2. 南京致润生物科技集团有限公司, 南京 210095
2. Nanjing Zhirun Biological Technology Group Co., Ltd., Nanjing 210095, China
早期断奶的仔猪由于胃肠道消化机能还未发育完全,不能很好地适应从食用母乳到采食固体饲料的过度阶段[1],加之受与母体和同窝仔猪分离、饲养环境和饲粮饮食突然改变等外部刺激,这一阶段仔猪易发生采食量下降、养分消化率降低、腹泻以及应激等“仔猪早期断奶综合征”。集约化养殖过程中,仔猪断奶后死亡率为6%~10%,有时甚至可能上升到20%[2]。因此,断奶是养猪业中最具挑战性的阶段,断奶阶段饲养水平将直接影响猪场的生产效益。如何缓解仔猪断奶应激导致的仔猪采食量和生长性能降低、发病率和死亡率增加等不良影响,一直以来都是集约化生猪养殖过程中的一大难题。
哺乳动物在胎儿时期,肠道内几乎没有微生物,母猪肠道中的微生物更容易在仔猪肠道中定植[3],而早期断奶由母乳向固体饲料的过渡阶段可能会影响仔猪肠道菌群结构。为了解决这一问题,我们提出一种富含益生菌的发酵酸奶使其功能更类似于母乳,试图通过其促进断奶仔猪的肠道建立有益的微生物群落。在全面禁饲抗生素的背景下,益生菌是目前研究最为广泛、应用前景较好的抗生素替代产品。益生菌通过饮食进入机体胃肠道时可以保持活力和数量充足,从而发挥其益生作用[4]。Chen等[5]研究发现,饲粮添加乳酸菌和芽孢杆菌对生长猪的平均日增重(average daily gain,ADG)有显著提高作用。研究发现,猪源乳酸菌相较于体外分离的乳酸菌更容易定植于肠道环境[6]。Huang等[7]研究发现,饲粮补饲猪源发酵乳酸菌可以显著改善断奶仔猪的生长性能和肠道微生态平衡。
仔猪早期断奶后易发生胃内pH升高,胃蛋白酶等消化酶分泌不足,易导致养分消化率下降。为了改进配方,我们选择添加复合型酸化剂降低发酵酸奶的pH。酸化剂作为一种安全、健康的替抗主流产品,一般可以用于胃肠道系统发育不完善的断奶仔猪。Heres等[8]进行体内和体外试验均发现,饲粮中添加5.7%的乳酸可有效降低肠道内有害菌的数量,从而防止腹泻。为进一步优化配方,相关文献指出母乳中富含寡糖且可以塑造仔猪的肠道微生物群落[3]。此外,人类婴儿配方奶粉通常会添加功能性的寡糖如半乳糖、果糖等。栾海宏等[9]研究发现,断奶仔猪饲粮中添加0.1%甘露寡糖可以改善仔猪生长性能、肠道微生物群落以及可以提高养分消化率。
近年来,将发酵酸奶作为益生菌载体以促进益生菌发挥最大效益成为研究热点[10]。Rateliffe等[11]研究发现,补饲酸奶可以用于畜牧生产中通过改善肠道菌群环境,减少仔猪腹泻,提高生长性能。不过,专门针对断奶仔猪的肠源乳酸菌发酵酸奶在畜牧生产上的应用报道很少。试验前期,本课题组以3种猪源乳酸菌作为菌种对牛奶进行复合发酵,并加入功能性寡糖和复合酸化剂等,开发了针对断奶仔猪的功能性发酵酸奶。在此基础上,本试验在饲喂教槽料的基础上通过补饲发酵酸奶,旨在评估其在仔猪断奶后2周内对其生长性能、养分消化和粪便菌群的影响,为发酵酸奶在猪生产上的应用提供依据。
1 材料与方法 1.1 发酵酸奶的制作以15%奶粉、8%蔗糖、功能性寡糖(低聚果糖和低聚半乳糖)为底物,以3种猪源乳酸菌(约氏乳杆菌、海氏肠球菌和粪肠球菌)为复合菌种,37 ℃下发酵12 h后,使用复合酸化剂调节酸奶pH至4.0左右,无菌封装后置于4 ℃冷藏备用。发酵酸奶的主要营养成分如下:干物质23.45%,乳蛋白质3.24%,乳脂3.12%,乳糖5.60%,活菌数4.50×109 CFU/mL。
