作为一种天然的瘤胃微生物代谢产物,共轭亚油酸(conjugated linoleic acid,CLA)是一系列具有共轭双键的十八碳二烯酸的位置和几何异构体的混合物,主要来源于瘤胃丁酸弧菌对亚油酸的生物转化作用[1]。饲粮组成、瘤胃pH和微生物群落活力是影响反刍动物瘤胃CLA合成的重要因素[2]。在人类等医学领域的相关研究中,已充分证明CLA由于与过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor,PPAR)配体具有高度的相似性,可以通过激活PPAR调控机体代谢和免疫稳态。PPAR属于核受体亚家族中的成员,通常表达于肠道、巨噬细胞和白色脂肪等组织,在炎症发生、脂质和碳水化合物代谢、细胞周期等多种信号通路的调控中发挥重要作用[3]。但是,CLA有益作用的发挥常存在一定的争议性,且具有物种、组织和细胞类型特异性[4-6]。瘤胃作为反刍动物最重要的消化器官,其免疫稳态的维持对动物机体健康和生产力的提高至关重要。瘤胃内环境稳态的失调会导致细菌毒素或组胺等有害物质的释放增加,刺激瘤胃上皮后导致上皮通透性增加,紧密连接破坏,自我修复能力减弱,屏障功能受损。瘤胃上皮屏障功能损伤后会导致瘤胃内毒素等有害物质易位入血,最终引发全身性病理反应[7]。目前,CLA在反刍动物上的应用主要集中于关注能量平衡、乳脂合成等方面[8-9],其在瘤胃上皮屏障功能稳态维持方面的报道还较少。因此,本文以反刍动物瘤胃上皮屏障功能损伤的危害为切入点,总结CLA调控瘤胃上皮屏障功能的可能生物学机制,为其在反刍动物健康养殖中的应用提供理论依据。
1 反刍动物瘤胃上皮屏障功能损伤的危害反刍动物生产中,常使用高谷物饲粮来满足高产奶量的能量需求。因此,在奶牛生产实践中,瘤胃酸中毒尤其是亚急性瘤胃酸中毒(subacute ruminal acidosis,SARA)时有发生[10]。SARA会导致有毒和有害物质如乳酸、乙醇、组胺、酪胺、色胺和细菌内毒素[如脂多糖(LPS)]等的释放增加[11]。LPS可以造成消化道上皮组织的损伤和通透性改变,进而易位进入血液中。血液循环中LPS含量的升高会引起全身性免疫反应,包括增加血液中性粒细胞和急性期蛋白如血清淀粉样蛋白A(serum amyloid A protein,SAA)、触珠蛋白(haptoglobin,Hp)、LPS结合蛋白(lipopolysaccharide binding protein,LBP)、C-反应蛋白(C-reactive protein,CRP)的浓度,刺激单核吞噬细胞释放白细胞介素-1(interleukin-1,IL-1)、白细胞介素-6(interleukin-6,IL-6)和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等炎症细胞因子等[12]。易位入血的LPS亦会导致代谢稳态的改变,表现为血糖和非酯化脂肪酸浓度的升高,动物采食量的下降[13-14]。细菌免疫原还会导致乳合成所需营养物质的供应减少,抑制乳腺上皮细胞的增殖和功能(脂肪酸的从头合成和吸收等),增加活性氧释放,造成氧化应激损伤[15]。研究表明,瘤胃LPS和血浆急性期蛋白浓度的增加与乳脂含量和产量、3.5%脂肪校正乳产量以及乳能量效率的下降密切相关[16]。
2 CLA调控瘤胃上皮屏障功能的生物学机制 2.1 通过缓解瘤胃上皮炎症发生调控瘤胃上皮屏障功能研究证实瘤胃上皮炎症发生与屏障结构和功能的破坏密切相关[17]。因此,通过缓解瘤胃上皮炎症发生是保护屏障功能的重要举措之一。Bayat等[18]和Gnott等[19]发现CLA的添加可以减少奶牛泌乳早期和晚期血液促炎因子和活性氧代谢物生成,具有降低全身性炎症反应和氧化应激发生的营养调控作用。但目前CLA在缓解瘤胃上皮炎症发生中的特异性作用报道还较少。本研究团队用cis-9, trans-11-CLA和trans-10, cis-12-CLA混合物(1:1)预处理瘤胃上皮细胞后发现,瘤胃上皮细胞可以抵抗LPS诱导的促炎细胞因子基因(如IL-6和TNF-α)的表达上调;基于转录组学测序发现,此CLA混合物抑制了肿瘤坏死因子(tumor necrosis factor,TNF)信号通路、细胞外基质受体(extracellular matrix receptor,ECM-receptor)相互作用途径等与炎症发生相关的分子信号通路,而上调了PPAR信号通路[20]。通过分别研究cis-9, trans-11-CLA和trans-10, cis-12-CLA的单独作用,发现trans-10, cis-12-CLA的抗炎效果较cis-9, trans-11-CLA更好,cis-9, trans-11-CLA单独作用时,并没有显著下调瘤胃上皮细胞受LPS刺激时IL-6和NF-κB的表达,而trans-10, cis-12-CLA的单独处理显著下调了IL-6和NF-κB的表达,转录组学测序结果进一步揭示trans-10, cis-12-CLA显著下调了NF-κB、趋化因子和NOD样受体(NOD-like receptor)等炎症发生信号通路[21]。研究发现trans-10, cis-12-CLA亦可通过下调瘤胃上皮细胞病原体识别受体如Toll样受体4(Toll-like receptor 4,TLR4)的表达来抑制LPS诱发的炎症反应[21]。CLA在抗炎作用中的同分异构体特异性亦被诸多试验证明。Jaudszus等[22]研究发现,与trans-10, cis-12-CLA相比,cis-9, trans-11-CLA在人支气管上皮细胞的炎症发生中表现出更好的抗炎效果。而Dipasquale等[23]研究发现,分别用cis-9, trans-11-CLA和trans-10, cis-12-CLA预处理牛乳腺上皮细胞后,trans-10, cis-12-CLA预处理的牛乳腺上皮细胞在受到LPS刺激后表现出更好的抗炎效果,对促炎细胞因子表达的下调作用更加均一化,且显著上调了PPARγ的表达。因此,CLA可以通过降低有害抗原刺激瘤胃上皮时炎症因子的生成来维持上皮屏障功能稳态,但具体机制仍需进一步的试验验证。
