2. 浙江大学教育部动物分子营养学重点实验室, 杭州 310058;
3. 农业农村部(华东)动物营养与饲料重点实验室, 杭州 310058;
4. 浙江省饲料与 动物营养重点实验室, 杭州 310058
2. Key Laboratory of Molecular Animal Nutrition of Zhejiang University, Ministry of Education, Hangzhou 310058, China;
3. Key Laboratory of Animal Nutrition and Feed Science (Eastern of China), Ministry of Agriculture and Rural Affairs, Hangzhou 310058, China;
4. Key Laboratory of Animal Feed and Nutrition of Zhejiang Province, Hangzhou 310058, China
抗生素禁用以来,探索新型饲料添加剂以改善畜禽生产的重要性已不言而喻。植物提取物因其天然来源、低毒性及多种生物学功能在医疗或食品行业受到了广泛关注,其在饲料添加剂领域内的应用前景也越来越宽阔。胡椒碱(piperine,PIP)作为生物碱的一种,广泛存在于胡椒科植物的根、茎、叶和果实中,其具有抗菌抗虫活性[1],并能调控机体氧化应激[2]、糖脂代谢[3-4]以及免疫[5]等。此外,PIP还能增加其他药物的治疗效果,因此往往和药物或其他植物提取物配伍使用[6-7]。关于PIP生物功能的研究揭示了其作为饲料添加剂促进动物生长性能的潜力,但目前关于PIP在畜牧科研领域上的研究相对较少,阻滞了其作为一种新型添加剂在生产中的普及。因此,本文就PIP的理化性质、生物学功能以及在畜牧上的研究应用进行了系统性综述,为日后PIP作为饲料添加剂调控畜禽生产提供一些理论参考。
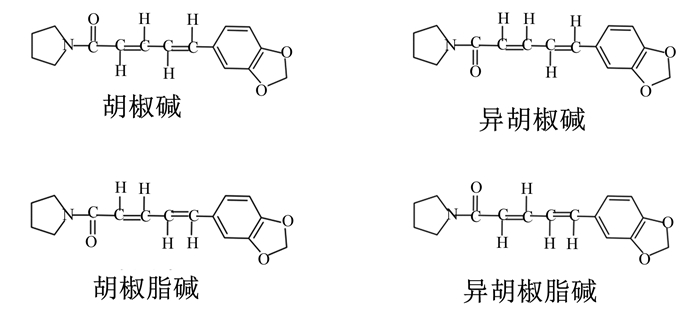
1 PIP的理化性质PIP又称为胡椒酰胺或胡椒辣碱,最早于1828年报道,是胡椒科植物胡椒(Piper nigrum L.)的主要有效成分[8],其在黑胡椒果实中含量可达5.0%~9.0%[9]。随后的研究发现PIP在多种胡椒科植物的根、茎、叶和果实中都广泛存在,包括荜茇(Piper longum)[10]、假荜拨(Piper Chaba)[11]、几内亚胡椒(Piper guineense)[12]和假蒟(Piper sarmentosum)[13]等。PIP属于生物碱类化合物,化学式为C17H19NO3,相对分子质量为285.34,为黄色结晶化合物,熔点为128~130 ℃,能溶于乙醇、乙酸、苯和氯仿,微溶于乙醚,但不溶于水和无机酸。PIP由3个部分组成——哌啶基、脂肪烃链和芳杂环,并具有3个同分异构体,分别是异胡椒碱(isopiperine)、胡椒脂碱(chavicine)、异胡椒脂碱(isochavicine)[14](图 1),但这些同分异构体均没有辛辣刺激性和药理活性。
2 PIP的生物学功能 2.1 增强化学药物的药效和一些生物分子的利用率生物分子在机体吸收代谢的过程中,可能会因为某些因素而影响其在体内的功能发挥,PIP作为一种生物增强剂(bio-enhancer),因其能增加体内药物及某些生物分子的利用率而被广泛关注。早在1985年,Atal等[15]就通过体内外试验发现PIP能以剂量依赖的方式,非竞争性抑制大鼠肝脏的芳香烃羟化酶(arylhydrocarbon hydroxylase,AHH)和二磷酸尿苷葡萄糖醛酸转移酶(uridine diphosphate-glucuronyltransferase,UDPGA)活性,从而延长药物在体内的半衰期,最终增加环己烯巴比妥和氯苯恶唑胺药物的安眠或麻痹效果。在随后的药物研究中,发现PIP能抑制多种参与药物转化的代谢酶,从而增加药物在体内的生物利用率。这些酶包括苯丙氨酸N-脱甲基酶[16]、氨基比林N-脱甲基酶[16]、苯胺羟化酶[16]、红霉素N-脱甲基酶[17]、7-甲氧基香豆素-O-脱甲基酶[17]、乙氧基甲红素-O-脱乙基酶[17]、还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)-细胞色素C还原酶[18]、5-脂氧合酶(5-lipoxygenase)[19]和细胞色素P450[20]等。由于PIP的非极性性质,其可以通过与小肠膜蛋白附近的脂质和疏水区域相互作用并改变小肠绒毛的胆固醇与磷脂比例,最终导致膜动力学和渗透能力的改变,促进小肠对生物分子的吸收[21-22]。转运蛋白对营养物质的吸收同样是十分重要的,目前关于PIP与转运蛋白相互作用的研究主要集中在P-糖蛋白(P-glycoprotein,P-gp)领域。在多项药物代谢研究中已经发现PIP作为P-gp底物,通过竞争性结合可以提高作为P-gp底物的其他药物的利用效率,包括多潘立酮[23]、阿霉素[24]和雷帕霉素[25]等。此外,PIP还可以通过抑制外排转运蛋白即乳腺癌耐药蛋白(breast cancer resistance protein,BCRP)和多药耐药相关蛋白2(multi-drug resistance protein 2,MRP2)来增强水飞蓟素(一种保肝剂)的吸收[26]。PIP还可以与血清白蛋白结合,增加生物分子的游离比例和溶解度,从而促进细胞对其的吸收[27-28]。除了药物外,PIP还表现出和植物提取物良好的配伍特性。植物提取物因其天然性、低毒性和多功能性被广泛用于食品、医学等领域的研究,然而其在机体内的低生物利用率是亟待解决的问题,研究发现,多种植物提取物与PIP的共同给药同样能增加其生物利用率并缓解多种疾病,如姜黄素[27]、熊果酸[29]、白藜芦醇[30]和槲皮素[31]等。