1.2 试验设计选取同批次36头30日龄初始体重为(9.83±0.07) kg的断奶“长×大”二元公猪,随机分为3个组,每组6个重复(栏),每个重复2头猪。对照组饲喂基础饲粮;低剂量发酵酸奶组在饲喂基础饲粮的基础上每头每日补饲3顿(07:00、12:00、17:00)酸奶,每顿40 mL;高剂量发酵酸奶组在基础饲粮的基础上每头每日补饲3顿(07:00、12:00、17:00)酸奶,每顿80 mL。试验期14 d。基础饲粮参照NRC(2012)配制,其组成及营养水平如表 1所示。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-day basis) |
试验前按照猪场的管理程序对猪舍进行杀菌清洁,试验猪每天早、中、晚(07:00、12:00、17:00)各喂料1次,投放饲粮充足,确保采食后有剩余;自动饮水设备保证充足饮水。猪舍温度控制在20~25 ℃,按猪场规定步骤实施免疫程序。
1.4 样品采集 1.4.1 粪便样品的采集于试验第1、3、7和14天07:00—08:00,每重复随机采集1头仔猪新鲜粪样置于自封袋中,4 ℃下运输至实验室,进行乳酸菌、大肠杆菌和沙门氏菌活菌计数。将第3天采样的粪便样品-20 ℃保存,用于菌群高通量测序分析。在试验结束前连续收集3 d各组仔猪粪便,混合均匀后-20 ℃密封保存,用于养分表观消化率分析。
1.4.2 血液样品的采集于试验第7和14天,每重复随机采集1头仔猪颈静脉血液5 mL,3 500 r/min离心10 min后,分装上清液于1.5 mL离心管中,-20 ℃保存,用于血清生化指标检测。
1.5 指标测定及方法 1.5.1 生长性能指标于试验第1、7和14天的清晨对仔猪进行空腹称重,记录每头猪的空腹体重,计算ADG;准确记录每重复投料量、剩料量,计算平均日采食量(average daily feed intake, ADFI);根据ADFI和ADG计算料重比(feed to gain ratio,F/G)。计算公式如下:

|
试验期每天09:00和17:00逐头检查仔猪肛门,观察并详细记录各仔猪腹泻情况,进行腹泻评分,评分标准见表 2;试验结束后计算腹泻指数。计算公式如下:

|
|
|
表 2 仔猪腹泻评分标准 Table 2 Scoring criteria for diarrhea of piglets |
将-20 ℃冰箱保存的血清样品放入4 ℃冰箱解冻,血清总胆固醇(TC)、甘油三酯(TG)、总蛋白(TP)、球蛋白(GLB)、白蛋白(ALB)、尿素氮(UN)、葡萄糖(GLU)、高密度脂蛋白(HDL)和低密度脂蛋白(LDL)含量采用全自动生化分析仪(SlectraE,VITALAB)测定。
1.5.3 养分表观消化率粪便样品于65 ℃恒温烘箱中烘至风干状态后粉碎过40目筛。采用内源指示剂酸不溶性灰分(acid insoluble ash, AIA)测定养分表观消化率。饲粮和粪便样品中的粗脂肪含量采用索氏提取法测定,粗蛋白质含量采用凯氏定氮法测定。养分表观消化率计算公式如下:

|
将粪便在无菌条件下取出1 g到灭菌离心管中,加入灭菌生理盐水进行梯度稀释,取稀释液100 μL,采用MRS培养基涂板对乳酸菌进行菌落计数,采用麦康凯培养基涂板对大肠杆菌进行菌落计数,以及采用HE培养基涂板对沙门氏菌进行菌落计数。乳酸菌37 ℃培养48 h,大肠杆菌和沙门氏菌37 ℃培养24 h,每个样品做4个平行重复。
