丝裂原活化蛋白激酶(MAPK)信号通路引起全身炎症反应,导致细胞因子和炎症介质的释放[5-6]。研究发现,高精料促进了瘤胃上皮细胞中TNF-α、IL-6、IL-1β mRNA的表达,提高了血清IL-1β、IL-6、TNF-α、IL-10含量,说明高精料破坏瘤胃上皮细胞功能,引起奶牛瘤胃炎症损伤[17-18]。
苦豆子中含有丰富的生物碱,苦豆碱的抗炎作用已在许多研究中得到验证。Jia等[19]发现,口服300、150或75 mg/kg苦豆子总碱均可显著抑制IL-1β、转化生长因子-β1(TGF-β1)表达,促进IL-10表达,改善由葡聚糖硫酸钠(DSS)引起的小鼠结肠炎和结肠损伤。Zhang等[20]研究发现,氧化苦参碱能通过抑制NF-κB和p38MAPK/细胞外信号调节激酶(Erk)的磷酸过程化减少TNF-α、IL-6、IL-1β表达,起到抗炎作用。本课题组前期研究发现,饲粮添加适量苦豆子降低了羔羊血清中IL-6、TNF-α含量,增加了血清IL-10、白细胞介素-22(IL-22)、转化生长因子-β(TGF-β)、IFN-γ含量[13]。本试验结果显示,饲粮添加0.1%苦豆子可降低羔羊瘤胃上皮IFN-γ、IL-6、TNF-α mRNA相对表达量,降低瘤胃上皮组织IFN-γ含量,增加瘤胃上皮IL-10 mRNA相对表达量及含量;饲粮添加0.3%苦豆子可降低羔羊瘤胃上皮IFN-γ、IL-1β、TNF-α mRNA相对表达量,增加瘤胃上皮IL-10 mRNA相对表达量。上述结果说明适量添加苦豆子可通过减少炎症因子的释放缓解高精料饲粮对羔羊瘤胃上皮造成的损伤,提高瘤胃上皮的免疫屏障功能。然而,饲粮添加0.5%苦豆子则降低了羔羊瘤胃上皮IFN-γ mRNA相对表达量,对其他炎症因子的影响不显著。这与课题组前期血清免疫检测及生长性能结果[13]表现相一致;而且,试验中还观察到添加0.5%苦豆子的试验组羔羔羊呈现轻微腹泻、排尿增多等症状。据药典记载,苦豆子性寒、有毒,家畜过量采食会引起消化不良造成腹泻,甚至引起死亡[21]。此外,前人试验结果表明过量苦豆碱可以损害肾脏组织[22]和鼠脑胶质瘤C6细胞[23],且呈剂量依赖性,这可能是本试验中添加高剂量苦豆子后羔羊呈现不良反应的原因。
紧密连接是胃肠道相邻上皮细胞间黏膜屏障重要性构成原件,在调节上皮屏障的通透性和防止LPS移位方面起着关键作用[24]。孙燕勇[2]研究发现,高NFC/NDF饲粮降低了萨能奶山羊瘤胃液pH,增加血液和瘤胃液LPS和组胺含量,降低瘤胃上皮ZO-1、occludin、claudin-1基因与蛋白相对表达量。李碧波[25]研究发现,高精料饲粮会增强瘤胃上皮IL-1β、TNF-α和IL-10等炎症因子mRNA相对表达量,降低瘤胃上皮occludin、ZO-1和claudin-1 mRNA相对表达量。紧密连接蛋白是评价瘤胃上皮屏障功能的重要指标,其相对表达量下降代表瘤胃上皮的结构和完整性受到破坏,瘤胃屏障功能受损[26]。本试验结果显示,与对照组相比,饲粮添加0.1%苦豆子可显著提高羔羊瘤胃上皮occludin、claudin-1 mRNA及蛋白相对表达量与ZO-1 mRNA相对表达量;而添加0.5%苦豆子则降低了羔羊瘤胃上皮ZO-1 mRNA及蛋白相对表达量与occludin mRNA相对表达量。上述结果说明羔羊饲粮中添加少量苦豆子可以调节瘤胃上皮ZO-1、occludin、claudin-1基因及蛋白表达,促进细胞间紧密连接结构的形成,降低瘤胃上皮通透性,增强瘤胃上皮屏障功能,而添加剂量过高可能会起到相反作用,可能是苦豆子添加过多引起瘤胃上皮炎症因子分泌增加,进一步破坏了瘤胃上皮的紧密连接。
MAPK是一类丝氨酸-苏氨酸蛋白激酶家族,是信号从细胞表面传导到细胞核内部重要的信号转导介质[27],该信号通路可被炎症细胞因子、LPS、氧化应激等激活[28],调节炎症、细胞分化、细胞生长和细胞紧密连接屏障等功能[29-30]。前人研究发现,高精料饲粮诱导亚急性瘤胃酸中毒产生时,胃肠道内LPS、组胺等有害物质大量积累,使瘤胃屏障功能受损,从而刺激免疫细胞及瘤胃上皮细胞释放促炎因子来破坏细胞间的紧密连接[4, 25]。近年来,一些研究证实了高精料饲粮可以通过MAPK信号通路影响紧密连接及炎症细胞因子的变化。Zhang等[31]研究发现,高精料饲粮可以通过p38MAPK/JNK/Erk信号通路来破坏瘤胃上皮屏障功能,降低claudin-1、封闭蛋白-4(claudin-4)、ocludin和ZO-1的表达。Guo等[32]发现,高精料饲粮可以激活奶牛肝脏中p38MAPK信号通路,增加外周血中TNF-α、IL-1β和IL-6的含量,引起全身炎症反应。同时,炎症细胞因子调控紧密连接蛋白的表达及分布也在前人研究中被证实。Gitter等[33]发现,TNF-α可以增加肠上皮细胞细胞旁离子的通透性,降低肠上皮细胞跨上皮电阻,通过干扰组装/拆卸机制引起紧密连接分布异常。而IFN-γ通过激活连接孔内的特定通路,选择性地增加大分子的跨上皮通量[34]。苦豆子作为传统的中草药,其含有的主要活性物质苦豆碱在小鼠的研究中被发现,其能降低LPS诱导的小肠Caco-2细胞中TNF-α和IL-6的表达,并恢复小肠Caco-2细胞中occludin、ZO-1和claudin-1的分布[35]。本研究发现,适量的苦豆子下调了羔羊瘤胃上皮JNK和p38MAPK蛋白磷酸化水平,抑制了IL-1β、IL-6、IFN-γ和TNF-α基因的表达,同时促进了ZO-1、occludin和claudin-1基因的表达。由此说明,苦豆子可能通过JNK/p38MAPK信号通路抑制炎症细胞因子的产生与释放以及促进紧密连接蛋白的表达来增强瘤胃屏障功能,缓解高精料饲粮对瘤胃上皮的损伤,从而发挥其抗炎作用。
4 结论① 适量苦豆子能够促进羔羊瘤胃乳头及肌层发育,缓解高精料饲粮对瘤胃上皮造成的损伤。
② 一方面,苦豆子通过抑制羔羊瘤胃上皮JNK、p38MAPK蛋白磷酸化,调控炎症细胞因子的产生与释放,缓解高精料饲粮对瘤胃造成的损伤;另一方面,苦豆子通过促进羔羊瘤胃上皮紧密连接蛋白的表达,降低瘤胃黏膜通透性,增强瘤胃上皮屏障功能。
| [1] |
PENNER G B, OBA M, GÄBEL G, et al. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term[J]. Journal of Dairy Science, 2010, 93(10): 4838-4845. DOI:10.3168/jds.2010-3406 |
