近几年,在人或动物消化道内发现了一类具有很大益生潜力但非常难培养的细菌,称之为下一代益生菌(next-generation probiotics,NGPs),比如嗜黏蛋白阿克曼菌(Akkermansia muciniphila,AKK)和产丁酸菌等。糖和脂肪代谢异常、炎症反应是分娩、泌乳、断奶期间宿主面临的主要健康问题。改善这些健康问题的最佳途径之一是通过益生菌调控肠道微生物区系,增强肠上皮细胞的完整性、增加最适免疫球蛋白A产量、调节体内胆汁酸的平衡以及增加抗菌肽的产量等[1]。目前,主要的传统益生菌比如乳杆菌属(厚壁菌门)和双歧杆菌属(放线菌门),是通过几个世纪以来在发酵食品中的广泛使用被选择出来的。虽然其有极高的安全性和广谱的促健康作用,但是在改善机体代谢障碍方面的总体效果和功能并不显著。其次,传统益生菌的使用并不能完全针对特定的健康问题[2]。基于上述情况,迫切需要对新的、功能特异性的NGPs进行鉴定和表征,对其作用机制和安全性进行探索和评估。本文就NGPs的候选菌群、作用机制、功能特性和安全评估进行综述,为NGPs的开发利用提供参考。
1 NGPs概述NGPs是指目前还未被确定,但是当给予足够剂量时,能通过特定作用对动物机体健康、生产性能等产生影响,适用于预防和治疗机体代谢障碍或改善宿主健康的活体微生物[2]。随着微生物分析技术的快速发展,使得鉴定具有潜在健康作用的未知肠道菌株成为可能[3],肠道微生物区系的组成、微生物门类的功能等神秘的面纱逐渐被揭开。肠道微生物区系是一个复杂的生态系统[4],是宿主健康和疾病的重要调节因子[5]。肠道微生物与宿主体内的各种器官和系统相互作用,形成了肠-脑轴、肠-肝轴、肠-血管轴等一系列调控机制,而且部分微生物的代谢产物是连接肠道微生物区系和动物机体生理功能的主要信号分子[5],例如短链脂肪酸(short chain fatty acids,SCFAs)等。因此,NGPs将以肠道共生菌作为主体,通过人工设计制成微生态制剂,作用于宿主重塑肠道微生物,以发挥其特定功能[6]。目前,开发利用NGPs的策略有2种:一种是将特定菌株的存在与否或者是丰度的差异与某一疾病状态联系起来,探索给予足够量菌株情况下,机体是否恢复健康;另一种是将一种特性良好的益生菌菌株作为某些生物活性分子的载体,运输该活性分子到特定部位发挥功能,从而起到靶向治疗的作用[7]。
NGPs来源于宿主共生菌[8],相比于传统益生菌,其属种范围都得到了扩展。新兴的NGPs开始成为新的预防和治疗工具,最近的研究揭示了许多潜在的NGPs,比如调控血糖代谢的普氏菌(Prevotella copri)和小克里斯滕森氏菌(Christensenella minuta),调节糖和脂肪代谢的金氏拟杆菌(Parabacteroides goldsteinii)和AKK,保护小鼠免受肠道疾病侵袭的普拉梭菌(Faecalibacterium prausnitzii),以及具有抗炎效果的脆弱类杆菌(Bacteroides fragilis)[2]。将获得的这些功能性微生物开发为活性生物制剂,在相关的监管框架下应用是NGPs的发展趋势。传统益生菌与NGPs在来源、范围、使用历史、目标效果、安全性等方面有着诸多差异,如表 1所示。NGPs可以说是一种新的尝试,标志着从具有长期安全使用历史的传统微生物到新兴的肠道微生物的进步[7]。
2 NGPs的候选菌群肠道微生物区系和粪菌移植的研究,已经证明肠道共生菌在调节机体健康方面的重要性,这也为NGPs的开发提供了方向,即在微生物区系失调中未得到充分表达的共生菌。比如拟杆菌属、产丁酸菌、AKK和其他一些肠道菌群(表 2)。拟杆菌属属于拟杆菌门,是一种严格的厌氧菌,其不同的菌株可能表现出完全不同的生理特征和病理表型。拟杆菌属占结肠微生物群的20%~40%,是乳杆菌属和双歧杆菌属的10 000倍。除结肠外,还可以从其他肠道、口腔、上呼吸道和生殖道等部位分离获得。其中具有潜在的益生功能的菌属有卵形拟杆菌(Bacteroides ovatus)、脆弱拟杆菌、解木聚糖拟杆菌(Bacteroides xylanisolvens)、多氏拟杆菌(Bacteroides dorei)、酸性拟杆菌(Bacteroides acidifaciens)等。产丁酸菌是通过降解碳水化合物产生丁酸的一类功能性菌群,其广泛存在于消化道的各个部位,包括瘤胃、口腔、结肠和盲肠等。大多数产丁酸菌是厚壁菌门,典型的菌种有丁酸梭菌(Clostridium butyricum)、普拉梭菌和霍氏真杆菌(Eubacterium hallii)。AKK属于疣微菌门,是一种丰富的肠道菌群,占肠道微生物区系的1%~3%,该菌呈椭圆形,不运动,不产芽孢,是一类厌氧的革兰氏阴性菌。Derrien等[9]2004年首次从粪便中分离得到一株优势菌MUCT,通过鉴定其为疣微菌门的一个新属,命名为阿克曼菌属,AKK为该菌的唯一菌种,模式菌株为MUCT。AKK最初被归类为一种严格厌氧菌,但最近研究表明,AKK能耐受少量的氧气,被重新归类为耐氧厌氧菌[10]。
|
|
表 2 NGPs主要候选菌群 Table 2 Main candidate flora of NGPs |
NGPs的作用机制主要分为3个方面,一是作为机体不可或缺的一部分,通过其自身结构和免疫特性发挥作用;二是通过分泌代谢产物在调节肠道微生态平衡和宿主健康方面起重要作用;三是通过提高肠道上皮细胞紧密连接蛋白的表达,增强肠道上皮屏障的功能而起作用。NGPs的作用机制见图 1。
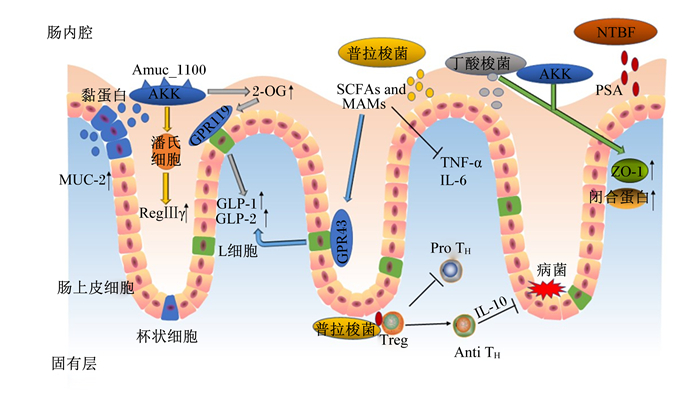
|