2.2 调节氧化应激PIP最初被认为是一种天然的抗氧化剂,可以清除过量自由基并减少致癌物诱导的肠黏膜氧化应激[32]。在体外试验中,PIP已被证明能抑制或淬灭自由基和活性氧(ROS),并通过抑制脂质过氧化来防止氧化损伤[33]。但随着研究的深入,人们逐渐发现PIP调节氧化应激的功能似乎随着处理模型和浓度的不同而表现出双向调节能力。大量研究已观察到,补充黑胡椒或PIP可降低代谢紊乱时脂质过氧化等氧化应激反应。在砷诱导的高脂饮食加重氧化应激介导的肝脏和心脏组织过程中,PIP(60 mg/kg BW)可通过降低抗氧化酶的降解,从而发挥其自由基猝灭能力,有效缓解氧化应激产生的损伤,这些抗氧化酶包括铜-锌超氧化物歧化酶(copper-zinc superoxide dismutase,Cu-Zn SOD)、锰超氧化物歧化酶(manganese superoxide dismutase,Mn-SOD)、过氧化氢酶(catalase,CAT)、和谷胱甘肽还原酶(glutathione reductase,GR)等[34]。类似的研究发现,30 mg/kg BW PIP能正常化血浆C反应蛋白浓度的增加从而减少炎症,同时通过降低血浆中尿酸浓度的增加和ROS的生成来减轻氧化应激,最终缓解大鼠在高碳水、高脂肪饮食下的代谢综合征[35]。由于其抗氧化和抗炎活性,PIP也具有保护肝脏的潜力。在小鼠肝损伤模型中,PIP(50 mg/kg BW)能通过抗氧化、抗炎和抗凋亡途径来逆转乙酰氨基酚诱导的肝脏损伤,从而对肝脏具有较好的保护作用,该项研究提出这种保护机制可能是由转化生长因子-β-受体相关结合蛋白1(transforming growth factor-β receptor-associated binding protein 1,TGFBRAP1)介导的[36]。而在另一些研究中发现,PIP能通过诱导氧化应激来发挥其积极作用。例如,PIP(1 μg/mL)能通过影响膜完整性从而诱导白色念珠菌的氧化应激反应,并最终促进其发生凋亡[37]。此外,在人乳腺癌细胞中,PIP(10 μg/mL)通过促进超氧化物歧化酶、CAT和GR的活性,增加脂质过氧化和羰基蛋白含量来诱导氧化应激,并在体内发挥抗肿瘤作用,这可能是由于过量ROS引发的肿瘤细胞凋亡[38]。尽管在不同模型中,PIP对氧化应激的调控作用存在差异化,但在总体上其趋于将氧化应激水平调整至一个有利于机体稳态的平衡状态。
2.3 调节糖、脂代谢目前研究认为,PIP能作为一个代谢稳定剂,平衡机体糖、脂代谢,这涉及到脂肪、肌肉、肠道和肝脏等。在代谢紊乱的情况下,PIP已被证实可以缓解肥胖[39]和2型糖尿病、改善非酒精性脂肪肝(nonalcoholic fatty liver disease,NAFLD)(和姜黄素联合使用时)[40]和注射味精的肥胖小鼠的葡萄糖耐受不良及胰岛素抵抗[41]等。在体外模型中,PIP可以通过拮抗3T3-L1前脂肪细胞中的成脂关键基因过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptors γ,PPARγ)的活性来抑制脂肪生成[42]。而在体外的高脂或高碳水化合物模型中,PIP已被证明可以通过调节氧化应激和减少炎症来缓解代谢综合征,包括肥胖、肝脏纤维化和胰岛素抵抗等[35, 41, 43]。每天口服PIP(15或30 mg/kg BW)并持续8周,可以通过抑制内脏脂肪组织、胰岛中巨噬细胞的募集和向促炎型的转化,显著改善高脂模型下小鼠胰岛β细胞的去分化和功能障碍,这对于全身的糖稳态是十分重要的[44]。体内外试验还表明,PIP可能通过修复肥胖引起的肠道紧密连接损伤,从而通过抑制脂肪酸吸收和增强肠道屏障功能来缓解肥胖[45]。也有研究认为,PIP对肥胖和糖尿病的缓解可以通过其上调静息肌纤维的代谢率来解释[39]。在小鼠模型中,Choi等[46]研究发现PIP(50 mg/kg BW)可逆转高脂饮食诱导的肝脏脂肪变性,而这一逆转可能是由脂联素(adiponectin)-单磷酸腺苷活化蛋白激酶(AMPK)信号通路介导的。而在一项针对NAFLD患者的临床试验中同样发现,每天补充500 mg姜黄素和5 mg PIP能显著缓解肝脏病理情况[40]。以上结果揭示了PIP在代谢紊乱过程中的促健康作用,这种糖、脂代谢调控能力可以靶向机体多个组织器官。
2.4 抗菌抗虫体内外的微生物对机体健康起着重要的调控作用,有害菌的过度定植会导致动物和人的健康受损。目前研究发现多种植物提取物表现出良好的抗菌活性,其中PIP的抗菌潜力也正逐渐被揭示,这一特性包含对细菌的直接抑制和激活机体免疫间接抑制的2个方面。首先,PIP可以直接作用于细菌的生物膜,抑制其生成从而发挥其抗菌功效[47];其次,PIP可以与细菌抗药性相关的外排蛋白直接结合并抑制其活性,增强细菌对药物的敏感性,如金黄色葡萄球菌中的外排泵NorA[48]和结核分枝杆菌中的外排蛋白Rv125c、Rv1258c、Rv1218c和Rv2942[49-50]等。除直接作用于细菌外,PIP还可以通过调控机体的免疫功能从而抑制有害菌在体内的增殖扩散。目前研究发现,PIP介导的代谢重编程可以促进机体内巨噬细胞抵抗细菌感染的能力。从机制上来讲,PIP通过向细胞膜上募集A类氨基酸转运蛋白(system L1 amino acid transporter),促进巨噬细胞的氨基酸代谢和哺乳动物雷帕霉素靶蛋白复合体C1(mammalian target of rapamycin protein complex C1,mTORC1)通路活性,从而保护巨噬细胞免受细菌诱导的凋亡[51]。