| [2] |
孙燕勇. 亚急性瘤胃酸中毒对奶山羊瘤胃上皮通透性的影响及其机制研究[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2017. SUN Y Y. Mechanism research on rumen epithelial permeability by subacute ruminal acidosis in dairy goats[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2017. (in Chinese) |
| [3] |
杨淑青. 亚急性瘤胃酸中毒对奶山羊瘤胃上皮屏障功能影响机制的研究[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2014. YANG S Q. Study on effect of subacute ruminal acidosis on rumen epithelial barrier function in dairy goats[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2014. (in Chinese) |
| [4] |
唐志文, 孙福昱, 杨亮, 等. 不同饲粮条件下奶牛胃肠道中内毒素浓度与炎症反应相关关系研究进展[J]. 家畜生态学报, 2018, 39(5): 6-10. TANG Z W, SUN F Y, YANG L, et al. Concentrations of endotoxin in gastrointestinal tract of dairy cows under different dietary conditions and the relationship with inflammation[J]. Acta Ecologae Animalis Domastici, 2018, 39(5): 6-10 (in Chinese). DOI:10.3969/j.issn.1673-1182.2018.05.002 |
| [5] |
OPAL S M. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis[J]. International Journal of Medical Microbiology, 2007, 297(5): 365-377. DOI:10.1016/j.ijmm.2007.03.006 |
| [6] |
LU Y C, YEH W C, OHASHI P S. LPS/TLR4 signal transduction pathway[J]. Cytokine, 2008, 42(2): 145-151. DOI:10.1016/j.cyto.2008.01.006 |
| [7] |
LI S, KHAFIPOUR E, KRAUSE D O, et al. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows[J]. Journal of Dairy Science, 2012, 95(1): 294-303. DOI:10.3168/jds.2011-4447 |
| [8] |
游菁菁, 李月英, 沙碧莹, 等. 中药苦豆子生物碱的研究进展[J]. 江西中医药大学学报, 2015, 27(2): 109-113, 116. YOU J J, LI Y Y, SHA B Y, et al. Advances in studies on the alkaloid of Sophora alopecuroides L.[J]. Journal of Jiangxi University of Traditional Chinese Medicine, 2015, 27(2): 109-113, 116 (in Chinese). |
| [9] |
ZHOU H F, LI J Y, SUN F, et al. A review on recent advances in aloperine research: pharmacological activities and underlying biological mechanisms[J]. Frontiers in Pharmacology, 2020, 11: 538137. DOI:10.3389/fphar.2020.538137 |
| [10] |
韩燕, 周娅, 刘泉. 苦豆子抗内毒素效应的实验研究[J]. 中药材, 2006, 29(10): 1066-1069. HAN Y, ZHOU Y, LIU Q. Antiendotoxic effects of Sophora alopecuroides L.[J]. Jorunal of Chinese Medicinal Materials, 2006, 29(10): 1066-1069 (in Chinese). DOI:10.3321/j.issn:1001-4454.2006.10.027 |
| [11] |
关亮俊. 苦豆子化学成分分析及槐定碱、13, 14-去氢槐定碱制备工艺研究[D]. 硕士学位论文. 北京: 北京中医药大学, 2019. GUAN L J. Chemical composition analysis of sophora alopecia and preparation of sophoridine, 13, 14-dehydrosophoridine[D]. Master's Thesis. Beijing: Beijing University of Chinese Medicine, 2019. (in Chinese) |
| [12] |
谢明欣. 苦豆子对蒙古羔羊体外发酵参数、微生物种群及血液中生理生化指标的影响[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2018. XIE M X. Effect of Sophora alopecuroides L. on rumen fermentation parameters, ruminal microbial population and blood physiological-biochemical indexes of Mongolian lamb[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2018. (in Chinese) |