2.2 通过调控瘤胃上皮损伤修复维持瘤胃上皮屏障功能瘤胃上皮屏障功能主要由完整的上皮细胞和细胞间紧密连接来维持[24]。研究发现诸多细胞因子、炎症介质和细菌毒素能够通过不同的信号途径作用于胃肠道上皮细胞或细胞间紧密连接,诱导上皮细胞凋亡,影响细胞间紧密连接的表达和分布,调控细胞骨架重组,使得上皮屏障通透性增加,进而引发胃肠道继发性损伤和功能紊乱[25]。上皮屏障损伤后会启动快速修复程序,上皮细胞的增殖、分化和移行在其中发挥重要作用[26]。本团队前期研究发现,用cis-9, trans-11-CLA和trans-10, cis-12-CLA的混合物(1:1)预处理瘤胃上皮细胞后,可以显著增加瘤胃上皮细胞受LPS刺激时细胞生长因子成纤维细胞生长因子7(fibroblast growth factor 7,FGF7)、成纤维细胞生长因子21(fibroblast growth factor 21,FGF21)、表皮调节素(epiregulin,EREG)、双调蛋白(amphiregulin,AREG)和肝素结合性表皮生长因子(heparin-binding epidermal growth factor,HBEGF)表达量,同时显著上调了与细胞增殖和迁移相关的ErbB信号通路[20],因此CLA可能通过促进瘤胃上皮细胞损伤后的增殖和移行缓解瘤胃上皮屏障损伤。Rahbar等[27]研究也发现,CLA的添加能够增加泌乳奶牛血液胰岛素样生长因子-1(IGF-1)含量,而IGF-1具有促进瘤胃上皮增殖的作用[28]。CLA的这种促细胞生长分化作用亦被一些其他研究证明。Della Casa等[29]发现cis-9, trans-11-CLA可以促进小鼠肌肉细胞分化。Lampen等[30]发现cis-9, trans-11-CLA能够诱导人肠道细胞Caco-2的分化。但是也有研究发现CLA异构体可以通过抑制细胞增殖来缓解癌症进展[31]。因此,CLA调控瘤胃上皮屏障功能损伤修复的机制还需进一步验证。
2.3 通过调控炎症性脂质介质生成缓解瘤胃上皮屏障功能损伤研究证明动物机体脂质代谢和免疫反应在多层面互相协调[32]。Kalucka等[33]发现内皮细胞脂肪酸氧化作用的缺失会通过增加氧化应激发生,促进白细胞浸润和内皮屏障功能损伤。CLA通过调控反刍动物脂质代谢和能量分配进而影响机体免疫稳态的作用已被证实[18-19]。Masur等[34]研究发现,CLA的添加可以增加瘤胃上皮细胞脂肪酸代谢通路关键调控因子PPARα的表达,而PPAR信号通路的激活能够抑制炎症因子表达。本团队前期研究发现cis-9, trans-11-CLA和trans-10, cis-12-CLA的单独或共同添加均能显著上调瘤胃上皮细胞中脂肪酸氧化功能关键基因肉碱脂酰转移酶1A(carnitine acyl transferase 1A,CPT1A)的表达,并且基于蛋白质-蛋白质互作(protein-protein interaction,PPI)分析发现瘤胃上皮细胞中CPT1A的表达量与促炎细胞因子的生成紧密相关[20-21]。Jung等[35]亦发现肉碱脂酰转移酶1(carnitine acyl transferase 1,CPT1)表达的上调可以缓解脂质介质诱发的炎症反应。此外,CLA异构体还能影响其他一些炎症性脂质介质代谢通路中关键因子的表达。例如,cis-9, trans-11-CLA和trans-10, cis-12-CLA(1:1)的混合添加可以增加瘤胃上皮细胞中脂解活化因子自水解酶结构域5(abhydrolase domain containing 5,ABHD5)和血管生成素样蛋白4(angiopoietin-like 4,ANGPTL4)的表达[20]。Lord等[36]发现ABHD5在缓解LPS诱导的炎症反应中发挥重要作用,ABHD5的缺乏会导致细胞中脂质过多积累,激活巨噬细胞NOD样受体家族pyrin域3(NOD like receptor family pyrin domain containing 3,NLRP3)炎症小体分泌白细胞介素-1β(interleukin-1β,IL-1β),上调IL-1β-细胞因子信号转导抑制因子3(suppressor of cytokine signaling 3,SOCS3)-叉头框蛋白O1(forkhead box O1,FOXO1)-IL-1β反馈回路,促进慢性炎症发生[37]。Phua等[38]发现ANGPTL4能够通过调控结肠上皮细胞趋化因子信号通路降低结肠炎症发生。但是,当cis-9, trans-11-CLA和trans-10, cis-12-CLA单独作用于瘤胃上皮细胞时,对于脂质代谢的调控作用亦会表现出一定的同分异构体特异性。例如,Yang等[21]研究发现,trans-10, cis-12-CLA降低了瘤胃上皮细胞受LPS刺激时脂质合成基因硬脂酰CoA去饱和酶(stearoyl-CoA desaturase,SCD)和脂肪酸去饱和酶2(fatty acid desaturase 2,FADS2)的表达,而cis-9, trans-11-CLA呈现出相反的作用,这可能与合成的脂肪酸类型(饱和或不饱和)有关。Masur等[34]研究却发现cis-9, trans-11-CLA或trans-10, cis-12-CLA的单独作用均下调了正常瘤胃上皮细胞SCD的表达。这种CLA对脂质代谢调控作用的同分异构体特异性在其他物种的研究中也有体现。Della Casa等[29]发现trans-10, cis-12-CLA有效减少了小鼠脂肪组织中的脂肪量,而cis-9, trans-11-CLA没有此作用。Evans等[39]等亦报道了CLA调控肥胖与脂质代谢的同分异构体特异性。