在感染结核分枝杆菌的小鼠模型中,PIP(1 mg/kg BW)同样可促进T细胞和B细胞的增殖以及巨噬细胞的活化,并导致肺脏中细菌感染量的减少,这可能是由于PIP刺激T细胞分化为Th-1亚群,由此产生的细胞因子(干扰素-γ和白细胞介素-2)引起的免疫增强[52]。随着PIP生物学功能的揭示,其在体内外的抗虫潜力也正被发掘,并且这一抗虫特性显示出从虫卵一直持续到成虫。在体外共孵育试验中Silva等[53]发现,PIP能抑制90%以上的胃肠道线虫虫卵的孵化率和25.29%的幼虫运动能力,其半最大效应浓度(concentration for 50% of maximal effect,EC50)为0.007 4 mM。在食物中添加PIP也可显著诱导阿拉伯按蚊幼虫的死亡,并且这一抗幼虫特性会随着PIP浓度的增加而升高[54]。Kancharana等[55]研究报道,纳米级氧化锌包被的PIP制剂可在极低的浓度(7 mg/L)下表现出良好的杀螨虫活性,可作为化学杀螨剂的替代剂。而在体内试验中,Ray等[56]将两性霉素B和PIP共同封装在肠溶包衣颗粒中,口服该组合药对仓鼠体内的利什曼原虫的抑制率高达96%。在水生动物中PIP也表现出了良好的抗虫特性,9 mg/L的PIP溶液可有效控制淡水鱼中的寄生甲壳动物鲺属类寄生虫[57]。
3 PIP在畜禽生产中的应用随着高铜、高锌、饲用抗生素在畜禽养殖中的禁用,寻找可改善畜禽免疫状况和提高生长性能的替代品是目前养殖生产中亟待解决的问题。而植物提取物因其天然来源和多种生物学功能,在畜禽饲料添加剂研究领域中正逐渐成为研究热点,以期其能在畜禽生产中发挥积极作用。目前,PIP作为一种多功能的天然提取物,已被广泛应用于医药和食品领域。畜禽生产中添加PIP或胡椒(PIP主要来源)已被证明具有广泛的应用价值,具有提高畜禽生长性能、改善肠道健康、预防疾病、改善产品品质等功能,而将其与其他活性物质联合添加则可能具有更好的饲喂效果,现将最新研究进展总结如下。
3.1 PIP在猪生产中的应用为追求母猪生长性能,早期断奶技术早已普及在生猪养殖过程中,但随之产生的仔猪断奶应激综合征会严重损害仔猪的健康状况和随后的生长性能,因此如何缓解仔猪断奶后的应激反应是生产中面临的一个问题。研究发现,断奶仔猪饲粮中补充PIP或与其他添加剂联合饲喂可改善断奶仔猪的肠道健康,促进生长性能。刘海隆等[58]在仔猪饲粮中添加1%的胡椒枝叶或果实,结果发现两者皆能改善仔猪的肠道功能,减少腹泻率并提高平均日增重(ADG)。Rodrigues等[59]在受到大肠杆菌感染的断奶仔猪饲粮中添加了苯甲酸和富含PIP的植物混合精油,结果显示PIP可能具有增加苯甲酸生物学效价的功能,饲粮中添加3 g/kg的苯甲酸和含PIP的混合精油能显著提高仔猪的ADG和饲料转化率,该添加剂可作为传统抗菌制剂黏菌素的替代品,有助于减少养殖中产生的细菌耐药性。在对中国地方猪种的研究中,Shi等[60]研究发现,姜黄素(200 mg/kg)和PIP(50 mg/kg)联合应用比单独使用更能维持五指山断奶仔猪的肠道健康,两者联合使用能改善五指山猪的肠道屏障,抑制肠道氧化应激,增加饲料转化率并提高生长性能。与上述结果类似的是,含有PIP的一种植物提取物添加剂(添加含量为0.3%,其中各活性成分占比为苯甲酸89.3%、百里酚1.8%、丁香酚和PIP 3.2%)可有效改善断奶后仔猪的生长性能、饲料消化率和肠道健康[61]。而在育肥猪饲粮中,胡椒添加物同样能改善育肥猪的生长性能。Sampath等[62]在育肥猪饲粮中分别添加0.025%、0.05%、0.1%、0.2%、0.4%的胡椒提取物,在育肥10周后发现添加胡椒提取物组的ADG均较对照组增加,且和胡椒提取物添加比例呈线性关系;在整个试验过程中没有观察到平均日采食量的差异,表明胡椒提取物可以改善料重比,并提高育肥猪的生长性能;此外,胡椒提取物对猪肉品质也有一定的积极作用,还增加了育肥猪粪便中乳酸杆菌的数量并减少氨气的排放,并对大肠杆菌有一定的抑制作用。除了能在生猪养殖饲粮中添加外,PIP在猪宰后胴体贮存环节也同样能发挥作用。脂质氧化会降低肉的营养价值并损害肉类制品的基本感官品质,是影响生肉化学变质的最重要问题之一。Zhang等[63]研究报道,在猪肉贮存过程不同时间点喷洒0.5%的黑胡椒精油能显著减少猪肉的脂质氧化,延缓肉品质的下降,抑制假单胞菌和肠杆菌的定植,最终增加猪肉的新鲜程度。
3.2 PIP在鸡生产中的应用多项养殖试验已证明肉鸡饲粮中补充PIP或含有胡椒的植物添加剂不会降低肉鸡的采食量,但对增重、饲料转化率、免疫和肉质等指标可能具有积极影响[64-66]。一项荟萃分析研究同样发现饲粮添加胡椒可能具有提高肉鸡生长效率的潜力[67]。Kirubakaran等[64]研究报道,黑胡椒粉(0.1%~0.2%)和大蒜粉(0.5%~1.0%)联合饲喂能增加肉鸡的增重和饲料转化率。另一项将植物提取物应用于肉鸡养殖中的研究发现,单独补充0.5%的黑胡椒同样能显著促进肉鸡的增重,但在此基础上额外添加0.5%的姜黄粉和2.0%的芫荽籽则能进一步改善肉鸡的健康状况[65]。近期一项研究表明,基础饲粮中联合添加精油(1.5 g/kg)和黑胡椒油(0.25或0.50 g/kg)能够显著提高肉仔鸡的体增重和饲料转化率[66]。除了调控肉鸡生长性能外,PIP还表现出改善禽类健康的应用潜力。在类固醇诱导的鸡胚和肝脏组织氧化应激中,PIP(100 mg/kg)能通过增加还原型谷胱甘肽(GSH)和总抗氧化状态(TAS)来减少氧化损伤[2, 68]。作为一种生物增强剂,PIP(10 mg/kg BW)还能通过抑制外排转运蛋白和肠道、肝脏部位药物代谢酶的表达来增强马波沙星(一种氟喹诺酮类抗菌药)在肉鸡体内的药效[69]。