1.5.5 粪便菌群的高通量测序分析基于粪便菌群平板计数结果,进一步对整个菌群进行分析,选取第3天粪菌进行高通量测序。取0.3 g粪便样品于2 mL灭菌离心管中,参照Dai等[12]的方法用用酚-氯仿-异戊醇提取微生物核酸。选用细菌通用引物515F和907R扩增细菌的16S rRNA基因V3~V4区。通过PicoGreen dsDNA Assay试剂盒,检测DNA的提取量,并按照既定流程在Illumina MiSeq平台上进行序列分析。使用UPARSE软件对己筛选序列进行操作分类单元(operational taxonomic units,OTU)聚类分析,菌群丰富度指标Chao1指数采用Mothur软件进行评价,菌群多样性指标采用Shannon和Simpson指数进行评价,比较组间菌群在门水平、属水平、种水平和OTU水平的丰度差异并制作韦恩图分析各组的核心OTU。
1.6 数据统计分析使用SPSS 21.0统计分析软件中的ANOVA程序对试验所测指标进行单因素方差分析,显著差异则采用Duncan氏法进行多重比较,P < 0.05表示差异显著,0.05≤P < 0.10表示有差异显著趋势,试验结果以“平均值±标准误”的形式表示。
2 结果与分析 2.1 补饲发酵酸奶对断奶仔猪生长性能的影响由表 3可见,第14天时,高剂量发酵酸奶组断奶仔猪体重显著高于对照组(P < 0.05)。第1~7天,与对照组相比,高剂量发酵酸奶组断奶仔猪ADG有提高趋势(P=0.058)。第8~14天,与对照组相比,高剂量发酵酸奶组断奶仔猪ADG显著提高(P < 0.05),同时,低剂量发酵酸奶组和高剂量发酵酸奶组断奶仔猪ADFI均显著提高(P < 0.05)。然而,补饲发酵酸奶对断奶仔猪各阶段F/G和腹泻指数均无显著影响(P>0.05)。
|
|
表 3 补饲发酵酸奶对断奶仔猪生长性能的影响 Table 3 Effects of supplementing fermented yoghurt on growth performance of weaned piglets |
由表 4可见,第7天时,与对照组相比,高剂量发酵酸奶组断奶仔猪血清白蛋白含量显著提高(P < 0.05);而在第14天时3组之间无显著差异(P < 0.05)。第14天时,与对照组相比,高剂量发酵酸奶组断奶仔猪血清尿素氮含量显著降低(P < 0.05)。补饲发酵酸奶对断奶仔猪第7和14天血清总蛋白、球蛋白、总胆固醇、甘油三酯、高密度脂蛋白和低密度脂蛋白含量均无显著影响(P>0.05)。
|
|
表 4 补饲发酵酸奶对断奶仔猪血清生化指标的影响 Table 4 Effects of supplementing fermented yoghurt on serum biochemical indices of weaned piglets |
由表 5可见,与对照组相比,高剂量发酵酸奶组断奶仔猪干物质表观消化率显著提高(P < 0.05);而3组之间粗蛋白质、粗脂肪、粗纤维和粗灰分的表观消化率没有显著差异(P>0.05)。
|
|
表 5 补饲发酵酸奶对断奶仔猪养分表观消化率的影响 Table 5 Effects of supplementing fermented yoghurt on nutrient apparent digestibility of weaned piglets |
由表 6可见,第3天时,与对照组相比,低剂量发酵酸奶组断奶仔猪粪便中沙门氏菌和大肠杆菌数量显著降低(P < 0.05),高剂量发酵酸奶组断奶仔猪粪便中乳酸菌数量呈现增高的趋势(P=0.08)。第1、7和14天时,3组断奶仔猪粪便中大肠杆菌、沙门氏菌以及乳酸菌数量没有显著差异(P>0.05)。