AKK:嗜黏蛋白阿克曼菌Akkermansia muciniphila;SCFAs and MAMs:短链脂肪酸及微生物抗炎性分子short chain fatty acids and microbial ant-inflammatory molecules;NTBF:非产毒素脆弱拟杆菌non-toxinogenic Bacteroides fragilis;Anti TH:产生抗炎因子的T细胞anti-inflammatory cytokines producing T cells;GPR119:G蛋白偶联受体119 G protein coupled receptor 119;GPR43:G蛋白偶联受体43 G protein coupled receptor 43;GLP-1:胰高血糖素样肽-1 glucagon-like peptide-1;GLP-2:胰高血糖素样肽-2 glucagon-like peptide-2;IL-6:白细胞介素-6 interleukin-6;IL-10:白细胞介素-10 interleukin-10;MUC-2:黏蛋白-2 mucin-2;2-OG:2-油酰甘油2-oleoylglycerol;Pro TH:产生促炎因子的T细胞pro-inflammatory cytokines producing T cells;PSA:多糖A polysaccharide A;RegⅢγ:再生胰岛源性3γ regenerating islet-derived 3 gamma;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;Treg:调节T细胞regulatory T cells;ZO-1:闭锁小带蛋白-1 zonula occludens-1。 图 1 NGPs的作用机制 Fig. 1 Mechanism of NGPs[39-41] |
NGPs对免疫的调节主要分为2部分:一是通过自身免疫调节,如激活巨噬细胞、B淋巴细胞和自然杀伤(NK)细胞对抗原刺激做出反应;与肠上皮和固有层细胞相互作用,诱导局部免疫反应;激活肠黏膜中的淋巴组织,提高分泌型免疫球蛋白含量。二是通过产生抗炎细胞因子来提高肠道免疫功能。NGPs与特定微生物或肠上皮细胞的相互作用诱导T、B淋巴细胞和其他免疫细胞产生特定的信号分子,包括细胞因子和趋化因子,通过调节细胞间信号传递和免疫细胞活力抑制Toll样受体4(TLR4)、肿瘤坏死因子-α(TNF-α)和白细胞介素-6(IL-6)等炎症因子活性,增强肠黏膜的免疫功能。非产毒素脆弱拟杆菌可以通过释放外囊膜泡,向免疫细胞传递免疫调节分子多糖A(polysaccharide A,PSA)。PSA可以刺激CD4+ T细胞,分泌白细胞介素-10(IL-10),进而发挥免疫保护作用[42]。普拉梭菌能够阻断核因子-κB(NF-κB)途径和诱导T细胞分化产生抗炎因子,发生免疫应答反应和清除有害物质从而保护机体免受攻击,在清除病原菌之后,调节性T细胞就会发出攻击停止信号,调节机体免疫稳态和阻止自身过度免疫[39]。
3.2 调节肠道稳态NGPs可通过产生SCFAs、抗菌肽、细菌素来抑制病原微生物生长,加快肠道上皮合成,保护胃肠黏膜。SCFAs是肠道微生物发酵纤维的终产物,能够促进糖异生、降血糖素合成。普拉梭菌和丁酸梭菌作为产丁酸菌的优势菌种,主要产生丁酸和部分其他SCFAs,这些SCFAs通过激活L细胞上的G蛋白偶联受体43(GPR43),促进胰高血糖素样肽-1(GLP-1)和胰高血糖素样肽-2(GLP-2)的分泌[41];此外,普拉梭菌和丁酸梭菌分泌的SCFAs及微生物抗炎性分子(MAMs)都能抑制炎性细胞因子的表达,如TNF-α、IL-6、白细胞介素-1β(IL-1β)等[43]。抗菌肽可以通过选择性地吸附在膜的结构上,与膜融合后产生跨膜通道,导致其内容物外泄致细菌死亡。其次,抗菌肽也可以迅速进入细菌内部,在特定的靶细胞位点发生作用、阻碍代谢和生长,有效地杀菌灭菌[44]。AKK通过潘氏细胞释放抗菌肽再生胰岛源性3γ(RegⅢγ)到肠内腔,协同增加肠道生态系统的抗炎环境[41]。而且AKK显著提高了肠道内大麻素系统中的生物活性脂质2-油酰甘油的含量[23],2-油酰甘油通过激活G蛋白偶联受体119(GPR119)刺激GLP-1和GLP-2的分泌[45],使得葡萄糖耐量增加和新陈代谢加快,进而影响肠道功能和能量稳态。细菌素通过特异性或非特异性受体与细胞壁成分如脂质或表面分子结合位点结合,引起细胞膜渗透而促使细胞内物质泄漏和质子原动力消失,导致细胞死亡,从而起到杀菌作用[46]。
3.3 增强肠道屏障功能肠上皮细胞的结构蛋白[闭锁小带蛋白(ZO)、封闭蛋白(claudin)等]和调节蛋白(肌动蛋白、E-钙黏蛋白等)用于连接相邻的肠上皮细胞,形成紧密连接并调节细胞间通透性。其中ZO属于细胞内的接头蛋白,主要负责将肌动蛋白纤维以及闭合蛋白(occludin)、claudin、连接黏附分子的胞内区连接起来。黏蛋白-2在小肠和结肠中表达,在肠道上形成一层黏液,以抑制病原菌的黏附和侵袭[47]。紧密连接可以限制脂质和膜蛋白在细胞不同液体空间的扩散,维持细胞极性;且相邻细胞的质膜紧密融合有利于维持肠黏膜通透性,防止氧化应激破坏屏障功能[48];调控因子通过激活p38和细胞外调节蛋白激酶(ERK)信号分子通路,可以增加紧密连接蛋白如claudin3、claudin7、claudin13的表达,从而改善肠上皮屏障的功能[49]。体外模型表明,在AKK表面有一种特殊的细菌结构成分蛋白Amuc_1100,不仅与菌毛的形成有关,而且被证实能激活肠上皮细胞Toll样受体2(TLR2)介导的细胞内信号,有助于增强肠道屏障[24]。其次,丁酸梭菌和AKK通过增加ZO-1和occludin的表达改善肠道屏障[39]。AKK可恢复肠道上皮杯状细胞数量,增加黏蛋白-2的表达,从而逆转肠黏膜损伤;同时杯状细胞分泌的黏蛋白被AKK降解形成一定量的醋酸盐和丁酸盐[40]。
4 主要NGPs的功能特性NGPs主要是严格的厌氧菌,其中部分菌株已经在机体代谢障碍、炎症性肠病、腹泻等疾病的动物模型中显示出效果,甚至NGPs能够通过调节肠道微生物区系或通过分泌代谢产物作用于肺部来增强免疫反应,对呼吸道病毒感染有一定的有益影响。研究表明,将NGPs作为治疗新型冠状病毒(SARS CoV-2)感染的药物具有一定的可能性。但面对当前需要所解决的新冠肺炎问题[39],需要更系统地研究来证实这些NGPs或其代谢物是否有效。下面将对主要的NGPs的功能特性作一阐述。