家禽中的黄曲霉毒素污染也是生产中的常见问题。Da Silva Cardoso等[70]研究发现,饲粮中添加安全剂量的PIP(0.6%)可以显著缓解肉鸡的黄曲霉毒素中毒症状,包括减少外周血细胞中的DNA损伤、降低微核红细胞数量以及多色红细胞与正常红细胞的比率等。Verlinden等[71]在体外筛选试验中发现,PIP对蛋鸡群中分离出来的中间短螺旋体(可诱发盲肠肠炎)具有一定的抑制作用,然而其最小抑制浓度较高,限制了生产中的应用,这可能是由于PIP本身生物学利用度低的原因[72]。饲粮中补充胡椒提取物已被证明能导致鸡蛋中PIP及其异构体浓度的增加[73],由于前期研究表明PIP可能引起生殖系统的障碍[74],其能否影响鸡的繁殖和产蛋性能也是个值得关注的问题。杨舒婷[75]研究发现,作为一种天然免疫调节剂,饲粮中添加适宜剂量的PIP(10 mg/kg BW)可以缓解海兰白鸡因炎症引起的产蛋性能下降和肠道炎症损伤,因此PIP对蛋鸡的产蛋性能可能不会产生负面影响,但对繁殖性能的影响目前还未有报道。在禽类肉产品加工过程中也发现了PIP的功能,Jaworska等[76]发现在禽类肉制品中添加黑胡椒(0.03%或0.30%)或其提取物能减少脂质氧化,抑制微生物生长,从而达到改善禽类肉制品感官品质的效果。
3.3 PIP在反刍动物生产中的应用PIP目前在反刍动物生产中的研究较少,仅有的几项研究结果表明,PIP可能具有调控瘤胃微环境并提高反刍动物生长性能的潜力;此外,PIP的抗氧化和抗寄生虫特性也被认为能改善反刍动物健康状况。假蒟是一种胡椒科、胡椒属植物,其根、茎、叶中含有大量PIP[13]。Cherdthong等[77]研究报道,每天补充2.4 g假蒟叶粉可以提高肉牛的总采食量和干物质消化率;进一步研究发现,假蒟叶能改善瘤胃生态,增加丙酸含量并降低甲烷的排放。另一项体外研究发现,黑胡椒和多种植物提取物相互作用,能提高瘤胃的发酵效率[78]。Hassan等[79]研究报道,含黑胡椒的草药混合剂(20 g/d)在不影响干物质摄入量和瘤胃发酵参数的情况下,有改变水牛瘤胃微生物丰度的趋势,并能增加乳中的乳脂率和不饱和脂肪酸含量。除了调控瘤胃微环境介导的生长性能改变,PIP也能被用于维持反刍动物的健康。在体外研究中发现,PIP可以通过其抗氧化活性保护山羊心脏线粒体免受抗坏血酸铜诱导的毒性损伤,这表明PIP可被视为未来的治疗性抗氧化剂[80]。胃肠道线虫经常在小型反刍动物消化道中出现,不当的使用抗生素驱虫药可能会导致抗药性和畜产品中药物残留等问题。生物碱已被证明具有广泛的抗寄生虫作用。Silva等[53]测试了多种生物碱对胃肠道线虫的驱虫活性,结果显示PIP在细胞低毒性剂量下能抑制90%以上的虫卵孵化并降低25.29%的幼虫运动能力,表现出较好的驱虫潜力。
4 小结在畜禽生产研究中,PIP因其多种生物学功能不仅能提高动物生长性能和改善畜产品品质,还能部分替代抗生素和抗菌抗虫药,因此在畜禽健康绿色发展中具有极大的应用前景。尽管我们已经发现了PIP的多种生物学功能,但是在很多领域内对其发挥作用的分子机制还不清晰,这可能会导致PIP应用过程中的脱靶效应,因此深入了解PIP调控功能的详细机制是十分必要的。而目前在养殖应用中,作为生物增强剂,PIP虽然能促进其他化学和生物分子在机体内的利用效率,但其自身的低溶解度和低生物利用率部分地限制了它的普及和应用。如何提高PIP的生物利用率也是一个重要的问题,酸化或包被处理或许能增加其在机体内的吸收率。反刍动物的瘤胃微环境可能会对PIP进行代谢,其在瘤胃中的代谢反应和代谢产物活性目前都是未知的,这也需要进一步加深理解PIP的代谢过程。PIP的高成本同样可能会限制其在畜牧业的推广,但PIP不仅存在于胡椒果实中,在胡椒科植物的根、茎、叶中也广泛存在。目前关于胡椒科经济作物副产品在养殖生产中的应用还较少,胡椒种植大省海南每年因修剪而产生的大量胡椒枝叶资源也未被很好利用,因此,未来的研究重点应该是合理开发利用此部分饲料资源[58]。除了开发含PIP的新型饲料原料外,筛选出能生产PIP的真菌也具有很大的生产潜力[81]。通过将PIP和药物共同给药,能在提高效价的情况下减少畜禽生产中的药物使用量,这对减少药物在产品中的残留和细菌的抗药性都是十分有价值的,深入研究PIP和各种药物的药代动力学,以调整生产中药物使用的最佳比例。除了常规药物外,PIP和多种植物提取物的共同使用还表现出更好的促生长和维持健康功能,开发包含PIP的植物添加剂也有助于绿色生态养殖的推广。
综上所述,PIP作为一种新型饲料添加剂,已展现出了巨大的应用潜力,但推广普及同样存在很多问题需要攻克。
| [1] |
MANJUNATH G B, AWASTHI S P, ZAHID M S H, et al. Piperine, an active ingredient of white pepper, suppresses the growth of multidrug-resistant toxigenic Vibrio cholerae and other pathogenic bacteria[J/OL]. Letters in Applied Microbiology, 2022. (2022-01-03)[2022-01-05]. https://pubmed.ncbi.nlm.nih.gov/34978719. DOI: 10.1111/lam.13646.