|
|
表 6 补饲发酵酸奶对断奶仔猪粪便菌群数量的影响 Table 6 Effects of supplementing fermented yoghurt on fecal flora count of weaned piglets |
本试验共测定了18个样本序列,获得843 379个序列(长度大于250 bp),序列的平均长度为255.93 bp。由表 7可见,3组之间断奶仔猪粪便菌群α多样性指数Chao1指数、Shannon指数和Simpson指数均无显著差异(P>0.05)。
|
|
表 7 补饲发酵酸奶对断奶仔猪粪便菌群α多样性的影响 Table 7 Effects of supplementing fermented yoghurt on fecal flora α diversity of weaned piglets |
由图 1可见,3组断奶仔猪粪便菌群之间在门水平上的相对丰度无显著差异(P>0.05)。由表 8可见,在属水平上,高剂量发酵酸奶组断奶仔猪粪便菌群中柯林斯菌属(Collinsella)、光冈菌属(Mitsuokella)的相对丰度显著高于对照组(P < 0.05),而孪生球菌属(Gemella)、不可培养肉杆菌科(uncultured Carnobacteriaceae)、韦荣氏球菌属(Veillonella)、Negativibacillus、副萨特氏菌属(Parasutterella)、理研菌科RC9肠道群(Rikenellaceae RC9 gut group)的相对丰度显著低于对照组(P < 0.05)。同时,高剂量发酵酸奶组和低剂量发酵酸奶组肠杆菌属(Enterorhabdus)、Paludicola和链球菌属(Streptococcus)的相对丰度显著高于对照组(P < 0.05),Muribaculum的相对丰度显著低于对照组(P < 0.05)。
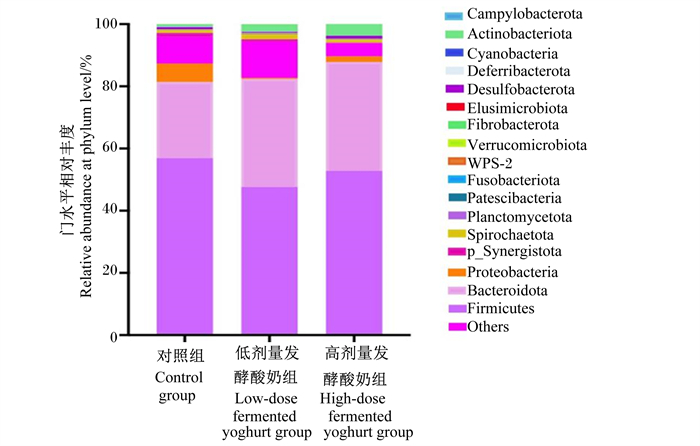
|
Campylobacterota:弯曲杆菌门;Actinobacteriota:放线菌门;Cyanobacteria:蓝菌门;Deferribacterota:脱铁杆菌门;Desulfobacterota:脱硫杆菌门;Elusimicrobiota:迷踪菌门;Fibrobacterota:纤维杆菌门;Verrucomicrobiota:疣微菌门;Fusobacteriota:梭杆菌门;Patescibacteria:髌骨细菌门;Planctomycetota:浮霉菌门;Spirochaetota:螺旋体门;p_Synergistota:p_联合菌门;Proteobacteria:变形菌门;Bacteroidota:拟杆菌门;Firmicutes:厚壁菌门;Others:其他。表 3同the same as Table 3。 图 1 补饲发酵酸奶对断奶仔猪粪便菌群门水平相对丰度的影响 Fig. 1 Effects of supplementing fermented yoghurt on relative abundance of fecal flora at phylum level of weaned piglets |
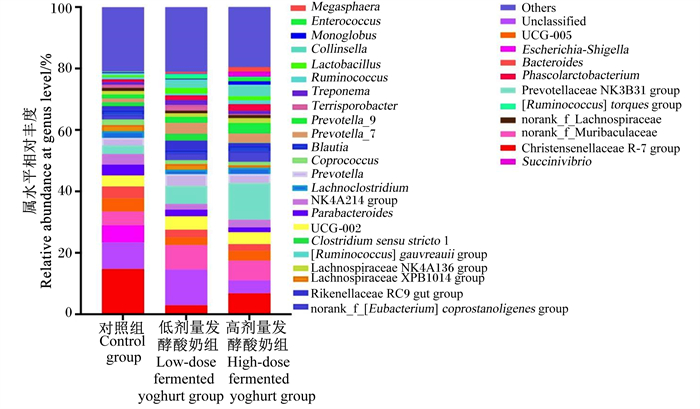
|