2.4 通过调节瘤胃微生态维持瘤胃上皮屏障功能稳态瘤胃微生物及其代谢产物通过与宿主互作维持和调控瘤胃上皮结构完整性和屏障功能的作用已被大量研究证实[40]。Yusuf等[41]研究发现,瘤胃内CLA含量的增加与纤维分解菌白色瘤胃球菌(Ruminococcus albus)、黄化瘤胃球菌(Ruminococcus flavefaciens)和产琥珀酸丝状杆菌(Fibrobacter succinogenes)丰富度的增加存在明显正相关。Pitta等[42]研究亦发现瘤胃trans-10, cis-12-CLA含量与丁酸生成菌丁酸弧菌属(Butyrivibrio)丰富度显著正相关。乙酸、丁酸等有益微生物代谢产物一方面能够为瘤胃上皮生理代谢提供能量来源,另一方面可以通过调节瘤胃上皮细胞膜蛋白受体如G蛋白偶联受体表达,进而调控瘤胃上皮紧密连接蛋白表达或上皮组织免疫细胞介导的免疫反应等来维持上皮屏障结构和功能稳态[43]。CLA通过增加益生菌嗜黏蛋白阿克曼氏菌(Akkermansia muciniphilia)与丁酸生成菌Butyrivibrio、罗氏菌属(Roseburia)和乳杆菌属(Lactobacillus)丰富度,提高短链脂肪酸生成,来维持肠道内环境和屏障功能稳态,缓解肠道炎症损伤的作用亦在小鼠研究中得到证实[44-45]。因此,CLA可能通过改善瘤胃微生态环境,增加微生物有益代谢产物生成来维持上皮屏障功能稳态,但具体机制需要进一步验证。
3 小结瘤胃上皮屏障功能稳态的维持对于反刍动物健康至关重要。研究证实瘤胃内代谢紊乱会导致LPS等细菌内毒素或有害物质的释放增加,进而引起瘤胃上皮细胞和细胞间紧密连接受损,造成上皮对营养素吸收率下降、通透性增加、屏障功能损伤、细菌毒素易位入血等,最终引起全身性病理反应发生,给动物生产性能和机体健康带来危害。CLA作为营养源保护胃肠道免疫,缓解肠道上皮屏障功能损伤的研究已在人类等医学领域大量被研究。作为瘤胃天然代谢产物,CLA对瘤胃上皮屏障功能的调控作用尚缺乏强有力证据,仅有少量报道证实其能够通过降低炎症反应、促进上皮屏障功能损伤修复、调控炎症性脂质介质代谢和瘤胃微生态来保护瘤胃上皮屏障功能,更深层次的作用机制尚未明确(图 1)。因此,CLA如何维持瘤胃上皮屏障功能的机制仍需更深入的研究,为其在反刍动物健康养殖中的应用提供更多的理论依据。
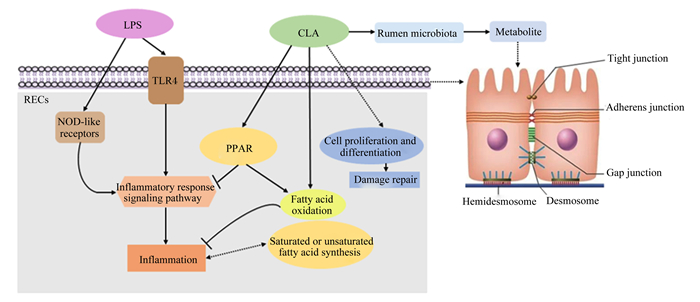
|
RECs:瘤胃上皮细胞rumen epithelial cells;LPS:脂多糖lipopolysaccharide;CLA:共轭亚油酸conjugated linoleic acid;TLR4:Toll样受体4 Toll-like receptor 4;NOD-like receptors:NOD样受体nucleotide-binding and oligomerization domain-like receptors;PPAR:过氧化物酶体增殖物激活受体peroxisome proliferator-activated receptor;Rumen microbiota:瘤胃微生物;Metabolite:代谢物;Inflammatory response signaling pathway:炎症反应信号通路;Inflammation:炎症;Fatty acid oxidation:脂肪酸氧化;Saturated or unsaturated fatty acid synthesis:饱和或不饱和脂肪酸合成;Hemidesmosome:半桥粒;Desmosome:桥粒;Tight junction:紧密连接;Adherens junction:黏合连接;Gap junction:间隙连接。 图 1 共轭亚油酸调控瘤胃上皮屏障功能的可能机制 Fig. 1 Potential mechanisms of conjugated linoleic acid in regulating ruminal epithelial barrier function[20-21] |
| [1] |
FERLAY A, BERNARD L, MEYNADIER A, et al. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: a review[J]. Biochimie, 2017, 141: 107-120. DOI:10.1016/j.biochi.2017.08.006 |
| [2] |
翁吉梅, 王松, 杨齐心, 等. 反刍动物合成共轭亚油酸的研究进展[J]. 饲料研究, 2021, 44(11): 149-151. WENG J M, WANG S, YANG Q X, et al. Research progress in synthesis of conjugated linoleic acid from ruminants[J]. Feed Research, 2021, 44(11): 149-151 (in Chinese). |
| [3] |
YUAN G F, CHEN X E, LI D. Modulation of peroxisome proliferator-activated receptor gamma (PPAR γ) by conjugated fatty acid in obesity and inflammatory bowel disease[J]. Journal of Agricultural and Food Chemistry, 2015, 63(7): 1883-1895. DOI:10.1021/jf505050c |
| [4] |