|
| [2] |
VURMAZ A, ATAY E. Antioxidant effects of piperine on steroid-induced hepatotoxicity[J]. European Review for Medical and Pharmacological Sciences, 2021, 25(17): 5500-5506. |
| [3] |
HOU X M, ZHANG C X, WANG L M, et al. Natural piperine improves lipid metabolic profile of high-fat diet-fed mice by upregulating SR-B1 and ABCG8 transporters[J]. Journal of Natural Products, 2021, 84(2): 373-381. DOI:10.1021/acs.jnatprod.0c01018 |
| [4] |
MAEDA A, SHIRAO T, SHIRASAYA D, et al. Piperine promotes glucose uptake through ROS-dependent activation of the CAMKK/AMPK signaling pathway in skeletal muscle[J]. Molecular Nutrition & Food Research, 2018, 62(11): e1800086. |
| [5] |
SAETANG J, TEDASEN A, SANGKHATHAT S, et al. Low piperine fractional Piper nigrum extract enhanced the antitumor immunity via regulating the Th1/Th2/Treg cell subsets on NMU-induced tumorigenesis rats[J/OL]. Planta Medica, 2021. (2021-04-26)[2022-01-05]. https://pubmed.ncbi.nlm.nih.gov/33902130. DOI: 10.1055/a-1458-5646.
|
| [6] |
BASU S, JANA S, PATEL V B, et al. Effects of piperine, cinnamic acid and gallic acid on rosuvastatin pharmacokinetics in rats[J]. Phytotherapy Research, 2013, 27(10): 1548-1556. |
| [7] |
PAWAR K S, MASTUD R N, PAWAR S K, et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19:a randomized clinical trial[J]. Frontiers in Pharmacology, 2021, 12: 669362. DOI:10.3389/fphar.2021.669362 |
| [8] |
CARPENTER G W. Observations on piperine, with the formula for its preparation, &c[J]. The London Medical and Physical Journal, 1828, 5(26): 117-122. |
| [9] |
EVANS W C. Trease and Evans' pharmacognosy[M]. 14th ed. London: W.B. Saunders, 1996.
|
| [10] |
MOHAPATRA M, BASAK U C. Evaluation of piperine content from roots of Piper longum Linn., originated from different sources with comparison of zonal variation in Odisha, India[J]. ResearchGate, 2015, 4(9): 1-8. |
| [11] |
KHAN M. Comparative physicochemical evaluation of fruits and anti depressant potential of volatile oils of fruits of local piper species[J]. Oriental Journal of Chemistry, 2015, 31(1): 541-545. DOI:10.13005/ojc/310167 |
| [12] |
JULIANI H R, KOROCH A R, GIORDANO L, et al. Piper guineense (Piperaceae): chemistry, traditional uses, and functional properties of west African black pepper[M]//JULIANI H R, SIMON J E, HO C T. African Natural Plant Products Volume Ⅱ: Discoveries and Challenges in Chemistry, Health, and Nutrition. Washington, D.C. : American Chemical Society, 2013: 33-48.