Megasphaera:巨球型菌属;Enterococcus:肠球菌属;Collinsella:柯林斯菌属;Lactobacillus:乳杆菌属;Ruminococcus:瘤胃球菌属;Treponema:密螺旋体属;Terrisporobacter:土孢杆菌属;Prevotella_9:普雷沃氏菌属9;Prevotella_7:普雷沃氏菌属7;Blautia:布劳特氏菌属;Coprococcus:粪球菌属;Prevotella:普雷沃氏菌属;NK4A214 group:NK4A214群;Parabacteroides:副拟杆菌属;Clostridium sensu stricto 1:狭义梭菌属1;[Ruminococcus] gauvreauii group:瘤胃球菌属gauvreauii群;Lachnospiraceae NK4A136 group:毛螺菌科NK4A136群;Lachnospiraceae XPB1014 group:毛螺菌科XPB101群;Rikenellaceae RC9 gut group:理研菌科RC9肠道群;norank_f_[Eubacterium] coprostanoligenes group:norank f_产粪甾醇真细菌群;Others:其他;Unclassified:未分类;Escherichia-Shigella:埃希氏-志贺氏菌属;Bacteroides:拟杆菌属;Phascolarctobacterium:考拉杆菌属;Prevotellaceae NK3B31 group:普雷沃氏菌科NK3B31群;[Ruminococcus] torques group:瘤胃球菌属扭矩群;norank_f_Lachnospiraceae:norank_f_毛螺菌科;Christensenellaceae R-7 group:克里斯滕森菌科R-7群;Succinivibrio:琥珀酸弧菌属。表 3同the same as Table 3。 图 2 补饲发酵酸奶对断奶仔猪粪便菌群属水平相对丰度的影响 Fig. 2 Effects of supplementing fermented yoghurt on relative abundance of fecal flora at genus level of weaned piglets |
|
|
表 8 断奶仔猪粪便菌群属水平上的差异菌群相对丰度 Table 8 Relative abundance of differential fecal flora at genus level of weaned piglets |
在OTU水平上,由表 9可见,与对照组相比,补饲高剂量发酵酸奶显著提高断奶仔猪粪便菌群中OTU450[厌氧棍状菌属(Anaerotruncus)]以及OTU1277[克里斯滕森菌科R-7群(Christensenellaceae R-7 group)]等12个OTU的相对丰度(P < 0.05);补饲低剂量发酵酸奶显著提高OTU391(Alloprevotella)等3个OTU的相对丰度(P < 0.05),显著降低了OTU302[瘤胃球菌属扭矩群([Ruminococcus] torques group)]的相对丰度(P < 0.05)。
|
|
表 9 断奶仔猪粪便菌群OTU水平上的差异菌群相对丰度(>1%) Table 9 Relative abundance of differential fecal flora at OTU level of weaned piglets (>1%) |