VILADOMIU M, HONTECILLAS R, BASSAGANYA-RIERA J. Modulation of inflammation and immunity by dietary conjugated linoleic acid[J]. European Journal of Pharmacology, 2016, 785: 87-95. DOI:10.1016/j.ejphar.2015.03.095 |
| [5] |
NICOD N, PARKER R S, GIORDANO E, et al. Isomer-specific effects of conjugated linoleic acid on HDL functionality associated with reverse cholesterol transport[J]. The Journal of Nutritional Biochemistry, 2015, 26(2): 165-172. DOI:10.1016/j.jnutbio.2014.10.002 |
| [6] |
AHN I S, CHOI B H, HA J H, et al. Isomer-specific effect of conjugated linoleic acid on inflammatory adipokines associated with fat accumulation in 3T3-L1 adipocytes[J]. Journal of Medicinal Food, 2006, 9(3): 307-312. DOI:10.1089/jmf.2006.9.307 |
| [7] |
MAO S Y, HUO W J, ZHU W Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model[J]. Environmental Microbiology, 2016, 18(2): 525-541. DOI:10.1111/1462-2920.12724 |
| [8] |
PELLATTIERO E, CECCHINATO A, TAGLIAPIETRA F, et al. The use of 2-dimensional gas chromatography to investigate the effect of rumen-protected conjugated linoleic acid, breed, and lactation stage on the fatty acid profile of sheep milk[J]. Journal of Dairy Science, 2015, 98(4): 2088-2102. DOI:10.3168/jds.2014-8395 |
| [9] |
QIN N B, BAYAT A R, TREVISI E, et al. Dietary supplement of conjugated linoleic acids or polyunsaturated fatty acids suppressed the mobilization of body fat reserves in dairy cows at early lactation through different pathways[J]. Journal of Dairy Science, 2018, 101(9): 7954-7970. |
| [10] |
NAGARAJA T G, TITGEMEYER E C. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook[J]. Journal of Dairy Science, 2007, 90(Suppl 1): E17-E38. |
| [11] |
PLAIZIER J C, KRAUSE D O, GOZHO G N, et al. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences[J]. The Veterinary Journal, 2008, 176(1): 21-31. DOI:10.1016/j.tvjl.2007.12.016 |
| [12] |
KHAFIPOUR E, KRAUSE D O, PLAIZIER J C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation[J]. Journal of Dairy Science, 2009, 92(3): 1060-1070. DOI:10.3168/jds.2008-1389 |
| [13] |
AMETAJ B N, EMMANUEL D G V, ZEBELI Q, et al. Feeding high proportions of barley grain in a total mixed ration perturbs diurnal patterns of plasma metabolites in lactating dairy cows[J]. Journal of Dairy Science, 2009, 92(3): 1084-1091. DOI:10.3168/jds.2008-1465 |
| [14] |
BROWN M S, KREHBIEL C R, GALYEAN M L, et al. Evaluation of models of acute and subacute acidosis on dry matter intake, ruminal fermentation, blood chemistry, and endocrine profiles of beef steers[J]. Journal of Animal Science, 2000, 78(12): 3155-3168. DOI:10.2527/2000.78123155x |