4.1 AKKAKK主要特征是能降解黏蛋白,以黏蛋白作为唯一的碳源和氮源,因此该菌能对其他降解黏蛋白的病原菌产生竞争抑制作用[50]。此外,巴氏杀菌的AKK对小鼠肥胖和胰岛素抵抗的预防能力增强[51],且单独纯化的膜蛋白Amuc_1100也显示了该菌的有益作用[52]:参与宿主肠道黏膜免疫稳态和肠道屏障功能的改善。各种疾病如代谢综合征和自身免疫性疾病也被报道与AKK的丰度紊乱有关,AKK可作为一种生物标志物,其丰度的降低标志着炎症和代谢疾病的发生[53]。此外,与严格的厌氧细菌相比,即使在纳摩尔浓度的氧气存在下,AKK也能够产生丁酸[54],AKK丰度的增加还与新陈代谢的改善和脂肪量发育的减少有关,但不会干扰宿主的总能量摄入,目前该菌被广泛认为在改善机体脂质代谢障碍和低度炎症方面具有极大潜力[22]。
4.2 拟杆菌属拟杆菌属通过调节淋巴细胞和细胞因子的表达,促进新陈代谢和缓解炎症。Ulsemer等[12]研究发现,用卵形拟杆菌D-6免疫的小鼠产生了特异性的抗TNF-α、免疫球蛋白M(lgM)和免疫球蛋白G(lgG)抗体,这些抗体能与携带TNF-α的人类癌细胞结合,增强了TNF-α的特异性免疫活性,因此,卵形芽孢杆菌D-6株是一种TNF-α特异性抗肿瘤疫苗的理想候选菌株。非产毒素脆弱拟杆菌与肠道黏蛋白之间的相互作用可能在宿主细菌共生过程中起着关键作用[55],但产肠毒素脆弱拟杆菌感染是引起宿主产生炎症性腹泻的原因[14]。Ulsemer等[15]研究发现,口服热处理的解木聚糖拟杆菌DSM 239964能够提高TNF-α特异性IgM血清抗体的水平,其安全性良好,已在小鼠和人类上面确定无致病性[18],被欧盟委员会批准为奶制品的补充剂。多氏拟杆菌和普通拟杆菌的序列相似度极高,有研究表明,肠道中高水平表达的多氏拟杆菌和机体血糖代谢障碍产生的自身免疫有关[56],而且普通拟杆菌和多氏拟杆菌都能够减少肠道微生物脂多糖的产生和改善脂质代谢异常[57]。
拟杆菌属的益生功能包括:1)与碳水化合物发酵有关的独特代谢活性,含氮物质的利用以及胆汁酸和其他类固醇的生物转化;2)防止病原微生物定植于睾丸素[58];3)产生可能具有降低胆固醇、刺激饱腹感的SCFAs;4)涉及宿主免疫系统发育和维持其抵抗病原体和疾病能力的免疫调节作用。因此,拟杆菌属在提高免疫、改善糖脂代谢障碍方面存在巨大潜力。
4.3 产丁酸菌产丁酸菌不是某一特定的菌属,是一类可以发酵碳水化合物产生丁酸的菌,比较具有代表性的有丁酸梭菌、普拉梭菌和霍氏真杆菌。丁酸盐宿主有几种有益的作用,包括为上皮细胞提供能量,诱导结肠调节性T细胞,抑制结肠上皮细胞的炎症反应,以及改善代谢综合征[59]。在当前的研究中,丁酸梭菌已被证明在动物生产中起到一定的作用。在断奶仔猪的饲粮中添加一定量的丁酸梭菌,能够增加其料重比,降低腹泻指数[60]。饲粮中添加丁酸梭菌能一定程度上提高犊牛的生长性能,降低腹泻率;提高血清葡萄糖和IgG含量;增强机体抗氧化能力和免疫功能,改善犊牛健康[61]。除此之外,丁酸梭菌还能缓解小鼠结肠炎,显著降低炎症性肠病(IBD)小鼠死亡率[62]。产丁酸菌通过形成不同的SCFAs,极大地影响代谢平衡以及肠道微生物区系和宿主的动态平衡,进而改善肠道健康。
5 NGPs的安全性评估虽然NGPs有益于动物健康,但是国际益生菌和益生元科学协会(International Scientific Association for Probiotics and Prebiotics,ISAPP)建议进行逐个菌株的评估,直至有足够的研究数据可以在物种水平上授予益生菌地位[63]。欧洲食品安全局引入了合格安全推定(qualified presumption of safety,QPS)的概念,旨在为饲料生产和食品中的微生物建立安全评估。评估的标准包括分类单元、知识体系、可能的致病性以及最终用途的说明,在饲料生产和食品中有悠久使用历史的微生物群体都涵盖在QPS清单中。对其安全性不完全了解的微生物要进行安全评估,需要包括对物种或菌株水平的明确分类,以及全面的菌株特征,包括全基因组序列分析,以确定潜在的毒力和抗生素耐药性基因及其水平转移能力[19]。丁酸梭菌通过抗生素耐药性图谱、毒素编码基因和动物研究等一系列安全问题测试,被授权为一种革兰氏阳性、产孢、专性需氧、非致病性的新食品添加剂,并且该产品的特征是白色或浅灰色的片剂,具有特殊的气味和甜味。丁酸梭菌每天的最大摄入剂量为1.35×108 CFU/g,在该菌的干预下,有助于预防溃疡性结肠炎的膀胱炎,减少接受根除幽门螺旋杆菌治疗的患者的副作用发生率[64]。2015年,用解木聚糖拟杆菌DSM-23964发酵的巴氏乳制品被批准为新型食品,但是该菌株作为乳制品的发酵剂的使用具有一定的局限性,必须经过热处理和灭活才能被添加到产品中[65]。益生菌的定义中要求菌株必须是活的微生物,在热处理和灭活后,不存在代谢活动。而且,解木聚糖拟杆菌无法在体外与上皮细胞结合,因此,我们应对活细菌进行研究,解决其活性和安全性的问题。药敏试验表明,解木聚糖拟杆菌DSM-23964对甲硝唑、美洛培南类药物和克林霉素敏感,且无毒力潜势,还能抵抗胃酶和低pH,是进一步研究其安全性和潜在的健康促进特性的候选菌株[18]。AKK菌从毒力检测来讲,没有什么特别的安全问题,但是如果作为添加剂产品应用在食品和饲料中,还需要包含该菌的新产品的详细说明,以及将该菌用于产品的生产工艺能否支持该菌的活性,实现潜在的益生菌作用。其次,该菌的毒理学和营养评估仅对动物模型进行,缺乏人体的临床试验以及最佳摄取量,还需进一步对该菌进行研究,了解其更多特性。AKK的优势在于经过巴氏杀菌后该菌也能够有好的效果,这也为其通过益生菌的授权增加了可能。普拉梭菌的培养条件极其严苛,即使是在厌氧的条件下都不一定能培养成功,而且在该菌株中观察到多重抗生素耐药基因,加快了该菌对抗生素的耐药性,如果要想获得监管部门的批准,还需要对该菌株进行全面的毒理学分析和鉴定。