基于上述菌群结构分析结果,进一步对高剂量发酵酸奶组粪便菌群中独有OTU进行了分析。由图 3可见,高剂量发酵酸奶组粪便菌群中有133个独有核心OTU。进一步对其门水平和属水平相对丰度进行分析发现,高剂量发酵酸奶组独有OTU的总相对丰度为5.60%,其中主要的菌门为厚壁菌门(Firmicutes)(4.02%)和拟杆菌门(Bacteroidota)(1.25%);在属水平上,厚壁菌门中的[Eubacterium] oxidoreducens group(1.04%)和瘤胃球菌属gauvreauii群([Ruminococcus] gauvreauii group)(0.94%)为优势菌属。
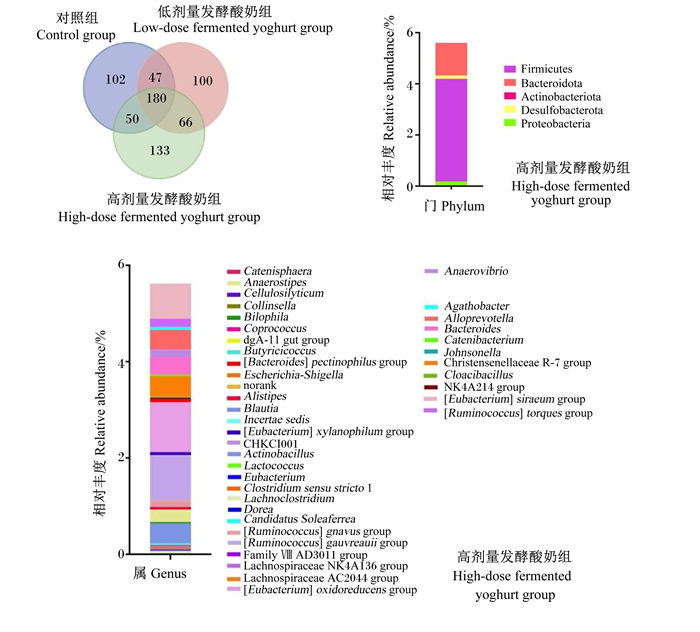
|
dgA-11 gut group:dgA-11肠道群;[Bacteroides] pectinophilus group:拟杆菌属pectinophilus群;Alistipes:另枝菌属;Incertae sedis:未定地位;Actinobacillus:放线杆菌属;Lactococcus:乳球菌属;Eubacterium:真杆菌属;[Ruminococcus] gnavus group:瘤胃球菌属gnavus群;Lachnospiraceae AC2044 group:毛螺菌科AC2044群;Anaerovibrio:厌氧弧菌属;Johnsonella:约翰森氏菌属。 图 3 高剂量发酵酸奶组粪便独有菌群的结构分析 Fig. 3 Structure analysis of fecal unique flora in high-dose fermented yoghurt group |
早期断奶的仔猪面临营养、心理和环境上的改变,饲粮由液体转为固体,加之此时胃肠道发育不全,都易影响机体对营养物质的消化吸收,造成营养摄入不足。乳酸菌发酵的酸奶具有浓郁酸甜香气味,对断奶仔猪有很好的诱食作用,从而增加断奶仔猪的采食量。在先前的研究中,Pollmann[13]对乳酸杆菌做了相关研究发现,仔猪的ADG和饲料转化率均显著提高。徐秀景等[14]研究发现,乳酸菌固态发酵饲料可以提高动物采食量从而提高ADG。张常明[15]研究发现,给每头断奶仔猪每天饲喂30 mL乳酸菌,能够显著提高断奶仔猪的ADG。本试验结果也表明,补饲乳酸菌发酵酸奶可以提高断奶仔猪的生长性能,这可能是因为补饲发酵酸奶提高了仔猪的采食量,同时发酵酸奶也额外提供了部分营养,这不仅增加了营养摄入,还提高了饲粮干物质的表观消化率。
经过乳酸菌发酵后的发酵酸奶中加入了一定剂量的酸化剂使得pH达到3.8~4.0,断奶仔猪因胃内盐酸分泌不足而无法激活胃蛋白酶等消化酶,发酵酸奶较低的pH可以促进激活胃蛋白酶的活性从而提高对营养物质的消化能力[16]。Collington等[17]认为有机酸可以通过乳酸菌发酵产生,从而促进仔猪的胃肠蠕动与消化功能。Xuan等[18]在断奶仔猪的饲粮中添加复合益生菌发现对养分表观消化率没有显著影响。Kornegay等[19]探究了2种益生菌产品对育肥猪养分表观消化率的影响,未发现显著作用。王志祥等[20]研究发现,饲粮添加乳酸杆菌显著提高了断奶仔猪对营养物质的表观消化率。本试验结果表明,补饲发酵酸奶对断奶仔猪的干物质的表观消化率有显著影响,但对粗蛋白质、粗脂肪、粗纤维和粗灰分的表观消化率无显著影响,这与上述结果不尽一致,原因可能是与试验动物的阶段、营养水平以及益生菌的种类不同有关。