| [13] |
杨晓东. 高精料日粮中添加苦豆子对蒙古羔羊生长性能、血清生化及免疫指标的影响. [D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2020. YANG X D. Effect of adding Sophora alopecuroides L. in high-concentration diet on growth performance, serum biochemical and immune indexes of Mongolian lambs[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2020. (in Chinese) |
| [14] |
STEELE M A, GREENWOOD S L, CROOM J, et al. An increase in dietary non-structural carbohydrates alters the structure and metabolism of the rumen epithelium in lambs[J]. Canadian Journal of Animal Science, 2012, 92(2): 123-130. DOI:10.4141/cjas2011-095 |
| [15] |
邬宇航. 亚急性瘤胃酸中毒对瘤胃、瓣胃上皮细胞增殖与凋亡的影响[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2013. WU Y H. Effects of subacute ruminal acidosis on proliferation and apoptosis of rumen and omasum epithelial cell[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2013. (in Chinese) |
| [16] |
高智雄. 日粮不同精粗比对羔羊生长性能和免疫机能的影响[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2020. GAO Z X. Effects of different dietary concentrate to forage ration on growth performance and immune function of lambs[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2020. (in Chinese) |
| [17] |
SUN X D, YUAN X, CHEN L, et al. Histamine induces bovine rumen epithelial cell inflammatory response via NF-κB pathway[J]. Cellular Physiology and Biochemistry, 2017, 42(3): 1109-1119. DOI:10.1159/000478765 |
| [18] |
许超. 烟酸调控高精料诱导瘤胃上皮细胞凋亡机制的研究[D]. 硕士学位论文. 南昌: 江西农业大学, 2017. XU C. Regulation mechanism of nicotinic acid on apoptosis of rumen epithelial cell induced by high concentrate diets in vivo[D]. Master's Thesis. Nanchang: Jiangxi Agricultural University, 2017. (in Chinese) |
| [19] |
JIA Y Q, YUAN Z W, ZHANG X S, et al. Total alkaloids of Sophora alopecuroides L. ameliorated murine colitis by regulating bile acid metabolism and gut microbiota[J]. Journal of Ethnopharmacology, 2020, 255: 112775. DOI:10.1016/j.jep.2020.112775 |
| [20] |
ZHANG Z Q, LIU Q F, ZANG H, et al. Oxymatrine protects against L-arginine-induced acute pancreatitis and intestine injury involving Th1/Th17 cytokines and MAPK/NF-κB signalling[J]. Pharmaceutical Biology, 2019, 57(1): 595-603. DOI:10.1080/13880209.2019.1657906 |
| [21] |
宁夏回族自治区卫生厅. 宁夏中药材标准[M]. 银川: 宁夏人民出版社, 1993: 88. Health Department of Ningxia Hui Autonomous Region. Ningxia medicinal material standard[M]. Yinchuan: Ningxia People's Publishing House, 1993: 88 (in Chinese). |
| [22] |
曹晓东. 苦豆子总碱的毒性及HPLC法测定绵羊血浆四种苦豆子碱的研究[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2014. CAO X D. TASA toxicology and by HPLC method to detect four kinds of alkaloids in plasma of sheep[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2014. (in Chinese) |
| [23] |