| [15] |
LEDBETTER T K, PAAPE M J, DOUGLASS L W. Cytotoxic effects of peroxynitrite, polymorphonuclear neutrophils, free-radical scavengers, inhibitors of myeloperoxidase, and inhibitors of nitric oxide synthase on bovine mammary secretory epithelial cells[J]. American Journal of Veterinary Research, 2001, 62(3): 286-293. DOI:10.2460/ajvr.2001.62.286 |
| [16] |
ZEBELI Q, AMETAJ B N. Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows[J]. Journal of Dairy Science, 2009, 92(8): 3800-3809. DOI:10.3168/jds.2009-2178 |
| [17] |
KLEVENHUSEN F, HOLLMANN M, PODSTATZKY-LICHTENSTEIN L, et al. Feeding barley grain-rich diets altered electrophysiological properties and permeability of the ruminal wall in a goat model[J]. Journal of Dairy Science, 2013, 96(4): 2293-2302. DOI:10.3168/jds.2012-6187 |
| [18] |
BAYAT A R, RAZZAGHI A, SARI M, et al. The effect of dietary rumen-protected trans-10, cis-12 conjugated linoleic acid or a milk fat-depressing diet on energy metabolism, inflammation, and oxidative stress of dairy cows in early lactation[J]. Journal of Dairy Science, 2022, 105(4): 3032-3048. DOI:10.3168/jds.2021-20543 |
| [19] |
GNOTT M, VOGEL L, KRÖGER-KOCH C, et al. Changes in fatty acids in plasma and association with the inflammatory response in dairy cows abomasally infused with essential fatty acids and conjugated linoleic acid during late and early lactation[J]. Journal of Dairy Science, 2020, 103(12): 11889-11910. DOI:10.3168/jds.2020-18735 |
| [20] |
YANG C L, LAN W, YE S J, et al. Transcriptomic analyses reveal the protective immune regulation of conjugated linoleic acids in sheep ruminal epithelial cells[J]. Frontiers in Physiology, 2020, 11: 588082. DOI:10.3389/fphys.2020.588082 |
| [21] |
YANG C L, ZHU B N, YE S J, et al. Isomer-specific effects of cis-9, trans-11- and trans-10, cis-12-CLA on immune regulation in ruminal epithelial cells[J]. Animals, 2021, 11(4): 1169. DOI:10.3390/ani11041169 |
| [22] |
JAUDSZUS A, FOERSTER M, KROEGEL C, et al. Cis-9, trans-11-CLA exerts anti-inflammatory effects in human bronchial epithelial cells and eosinophils: comparison to trans-10, cis-12-CLA and to linoleic acid[J]. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 2005, 1737(2/3): 111-118. |
| [23] |
DIPASQUALE D, BASIRICÒ L, MORERA P, et al. Anti-inflammatory effects of conjugated linoleic acid isomers and essential fatty acids in bovine mammary epithelial cells[J]. Animal, 2018, 12(10): 2108-2114. DOI:10.1017/S1751731117003676 |