3.2 补饲发酵酸奶对断奶仔猪血清生化指标的影响血清生化指标与机体营养物质的消化吸收代谢有着重要的相关性。白蛋白具有调节血液胶体渗透压的作用,血清白蛋白含量升高说明肝脏合成蛋白质以及代谢能力的增强[21]。杜泓明等[22]研究发现,饲粮中添加益生菌能够一定程度上提高断奶仔猪血清中的总蛋白和白蛋白含量。本试验结果也发现,补饲发酵酸奶后断奶仔猪血清中白蛋白含量显著提高,这表明补饲发酵酸奶可以增强仔猪的蛋白质合成代谢。血清中尿素氮的含量与动物体内氨基酸和蛋白质的平衡有关,血清尿素氮含量低说明氨基酸在仔猪体内合成蛋白质的效率较高。本试验结果表明,补饲发酵酸奶能够显著降低断奶仔猪血清尿素氮含量,这与刘辉等[23]在生长猪上的研究结果一致,说明发酵酸奶可以有效提高仔猪体内蛋白质的合成代谢效率,从而增强机体免疫。补饲发酵酸奶对断奶仔猪免疫功能的积极作用可能与发酵酸奶中添加的寡糖有关,寡糖可以提高断奶仔猪生长性能、免疫功能以及可以被乳酸菌吸收利用并产生益生作用。
3.3 补饲发酵酸奶对断奶仔猪粪便菌群的影响通常情况下,粪便中乳酸菌和大肠杆菌的数量可以用来评判肠道微生态健康的状态[24]。肠道pH在低于5.5时不利于大肠杆菌的生存[25],发酵酸奶经过乳酸菌发酵后且加入酸化剂使pH达到3.8~4.0时可以保证仔猪胃肠道内适宜的pH,以保证肠道内有益菌的合理生长,从而抵御有害菌在肠道内的繁殖[26-27]。此外,发酵酸奶中的功能性寡糖可作为乳酸菌的碳源,同时可以抑制大肠杆菌等病原菌的增殖;而且,补饲乳酸菌发酵酸奶后使得肠道内的乳酸含量增加,使得肠道内pH下降,可使乳酸菌成为优势菌从而降低有害菌的数量。Pieper等[28]研究表明,饲喂乳酸杆菌可通过增加肠道内的有益菌群从而保证仔猪胃肠的微生态健康。蔡艳等[29]研究表明,乳酸菌可以提高猪肠道中有益菌的数量,并且降低大肠杆菌和梭菌等病原菌的生长繁殖。本试验结果表明,与对照组相比,低剂量发酵酸奶组断奶仔猪粪便中沙门氏菌和大肠杆菌数量显著降低,高剂量发酵酸奶组断奶仔猪粪便中乳酸菌数量呈现增高的趋势,这与前人研究结果一致。由此可见,补饲发酵酸奶可通过促进仔猪肠道中有益菌群的生长,同时抑制有害菌群的生长,从而维持仔猪肠道的微生态平衡,而断奶仔猪生长性能和养分表观消化率的提高可能与肠道菌群稳态有关。孙玉亭[25]使用芽孢乳杆菌S1饲喂断奶仔猪发现粪便菌群的多样性和丰富度没有显著差异。杜泓明[30]也发现,益生菌培养物对断奶仔猪盲肠微生物多样性无显著影响。本试验结果与上述研究类似。补饲乳酸菌发酵酸奶增加了乳酸菌的数量但是没有增加菌群多样性,造成这一结果的原因有可能是因为随着肠段的后移微生物区系的结构和组成趋于复杂[31],在动物采食发酵酸奶后乳酸菌到达大肠需要几个小时的时间[32],而大肠处于持续发酵的状态[33],导致各组之间的粪便菌群多样性差异不显著;还有可能与乳酸菌的添加量、种类、产品不同以及试验动物的品种、日龄等因素不同有关[34]。
Park等[35]研究表明,粪便的优势菌群反映着肠道菌群的平衡以及肠道功能是否正常。Kim等[36]对猪粪便微生物区系进行分析也得到相同的结果。本试验中,在属水平上,高剂量发酵酸奶组断奶仔猪粪便菌群中柯林斯菌属和光冈菌属的相对丰度显著高于对照组。据报道,柯林斯菌属具有防止玉米中有氧雪腐镰刀菌烯醇对肠道菌群毒害的作用[37],还可避免肠道功能紊乱从而保持肠道健康,提高饲粮养分利用率[30]。光冈菌属可通过促进植酸酶的生成,加速对饲粮中抗营养因子“植酸”的代谢[38],从而提高仔猪对营养物质的利用,进而提高生长性能。此外,本试验中,高剂量发酵酸奶组断奶仔猪粪便菌群中韦荣氏球菌属和副萨特氏菌属的相对丰度显著低于对照组。