6 小结综上所述,NGPs作为新一代益生菌,多来源于肠道共生菌,其通过特定作用和调节特性对于肠道炎症等代谢失调具有预防和治疗作用。NGPs是益生菌向功能性发展的新阶段。目前已经确定了部分潜在的NGPs候选菌株,阐明了其作用机制,但相比传统益生菌而言,其安全性评价复杂且重要。对氧气的极端脆弱性是NGPs需解决的重要问题。除此之外,对胃pH和胆盐的敏感性以及益生菌的工业生产等因素需要优化,毒理学测试和抗生素耐药性测试也应该在菌株上重复进行。NGPs在调控机体代谢、缓解炎症反应、改善动物腹泻方面存在极大潜力,将其作为微生态制剂应用在畜牧业以及其他领域有巨大的发展前景。
| [1] |
OLVEIRA G, GONZÁLEZ-MOLERO I. An update on probiotics, prebiotics and symbiotics in clinical nutrition[J]. Endocrinologia y Nutricion, 2016, 63(9): 482-494. DOI:10.1016/j.endonu.2016.07.006 |
| [2] |
CHANG C J, LIN T L, TSAI Y L, et al. Next generation probiotics in disease amelioration[J]. Journal of Food and Drug Analysis, 2019, 27(3): 615-622. DOI:10.1016/j.jfda.2018.12.011 |
| [3] |
BUI T P N, DE VOS W M. Next-generation therapeutic bacteria for treatment of obesity, diabetes, and other endocrine diseases[J]. Best Practice & Research.Clinical Endocrinology & Metabolism, 2021, 35(3): 101504. |
| [4] |
SATOKARI R. Modulation of gut microbiota for health by current and next-generation probiotics[J]. Nutrients, 2019, 11(8): 1921. DOI:10.3390/nu11081921 |
| [5] |
FENG Q Q, CHEN W D, WANG Y D. Gut microbiota: an integral moderator in health and disease[J]. Frontiers in Microbiology, 2018, 9: 151. DOI:10.3389/fmicb.2018.00151 |
| [6] |
SINGH T P, NATRAJ B H. Next-generation probiotics: a promising approach towards designing personalized medicine[J]. Critical Reviews in Microbiology, 2021, 47(4): 479-498. DOI:10.1080/1040841X.2021.1902940 |
| [7] |
O'TOOLE P W, MARCHESI J R, HILL C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics[J]. Nature Microbiology, 2017, 2: 17057. DOI:10.1038/nmicrobiol.2017.57 |
| [8] |
LIN T L, SHU C C, LAI W F, et al. Investiture of next generation probiotics on amelioration of diseases-strains do matter[J]. Medicine in Microecology, 2019, 1/2: 100002. DOI:10.1016/j.medmic.2019.100002 |
| [9] |
DERRIEN M, VAUGHAN E E, PLUGGE C M, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium[J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54(Pt.5): 1469-1476. |
| [10] |
REUNANEN J, KAINULAINEN V, HUUSKONEN L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer[J]. Applied and Environmental Microbiology, 2015, 81(11): 3655-3662. DOI:10.1128/AEM.04050-14 |
| [11] |
闫丹丽, 武俊瑞, 史海粟, 等. 下一代益生菌——卵形拟杆菌研究进展[J]. 乳业科学与技术, 2020, 43(1): 50-54. YAN D L, WU J R, SHI H S, et al. Recent progress in a next generation probiotic, Bacteroides ovatus[J]. Journal of Dairy Science and Technology, 2020, 43(1): 50-54 (in Chinese). |
| [12] |
ULSEMER P, HENDERSON G, TOUTOUNIAN K, et al. Specific humoral immune response to the Thomsen-Friedenreich tumor antigen (CD176) in mice after vaccination with the commensal bacterium Bacteroides ovatus D-6[J]. Cancer Immunology, Immunotherapy, 2013, 62(5): 875-887. DOI:10.1007/s00262-013-1394-x |