|
| [13] |
HUSSAIN K, ISMAIL Z, SADIKUN A, et al. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb[J]. Natural Product Research, 2009, 23(3): 238-249. DOI:10.1080/14786410801987597 |
| [14] |
HAQ I U, IMRAN M, NADEEM M, et al. Piperine: a review of its biological effects[J]. Phytotherapy Research, 2021, 35(2): 680-700. DOI:10.1002/ptr.6855 |
| [15] |
ATAL C K, DUBEY R K, SINGH J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism[J]. Journal of Pharmacology and Experimental Therapeutics, 1985, 232(1): 258-262. |
| [16] |
DALVI R R, DALVI P S. Comparison of the effects of piperine administered intragastrically and intraperitoneally on the liver and liver mixed-function oxidases in rats[J]. Drug Metabolism and Drug Interactions, 1991, 9(1): 23-30. |
| [17] |
NAJAR I A, SHARMA S C, SINGH G D, et al. Involvement of P-glycoprotein and CYP 3A4 in the enhancement of etoposide bioavailability by a piperine analogue[J]. Chemico-Biological Interactions, 2011, 190(2/3): 84-90. |
| [18] |
DALVI R R, DALVI P S. Differences in the effects of piperine and piperonyl butoxide on hepatic drug-metabolizing enzyme system in rats[J]. Drug and Chemical Toxicology, 1991, 14(1/2): 219-229. |
| [19] |
PRASAD N S, RAGHAVENDRA R, LOKESH B R, et al. Spice phenolics inhibit human PMNL 5-lipoxygenase[J]. Prostaglandins Leukotrienes and Essential Fatty Acids, 2004, 70(6): 521-528. DOI:10.1016/j.plefa.2003.11.006 |
| [20] |
SHAMSI S, TRAN H, TAN R S J, et al. Curcumin, piperine, and capsaicin: a comparative study of spice-mediated inhibition of human cytochrome P450 isozyme activities[J]. Drug Metabolism and Disposition, 2017, 45(1): 49-55. DOI:10.1124/dmd.116.073213 |
| [21] |
KHAJURIA A, THUSU N, ZUTSHI U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics[J]. Phytomedicine, 2002, 9(3): 224-231. DOI:10.1078/0944-7113-00114 |
| [22] |
PRAKASH U N S, SRINIVASAN K. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats[J]. British Journal of Nutrition, 2010, 104(1): 31-39. DOI:10.1017/S0007114510000334 |
| [23] |
ISLAM N, IRFAN M, HUSSAIN T, et al. Piperine phytosomes for bioavailability enhancement of domperidone[J/OL]. Journal of Liposome Research, 2021: 1-9. (2021-05-04)[2022-01-05]. https://pubmed.ncbi.nlm.nih.gov/33944662/. DOI: 10.1080/08982104.2021.1918153.
|
| [24] |
LI H M, KRSTIN S, WANG S H, et al. Capsaicin and piperine can overcome multidrug resistance in cancer cells to doxorubicin[J]. Molecules, 2018, 23(3): 557. DOI:10.3390/molecules23030557 |
| [25] |
KATIYAR S S, MUNTIMADUGU E, RAFEEQI T A, et al. Co-delivery of rapamycin-and piperine-loaded polymeric nanoparticles for breast cancer treatment[J]. Drug Delivery, 2016, 23(7): 2608-2616. DOI:10.3109/10717544.2015.1039667 |
| [26] |
BI X L, YUAN Z W, QU B, et al. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2[J]. Phytomedicine, 2019, 54: 98-108. DOI:10.1016/j.phymed.2018.09.217 |
| [27] |
ITAYA M, MIYAZAWA T, KHALIFA S, et al. The inhibition of interaction with serum albumin enhances the physiological activity of curcumin by increasing its cellular uptake[J]. Food & Function, 2022, 13(2): 639-648. |
| [28] |
FLORY S, SUS N, HAAS K, et al. Increasing post-digestive solubility of curcumin is the most successful strategy to improve its oral bioavailability: a randomized cross-over trial in healthy adults and in vitro bioaccessibility experiments[J]. Molecular Nutrition & Food Research, 2021, 65(24): e2100613. |
| [29] |
BISWAS S, KAR A, SHARMA N, et al. Synergistic effect of ursolic acid and piperine in CCl4 induced hepatotoxicity[J]. Annals of Medicine, 2021, 53(1): 2009-2017. DOI:10.1080/07853890.2021.1995625 |
| [30] |
XU Y, ZHANG C, WU F Y, et al. Piperine potentiates the effects of trans-resveratrol on stress-induced depressive-like behavior: involvement of monoaminergic system and cAMP-dependent pathway[J]. Metabolic Brain Disease, 2016, 31(4): 837-848. DOI:10.1007/s11011-016-9809-y |
| [31] |
LI H W, LI M Z, FU J X, et al. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions[J]. Drug Delivery, 2021, 28(1): 1226-1236. DOI:10.1080/10717544.2021.1927244 |
| [32] |
KHAJURIA A, THUSU N, ZUTSHI U, et al. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa[J]. Molecular and Cellular Biochemistry, 1998, 189(1/2): 113-118. DOI:10.1023/A:1006877614411 |
| [33] |
MITTAL R, GUPTA R L. In vitro antioxidant activity of piperine[J]. Methods and Findings in Experimental and Clinical Pharmacology, 2000, 22(5): 271-274. DOI:10.1358/mf.2000.22.5.796644 |
| [34] |
DEY T, GHOSH A, MISHRA S, et al. Attenuation of arsenic induced high fat diet exacerbated oxidative stress mediated hepatic and cardiac injuries in male Wistar rats by piperine involved antioxidative mechanisms[J]. Food and Chemical Toxicology, 2020, 142: 111477. DOI:10.1016/j.fct.2020.111477 |
| [35] |
DIWAN V, POUDYAL H, BROWN L. Piperine attenuates cardiovascular, liver and metabolic changes in high carbohydrate, high fat-fed rats[J]. Cell Biochemistry and Biophysics, 2013, 67(2): 297-304. DOI:10.1007/s12013-011-9306-1 |
| [36] |
MORSY M A, YOUNIS N S, EL-SHEIKH A A K, et al. Protective mechanisms of piperine against acetaminophen-induced hepatotoxicity may be mediated through TGFBRAP1[J]. European Review for Medical and Pharmacological Sciences, 2020, 24(19): 10169-10180. |