| [24] |
ODENWALD M A, TURNER J R. The intestinal epithelial barrier: a therapeutic target?[J]. Nature Reviews.Gastroenterology & Hepatology, 2017, 14(1): 9-21. |
| [25] |
LUISSINT A C, PARKOS C A, NUSRAT A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair[J]. Gastroenterology, 2016, 151(4): 616-632. DOI:10.1053/j.gastro.2016.07.008 |
| [26] |
BANKAITIS E D, HA A, KUO C J, et al. Reserve stem cells in intestinal homeostasis and injury[J]. Gastroenterology, 2018, 155(5): 1348-1361. DOI:10.1053/j.gastro.2018.08.016 |
| [27] |
RAHBAR B, TAGHIZADEH A, PAYA H, et al. Conjugated linoleic acid (CLA) supplementation effects on performance, metabolic parameters and reproductive traits in lactating Holstein dairy cows[J]. Veterinary Research Forum, 2021, 12(3): 297-304. |
| [28] |
LU J, ZHAO H, XU J, et al. Elevated cyclin D1 expression is governed by plasma IGF-1 through Ras/Raf/MEK/ERK pathway in rumen epithelium of goats supplying a high metabolizable energy diet[J]. Journal of Animal Physiology and Animal Nutrition, 2013, 97(6): 1170-1178. DOI:10.1111/jpn.12026 |
| [29] |
DELLA CASA L, ROSSI E, ROMANELLI C, et al. Effect of diets supplemented with different conjugated linoleic acid (CLA) isomers on protein expression in C57/BL6 mice[J]. Genes and Nutrition, 2016, 11: 26. DOI:10.1186/s12263-016-0542-2 |
| [30] |
LAMPEN A, LEIFHEIT M, VOSS J, et al. Molecular and cellular effects of cis-9, trans-11-conjugated linoleic acid in enterocytes: effects on proliferation, differentiation, and gene expression[J]. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 2005, 1735(1): 30-40. DOI:10.1016/j.bbalip.2005.01.007 |
| [31] |
KORONOWICZ A A, BANKS P. Antitumor properties of CLA-enriched food products[J]. Nutrition and Cancer, 2018, 70(4): 529-545. DOI:10.1080/01635581.2018.1460684 |
| [32] |
DI CARA F, SHESHACHALAM A, BRAVERMAN N E, et al. Peroxisome-mediated metabolism is required for immune response to microbial infection[J]. Immunity, 2017, 47(1): 93-106. DOI:10.1016/j.immuni.2017.06.016 |
| [33] |
KALUCKA J, BIERHANSL L, CONCHINHA N V, et al. Quiescent endothelial cells upregulate fatty acid β-oxidation for vasculoprotection via redox homeostasis[J]. Cell Metabolism, 2018, 28(6): 881-894. DOI:10.1016/j.cmet.2018.07.016 |
| [34] |
MASUR F, BENESCH F, PFANNKUCHE H, et al. Conjugated linoleic acids influence fatty acid metabolism in ovine ruminal epithelial cells[J]. Journal of Dairy Science, 2016, 99(4): 3081-3095. DOI:10.3168/jds.2015-10042 |