| [13] |
ROUND J L, MAZMANIAN S K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(27): 12204-12209. DOI:10.1073/pnas.0909122107 |
| [14] |
SEARS C L, ISLAM S, SAHA A, et al. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea[J]. Clinical Infectious Diseases, 2008, 47(6): 797-803. DOI:10.1086/591130 |
| [15] |
ULSEMER P, TOUTOUNIAN K, KRESSEL G, et al. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults[J]. Beneficial Microbes, 2016, 7(4): 485-500. DOI:10.3920/BM2015.0143 |
| [16] |
ULSEMER P, TOUTOUNIAN K, KRESSEL G, et al. Safety and tolerance of Bacteroides xylanisolvens DSM 23964 in healthy adults[J]. Beneficial Microbes, 2012, 3(2): 99-111. DOI:10.3920/BM2011.0051 |
| [17] |
ULSEMER P, TOUTOUNIAN K, SCHMIDT J, et al. Preliminary safety evaluation of a new Bacteroides xylanisolvens isolate[J]. Applied and Environmental Microbiology, 2012, 78(2): 528-535. DOI:10.1128/AEM.06641-11 |
| [18] |
ULSEMER P, TOUTOUNIAN K, SCHMIDT J, et al. Safety assessment of the commensal strain Bacteroides xylanisolvens DSM 23964[J]. Regulatory Toxicology and Pharmacology, 2012, 62(2): 336-346. DOI:10.1016/j.yrtph.2011.10.014 |
| [19] |
BRODMANN T, ENDO A, GUEIMONDE M, et al. Safety of novel microbes for human consumption: practical examples of assessment in the European Union[J]. Frontiers in Microbiology, 2017, 8: 1725. DOI:10.3389/fmicb.2017.01725 |
| [20] |
GÉRARD P, LEPERCQ P, LECLERC M, et al. Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces[J]. Applied and Environmental Microbiology, 2007, 73(18): 5742-5749. DOI:10.1128/AEM.02806-06 |
| [21] |
PEDERSEN H K, GUDMUNDSDOTTIR V, NIELSEN H B, et al. Human gut microbes impact host serum metabolome and insulin sensitivity[J]. Nature, 2016, 535(7612): 376-381. DOI:10.1038/nature18646 |
| [22] |
CANI P D, DE VOS W M. Next-generation beneficial microbes: the case of Akkermansia muciniphila[J]. Frontiers in Microbiology, 2017, 8: 1765. DOI:10.3389/fmicb.2017.01765 |
| [23] |
EVERARD A, BELZER C, GEURTS L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(22): 9066-9071. DOI:10.1073/pnas.1219451110 |
| [24] |
PLOVIER H, EVERARD A, DRUART C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice[J]. Nature Medicine, 2017, 23(1): 107-113. DOI:10.1038/nm.4236 |
| [25] |
CANI P D, GEURTS L, MATAMOROS S, et al. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond[J]. Diabetes & Metabolism, 2014, 40(4): 246-257. |
| [26] |