| [37] |
THAKRE A, JADHAV V, KAZI R B A, et al. Oxidative stress induced by piperine leads to apoptosis in Candida albicans[J]. Medical Mycology, 2021, 59(4): 366-378. DOI:10.1093/mmy/myaa058 |
| [38] |
DE SOUZA GRINEVICIUS V M A, KVIECINSKI M R, SANTOS MOTA N S R, et al. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells[J]. Journal of Ethnopharmacology, 2016, 189: 139-147. DOI:10.1016/j.jep.2016.05.020 |
| [39] |
NOGARA L, NABER N, PATE E, et al. Piperine's mitigation of obesity and diabetes can be explained by its up-regulation of the metabolic rate of resting muscle[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(46): 13009-13014. DOI:10.1073/pnas.1607536113 |
| [40] |
PANAHI Y, VALIZADEGAN G, AHAMDI N, et al. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: a clinical trial[J]. Journal of Cellular Biochemistry, 2019, 120(9): 15989-15996. DOI:10.1002/jcb.28877 |
| [41] |
LIU C L, YUAN Y T, ZHOU J, et al. Piperine ameliorates insulin resistance via inhibiting metabolic inflammation in monosodium glutamate-treated obese mice[J]. BMC Endocrine Disorders, 2020, 20(1): 152. DOI:10.1186/s12902-020-00617-1 |
| [42] |
PARK U H, JEONG H S, JO E Y, et al. Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARγ activity in 3T3-L1 cells[J]. Journal of Agricultural and Food Chemistry, 2012, 60(15): 3853-3860. DOI:10.1021/jf204514a |
| [43] |
BRAHMANAIDU P, NEMANI H, MERIGA B, et al. Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats[J]. Chemico-Biological Interactions, 2014, 221: 42-51. DOI:10.1016/j.cbi.2014.07.008 |
| [44] |
YUAN Y T, ZHOU J, HU R X, et al. Piperine protects against pancreatic β-cell dysfunction by alleviating macrophage inflammation in obese mice[J]. Life Sciences, 2021, 274: 119312. DOI:10.1016/j.lfs.2021.119312 |
| [45] |
WANG W L, ZHANG Y H, WANG X, et al. Piperine improves obesity by inhibiting fatty acid absorption and repairing intestinal barrier function[J]. Plant Foods for Human Nutrition, 2021, 76(4): 410-418. DOI:10.1007/s11130-021-00919-2 |
| [46] |
CHOI S, CHOI Y, CHOI Y, et al. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice[J]. Food Chemistry, 2013, 141(4): 3627-3635. DOI:10.1016/j.foodchem.2013.06.028 |
| [47] |
DWIVEDI D, SINGH V. Effects of the natural compounds embelin and piperine on the biofilm-producing property of Streptococcus mutans[J]. Journal of Traditional and Complementary Medicine, 2016, 6(1): 57-61. DOI:10.1016/j.jtcme.2014.11.025 |
| [48] |
SANGWAN P L, KOUL J L, KOUL S, et al. Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors[J]. Bioorganic & Medicinal Chemistry, 2008, 16(22): 9847-9857. |
| [49] |
SHARMA S, KUMAR M, SHARMA S, et al. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis[J]. Journal of Antimicrobial Chemotherapy, 2010, 65(8): 1694-1701. DOI:10.1093/jac/dkq186 |
| [50] |
CALSAVARA L L, HEGETO L A, SAMPIRON E G, et al. Rescue of streptomycin activity by piperine in Mycobacterium tuberculosis[J]. Future Microbiology, 2021, 16: 623-633. DOI:10.2217/fmb-2020-0124 |
| [51] |
PAN H, XU L H, HUANG M Y, et al. Piperine metabolically regulates peritoneal resident macrophages to potentiate their functions against bacterial infection[J]. Oncotarget, 2015, 6(32): 32468-32483. DOI:10.18632/oncotarget.5957 |
| [52] |
SHARMA S, KALIA N P, SUDEN P, et al. Protective efficacy of piperine against Mycobacterium tuberculosis[J]. Tuberculosis, 2014, 94(4): 389-396. DOI:10.1016/j.tube.2014.04.007 |
| [53] |
SILVA G D, DE LIMA H G, DE SOUSA N B, et al. In vitro anthelmintic evaluation of three alkaloids against gastrointestinal nematodes of goats[J]. Veterinary Parasitology, 2021, 296: 109505. DOI:10.1016/j.vetpar.2021.109505 |
| [54] |
SAMUEL M, OLIVER S V, COETZEE M, et al. The larvicidal effects of black pepper (Piper nigrum L.) and piperine against insecticide resistant and susceptible strains of Anopheles malaria vector mosquitoes[J]. Parasites & Vectors, 2016, 9: 238. |
| [55] |
KANCHARANA S, CHENGALVA R V, KOTHAPALLI S R, et al. Assessment of acaricidal activity of nanoscale ZnO encapsulated piperine formulation against Rhipicephalus microplus[J]. IET Nanobiotechnology, 2020, 14(8): 722-731. DOI:10.1049/iet-nbt.2020.0159 |
| [56] |
RAY L, KARTHIK R, SRIVASTAVA V, et al. Efficient antileishmanial activity of amphotericin B and piperine entrapped in enteric coated guar gum nanoparticles[J]. Drug Delivery and Translational Research, 2021, 11(1): 118-130. DOI:10.1007/s13346-020-00712-9 |
| [57] |
KUMAR A, RAMAN R P, KUMAR K, et al. Antiparasitic efficacy of piperine against Argulus spp. on Carassius auratus (Linn. 1758): in vitro and in vivo study[J]. Parasitology Research, 2012, 111(5): 2071-2076. DOI:10.1007/s00436-012-3054-z |
| [58] |
刘海隆, 曹宗喜, 晁哲, 等. 日粮中添加胡椒枝叶及果实对仔猪生长性能和肠道发育的影响[J]. 饲料工业, 2021, 42(9): 21-24. LIU H L, CAO Z X, CHAO Z, et al. Effects of branches, leaves and fruits of pepper on growth performance and intestinal development of piglets[J]. Feed Industry, 2021, 42(9): 21-24 (in Chinese). |
| [59] |
RODRIGUES L M, NETO T O D A L, GARBOSSA C A P, et al. Benzoic acid combined with essential oils can be an alternative to the use of antibiotic growth promoters for piglets challenged with E. coli F4[J]. Animals, 2020, 10(11): 1978. DOI:10.3390/ani10111978 |
| [60] |