姚雯, 孙培环, 徐晓敏, 等. 苦豆子种子生物碱类化学成分及细胞毒活性[J]. 中国实验方剂学杂志, 2014, 20(22): 95-99. YAO W, SUN P H, XU X M, et al. Alkaloids constituents of sophora alopecuroides seed and their cytotoxic activity[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2014, 20(22): 95-99 (in Chinese). |
| [24] |
TURNER J R. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application[J]. The American Journal of Pathology, 2006, 169(6): 1901-1909. DOI:10.2353/ajpath.2006.060681 |
| [25] |
李碧波. 绒山羊胃肠道微生物区系及其对日粮响应的研究[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2020. LI B B. Study on gastro-intestinal microbial flora and its response to diet in cashmere goat[D]. Ph. D. Thesis. Yangling: Northwest A & F University, 2020. (in Chinese) |
| [26] |
邓波波, 冯宝宝, 詹康, 等. 短链脂肪酸对奶牛瘤胃上皮细胞促炎因子、趋化因子和紧密连接蛋白表达量的影响[J]. 甘肃农业大学学报, 2020, 55(4): 22-28. DENG B B, FENG B B, ZHAN K, et al. Effects of short-chain fatty acids on the expression of pro-inflammatory factors, chemokines and tight junction proteins in dairy cow rumen epithelial cells[J]. Journal of Gansu Agricultural University, 2020, 55(4): 22-28 (in Chinese). |
| [27] |
姜勇, 罗深秋. 细胞信号转导的分子基础与功能调控[M]. 北京: 科学出版社, 2005. JIANG Y, LUO S Q. Molecular basis and functional regulation of cellular signal transduction[M]. Beijing: Science Press, 2005 (in Chinese). |
| [28] |
JOHNSON G L, LAPADAT R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases[J]. Science, 2002, 298(5600): 1911-1912. DOI:10.1126/science.1072682 |
| [29] |
ONO K, HAN J. The p38 signal transduction pathway: activation and function[J]. Cellular Signalling, 2000, 12(1): 1-13. DOI:10.1016/S0898-6568(99)00071-6 |
| [30] |
KIM B, BRETON S. The MAPK/ERK-signaling pathway regulates the expression and distribution of tight junction proteins in the mouse proximal epididymis[J]. Biology of Reproduction, 2016, 94(1): 22. |
| [31] |
ZHANG K, MENG M J, GAO L P, et al. Rumen-derived lipopolysaccharide induced ruminal epithelium barrier damage in goats fed a high-concentrate diet[J]. Microbial Pathogenesis, 2019, 131: 81-86. |
| [32] |
GUO J F, CHANG G J, ZHANG K, et al. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet[J]. Oncotarget, 2017, 8(29): 46769-46780. |
| [33] |
GITTER A H, BENDFELDT K, SCHMITZ H, et al. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-α[J]. Annals of the New York Academy of Sciences, 2000, 915(1): 193-203. |
| [34] |
WATSON C J, HOARE C J, GARROD D R, et al. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores[J]. Journal of Cell Science, 2005, 118(Pt.22): 5221-5230. |
| [35] |
许霓珊, 曹惠慧, 卢子滨, 等. 苦豆碱激活胆碱能通路发挥抗结肠炎作用[J]. 中国药理学与毒理学杂志, 2021, 35(10): 752. XU N S, CAO H H, LU Z B, et al. Oxymatrine activates cholinergic pathway to play an anti-colitis role[J]. Chinese Journal of Pharmacology and Toxicology, 2021, 35(10): 752 (in Chinese). |