WANG Y M, MA R N, LIU F, et al. Modulation of gut microbiota: a novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy[J]. Frontiers in Immunology, 2018, 9: 374. DOI:10.3389/fimmu.2018.00374 |
| [27] |
CHEN D F, JIN D C, HUANG S M, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota[J]. Cancer Letters, 2020, 469: 456-467. DOI:10.1016/j.canlet.2019.11.019 |
| [28] |
DUNCAN S H, HOLD G L, HARMSEN H J M, et al. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(Pt 6): 2141-2146. |
| [29] |
WRZOSEK L, MIQUEL S, NOORDINE M L, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent[J]. BMC Biology, 2013, 11: 61. DOI:10.1186/1741-7007-11-61 |
| [30] |
PARK Y J, KUEN D S, CHUNG Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance[J]. Experimental & Molecular Medicine, 2018, 50(8): 1-13. |
| [31] |
LI C, CHEN X, KOU L, et al. Selenium-Bifidobacterium longum as a delivery system of endostatin for inhibition of pathogenic bacteria and selective regression of solid tumor[J]. Experimental and Therapeutic Medicine, 2010, 1(1): 129-135. DOI:10.3892/etm_00000022 |
| [32] |
FU G F, LI X, HOU Y Y, et al. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer[J]. Cancer Gene Therapy, 2005, 12(2): 133-140. DOI:10.1038/sj.cgt.7700758 |
| [33] |
CHANG C J, LIN C S, LU C C, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota[J]. Nature Communications, 2015, 6: 7489. DOI:10.1038/ncomms8489 |
| [34] |
GOPALAKRISHNAN V, SPENCER C N, NEZI L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients[J]. Science, 2018, 359(6371): 97-103. DOI:10.1126/science.aan4236 |
| [35] |
DASGUPTA S, ERTURK-HASDEMIR D, OCHOA-REPARAZ J, et al. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms[J]. Cell Host & Microbe, 2014, 15(4): 413-423. |
| [36] |
LUKIW W J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's disease[J]. Frontiers in Microbiology, 2016, 7: 1544. |
| [37] |
UDAYAPPAN S, MANNERAS-HOLM L, CHAPLIN-SCOTT A, et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice[J]. NPJ Biofilms and Microbiomes, 2016, 2: 16009. DOI:10.1038/npjbiofilms.2016.9 |
| [38] |
ENGELS C, RUSCHEWEYH H J, BEERENWINKEL N, et al. The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation[J]. Frontiers in Microbiology, 2016, 7: 713. |
| [39] |
GAUTIER T, DAVID-LE GALL S, SWEIDAN A, et al. Next-generation probiotics and their metabolites in COVID-19[J]. Microorganisms, 2021, 9(5): 941. DOI:10.3390/microorganisms9050941 |
| [40] |
ALMEIDA D, MACHADO D, ANDRADE J C, et al. Evolving trends in next-generation probiotics: a 5W1H perspective[J]. Critical Reviews in Food Science and Nutrition, 2020, 60(11): 1783-1796. DOI:10.1080/10408398.2019.1599812 |