SHI L G, XUN W J, PENG W Q, et al. Effect of the single and combined use of curcumin and piperine on growth performance, intestinal barrier function, and antioxidant capacity of weaned Wuzhishan piglets[J]. Frontiers in Veterinary Science, 2020, 7: 418. DOI:10.3389/fvets.2020.00418 |
| [61] |
SILVA JÚNIOR C D, MARTINS C C S, DIAS F T F, et al. The use of an alternative feed additive, containing benzoic acid, thymol, eugenol, and piperine, improved growth performance, nutrient and energy digestibility, and gut health in weaned piglets[J]. Journal of Animal Science, 2020, 98(5): skaa119. DOI:10.1093/jas/skaa119 |
| [62] |
SAMPATH V, SHANMUGAM S, PARK J H, et al. The effect of black pepper (piperine) extract supplementation on growth performance, nutrient digestibility, fecal microbial, fecal gas emission, and meat quality of finishing pigs[J]. Animals, 2020, 10(11): 1965. DOI:10.3390/ani10111965 |
| [63] |
ZHANG J, WANG Y, PAN D D, et al. Effect of black pepper essential oil on the quality of fresh pork during storage[J]. Meat Science, 2016, 117: 130-136. DOI:10.1016/j.meatsci.2016.03.002 |
| [64] |
KIRUBAKARAN A, MOORTHY M, CHITRA R, et al. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers[J]. Veterinary World, 2016, 9(5): 470-474. DOI:10.14202/vetworld.2016.470-474 |
| [65] |
ABOU-ELKHAIR R, AHMED H A, SELIM S. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens[J]. Asian-Australasian Journal of Animal Sciences, 2014, 27(6): 847-854. DOI:10.5713/ajas.2013.13644 |
| [66] |
KISHAWY A T Y, AL-KHALAIFAH H S, NADA H S, et al. Black pepper or radish seed oils in a new combination of essential oils modulated broiler chickens' performance and expression of digestive enzymes, lipogenesis, immunity, and autophagy-related genes[J]. Veterinary Sciences, 2022, 9(2): 43. DOI:10.3390/vetsci9020043 |
| [67] |
OGBUEWU I P, OKORO V M, MBAJIORGU C A. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance[J]. Tropical Animal Health and Production, 2020, 52(1): 17-30. DOI:10.1007/s11250-019-02118-3 |
| [68] |
VURMAZ A, DUMAN R, SABANER M C, et al. Antioxidant effects of piperine in in-vivo chick embryo cataract model induced by steroids[J]. Cutaneous and Ocular Toxicology, 2019, 38(2): 182-189. DOI:10.1080/15569527.2019.1570521 |
| [69] |
PATEL H B, PATEL U D, MATHAPATI B S, et al. Effect of piperine and quercetin alone or in combination with marbofloxacin on CYP3A37 and MDR1 mRNA expression levels in broiler chickens[J]. Research in Veterinary Science, 2019, 126: 178-183. DOI:10.1016/j.rvsc.2019.09.005 |
| [70] |
DA SILVA CARDOSO V, VERMELHO A B, RIBEIRO DE LIMA C A, et al. Antigenotoxic effect of piperine in broiler chickens intoxicated with aflatoxin B1[J]. Toxins, 2016, 8(11): 316. DOI:10.3390/toxins8110316 |
| [71] |
VERLINDEN M, PASMANS F, MAHU M, et al. In vitro sensitivity of poultry Brachyspira intermedia isolates to essential oil components and in vivo reduction of Brachyspira intermedia in rearing pullets with cinnamaldehyde feed supplementation[J]. Poultry Science, 2013, 92(5): 1202-1207. DOI:10.3382/ps.2012-02690 |
| [72] |
REN T J, HU M Y, CHENG Y, et al. Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control[J]. European Journal of Pharmaceutical Sciences, 2019, 137: 104988. DOI:10.1016/j.ejps.2019.104988 |
| [73] |
TERNES W, KRAUSE E L. Characterization and determination of piperine and piperine isomers in eggs[J]. Analytical and Bioanalytical Chemistry, 2002, 374(1): 155-160. DOI:10.1007/s00216-002-1416-6 |
| [74] |
CHEN X W, GE F, LIU J P, et al. Diverged effects of piperine on testicular development: stimulating leydig cell development but inhibiting spermatogenesis in rats[J]. Frontiers in Pharmacology, 2018, 9: 244. DOI:10.3389/fphar.2018.00244 |
| [75] |
杨舒婷. 胡椒碱对炎症引起的高产蛋鸡减产效应的防御作用[D]. 硕士学位论文. 杭州: 浙江大学, 2021. YANG S T. Protective effect of piperine on the decreased laying performance of high-laying hens caused by inflammation[D]. Master's Thesis. Hangzhou: Zhejiang University, 2021. (in Chinese) |
| [76] |
JAWORSKA D, ROSIAK E, KOSTYRA E, et al. Effect of herbal addition on the microbiological, oxidative stability and sensory quality of minced poultry meat[J]. Foods, 2021, 10(7): 1537. DOI:10.3390/foods10071537 |
| [77] |
CHERDTHONG A, KHONKHAENG B, FOIKLANG S, et al. Effects of supplementation of Piper sarmentosum leaf powder on feed efficiency, rumen ecology and rumen protozoal concentration in Thai native beef cattle[J]. Animals, 2019, 9(4): 130. DOI:10.3390/ani9040130 |
| [78] |
TEMMAR R, RODRÍGUEZ-PRADO M, FORGEARD G, et al. Interactions among natural active ingredients to improve the efficiency of rumen fermentation in vitro[J]. Animals, 2021, 11(5): 1205. DOI:10.3390/ani11051205 |
| [79] |
HASSAN F, TANG Z H, EBEID H M, et al. Consequences of herbal mixture supplementation on milk performance, ruminal fermentation, and bacterial diversity in water buffaloes[J]. PeerJ, 2021, 9: e11241. DOI:10.7717/peerj.11241 |
| [80] |
DUTTA M, GHOSH A K, MISHRA P, et al. Protective effects of piperine against copper-ascorbate induced toxic injury to goat cardiac mitochondria in vitro[J]. Food & Function, 2014, 5(9): 2252-2267. |
| [81] |
CHITHRA S, JASIM B, SACHIDANANDAN P, et al. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum[J]. Phytomedicine, 2014, 21(4): 534-540. DOI:10.1016/j.phymed.2013.10.020 |