| [35] |
JUNG T W, LEE S H, KIM H C, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice[J]. Experimental and Molecular Medicine, 2018, 50(9): 1-11. |
| [36] |
LORD C C, BETTERS J L, IVANOVA P T, et al. CGI-58/ABHD5-derived signaling lipids regulate systemic inflammation and insulin action[J]. Diabetes, 2012, 61(2): 355-363. DOI:10.2337/db11-0994 |
| [37] |
MIAO H M, OU J J, ZHANG X, et al. Macrophage CGI-58 deficiency promotes IL-1β transcription by activating the SOCS3-FOXO1 pathway[J]. Clinical Science, 2015, 128(8): 493-506. DOI:10.1042/CS20140414 |
| [38] |
PHUA T, SNG M K, TAN E H P, et al. Angiopoietin-like 4 mediates colonic inflammation by regulating chemokine transcript stability via tristetraprolin[J]. Scientific Reports, 2017, 7(1): 44351. DOI:10.1038/srep44351 |
| [39] |
EVANS M E, BROWN J M, MCLNTOSH M K. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism[J]. The Journal of Nutritional Biochemistry, 2002, 13(9): 508-516. DOI:10.1016/S0955-2863(02)00211-5 |
| [40] |
CHAI J M, LV X K, DIAO Q Y, et al. Solid diet manipulates rumen epithelial microbiota and its interactions with host transcriptomic in young ruminants[J]. Environmental Microbiology, 2021, 23(11): 6557-6568. DOI:10.1111/1462-2920.15757 |
| [41] |
YUSUF A L, ADEYEMI K D, SAMSUDIN A A, et al. Effects of dietary supplementation of leaves and whole plant of Andrographis paniculata on rumen fermentation, fatty acid composition and microbiota in goats[J]. BMC Veterinary Research, 2017, 13(1): 349. DOI:10.1186/s12917-017-1223-0 |
| [42] |
PITTA D W, INDUGU N, VECCHIARELLI B, et al. Alterations in ruminal bacterial populations at induction and recovery from diet-induced milk fat depression in dairy cows[J]. Journal of Dairy Science, 2018, 101(1): 295-309. DOI:10.3168/jds.2016-12514 |
| [43] |
ZHAN K, GONG X X, CHEN Y Y, et al. Short-chain fatty acids regulate the immune responses via G protein-coupled receptor 41 in bovine rumen epithelial cells[J]. Frontiers in Immunology, 2019, 10: 2042. DOI:10.3389/fimmu.2019.02042 |
| [44] |
CHAPLIN A, PARRA P, SERRA F, et al. Conjugated linoleic acid supplementation under a high-fat diet modulates stomach protein expression and intestinal microbiota in adult mice[J]. PLoS One, 2015, 10(4): e0125091. DOI:10.1371/journal.pone.0125091 |
| [45] |
MARQUES T M, WALL R, O'SULLIVAN O, et al. Dietary trans-10, cis-12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice[J]. British Journal of Nutrition, 2015, 113(5): 728-738. DOI:10.1017/S0007114514004206 |