| [41] |
CANI P D, VAN HUL M. Novel opportunities for next-generation probiotics targeting metabolic syndrome[J]. Current Opinion in Biotechnology, 2015, 32: 21-27. DOI:10.1016/j.copbio.2014.10.006 |
| [42] |
ZHANG W D, ZHU B, XU J H, et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses[J]. Frontiers in Immunology, 2018, 9: 1040. DOI:10.3389/fimmu.2018.01040 |
| [43] |
ALAMEDDINE J, GODEFROY E, PAPARGYRIS L, et al. Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction[J]. Frontiers in Immunology, 2019, 10: 143. DOI:10.3389/fimmu.2019.00143 |
| [44] |
赵雪娇, 李文茜. 抗菌肽的生物学功能和作用机制[J]. 畜牧兽医科技信息, 2015(8): 15-16. ZHAO X J, LI W Q. Biological function and mechanism of antimicrobial peptides[J]. Chinese Journal of Animal Husbandry and Veterinary Medicine, 2015(8): 15-16 (in Chinese). DOI:10.3969/J.ISSN.1671-6027.2015.08.007 |
| [45] |
HANSEN K B, ROSENKILDE M M, KNOP F K, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans[J]. The Journal of Clinical Endocrinology and Metabolism, 2011, 96(9): E1409-E1417. DOI:10.1210/jc.2011-0647 |
| [46] |
陈鹏. 细菌素在动物饲料中的替抗应用[J]. 饲料研究, 2021, 44(18): 150-153. CHEN P. Antibiotic replacement application of bacteriocins in animal feed[J]. Feed Research, 2021, 44(18): 150-153 (in Chinese). |
| [47] |
QUINTANA-HAYASHI M P, PADRA M, PADRA J T, et al. Mucus-pathogen interactions in the gastrointestinal tract of farmed animals[J]. Microorganisms, 2018, 6(2): 55. DOI:10.3390/microorganisms6020055 |
| [48] |
周洁, 朱俊萍, 诸欣平. 益生菌对肠道疾病的作用机制及应用进展[J]. 中国新药杂志, 2015, 24(13): 1484-1487. ZHOU J, ZHU J P, ZHU X P. Action mechanisms and application of probiotics in intestinal diseases[J]. Chinese Journal of New Drugs, 2015, 24(13): 1484-1487 (in Chinese). |
| [49] |
DAI C, ZHAO D H, JIANG M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways[J]. International Journal of Molecular Medicine, 2012, 29(2): 202-208. |
| [50] |
BELZER C, DE VOS W M. Microbes inside—from diversity to function: the case of Akkermansia[J]. The ISME Journal, 2012, 6(8): 1449-1458. DOI:10.1038/ismej.2012.6 |
| [51] |
DEPOMMIER C, VAN HUL M, EVERARD A, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice[J]. Gut Microbes, 2020, 11(5): 1231-1245. DOI:10.1080/19490976.2020.1737307 |
| [52] |
OTTMAN N, REUNANEN J, MEIJERINK M, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function[J]. PLoS One, 2017, 12(3): e0173004. DOI:10.1371/journal.pone.0173004 |
| [53] |
ZHAI Q X, FENG S S, ARJAN N, et al. A next generation probiotic, Akkermansia muciniphila[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(19): 3227-3236. DOI:10.1080/10408398.2018.1517725 |
| [54] |
OUWERKERK J P, VAN DER ARK K C H, DAVIDS M, et al. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer[J]. Applied and Environmental Microbiology, 2016, 82(23): 6983-6993. DOI:10.1128/AEM.01641-16 |
| [55] |
HUANG J Y, LEE S M, MAZMANIAN S K. The human commensal Bacteroides fragilis binds intestinal mucin[J]. Anaerobe, 2011, 17(4): 137-141. DOI:10.1016/j.anaerobe.2011.05.017 |
| [56] |
DAVIS-RICHARDSON A G, ARDISSONE A N, DIAS R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes[J]. Frontiers in Microbiology, 2014, 5: 678. |
| [57] |
YOSHIDA N, EMOTO T, YAMASHITA T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis[J]. Circulation, 2018, 138(22): 2486-2498. DOI:10.1161/CIRCULATIONAHA.118.033714 |
| [58] |
TAN H Z, ZHAI Q X, CHEN W. Investigations of Bacteroides spp. towards next-generation probiotics[J]. Food Research International, 2019, 116: 637-644. |
| [59] |
陈映宇, 毛联智, 刘华缓, 等. 肠道产丁酸菌防治炎症性肠病的机制研究进展[J]. 世界华人消化杂志, 2019, 27(14): 907-912. CHEN Y Y, MAO L Z, LIU H H, et al. Mechanism of gut butyric acid producing bacteria for prevention and treatment of inflammatory bowel disease[J]. World Chinese Journal of Digestology, 2019, 27(14): 907-912 (in Chinese). |
| [60] |
CAO G T, TAO F, HU Y H, et al. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets[J]. Food & Function, 2019, 10(5): 2926-2934. |
| [61] |
何家俊, 吴汉葵, 杨昕涧, 等. 丁酸梭菌对犊牛生长性能和血清生化、抗氧化、免疫指标及粪便微生物数量的影响[J]. 动物营养学报, 2021, 33(9): 5076-5085. HE J J, WU H K, YANG X J, et al. Effects of Clostridium butyricum on growth performance, serum biochemical, antioxidant, immune indexes and fecal microorganism number of calves[J]. Chinese Journal of Animal Nutrition, 2021, 33(9): 5076-5085 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.09.028 |
| [62] |
ARAKI Y, ANDOH A, TAKIZAWA J, et al. Clostridium butyricum, a probiotic derivative, suppresses dextran sulfate sodium-induced experimental colitis in rats[J]. International Journal of Molecular Medicine, 2004, 13(4): 577-580. |
| [63] |
HILL C, GUARNER F, REID G, et al. Expert consensus document.The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic[J]. Nature Reviews.Gastroenterology & Hepatology, 2014, 11(8): 506-514. |
| [64] |
YASUEDA A, MIZUSHIMA T, NEZU R, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis[J]. Surgery Today, 2016, 46(8): 939-949. |
| [65] |
AGOSTONI C, CANANI R B, FAIRWEATHER-TAIT S, et al. Scientific opinion on the safety of'heat-treated milk products fermented with Bacteroides xylanisolvens DSM 23964'as a novel food[J]. EFSA Journal, 2015, 13(1): 3956. |