研究发现,韦荣氏球菌属可寄生在肠道中产生内毒素,常在混合感染中起作用[39];而副萨特氏菌属是一种容易引起肠炎和败血症的细菌[40]。由此可见,高剂量发酵酸奶可以通过降低潜在致病菌的相对丰度从而降低肠道发病率,从而保障肠道菌群稳态。本试验中,克里斯滕森菌科R-7群是补饲发酵酸奶组断奶仔猪粪便菌群中属水平相对丰度较高的优势菌属,研究表明其相对丰度与仔猪的炎症和肥胖等症状呈负相关[41]。此外,在高剂量发酵酸奶组的133个独有OTU中,瘤胃球菌属gauvreauii群作为仔猪肠道内的有益优势菌属,其能够在肠道中发酵产生乙酸[42],而乙酸作为碳水化合物发酵的主要最终产物,可为肠道上皮和宿主机体提供能量,进而保障肠道的稳态以及机体对营养物质的利用率。综上所述,高剂量发酵酸奶能够有效促进肠道有益菌属相对丰度的提高,降低潜在的可能会引起肠道菌群紊乱的致病菌属相对丰度,从而改善断奶仔猪肠道内环境。肠道中的有益菌可促进断奶仔猪对营养物质的消化吸收,这也解释了之前高剂量发酵酸奶对断奶仔猪对生长性能指标影响的试验结果。
4 结论在饲喂断奶饲粮基础上补饲发酵酸奶可提高断奶仔猪采食量,改善断奶仔猪对干物质的表观消化率,提高断奶后3 d粪便中柯林斯菌属和光冈菌属等有益菌属的相对丰度,降低韦荣氏球菌属和副萨特氏菌属等潜在致病菌的相对丰度以及沙门氏菌和大肠杆菌数量,进而提高断奶仔猪的生长性能。
| [1] |
GRESSE R, CHAUCHEYRAS-DURAND F, FLEURY M A, et al. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health[J]. Trends in Microbiology, 2017, 25(10): 851-873. DOI:10.1016/j.tim.2017.05.004 |
| [2] |
XIONG X, TAN B, SONG M, et al. Nutritional intervention for the intestinal development and health of weaned pigs[J]. Frontiers in Veterinary Science, 2019, 6: 46. DOI:10.3389/fvets.2019.00046 |
| [3] |
ROGGERO P, LIOTTO N, POZZI C, et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula[J]. Nature Communications, 2020, 11(1): 2703. DOI:10.1038/s41467-020-16582-1 |
| [4] |
SAKANDAR H A, ZHANG H P. Trends in probiotic(s)-fermented milks and their in vivo functionality: a review[J]. Trends in Food Science & Technology, 2021, 110: 55-65. |
| [5] |
CHEN Y J, MIN B J, CHO J H, et al. Effects of dietary bacillus-based probiotic on growth performance, nutrients digestibility, blood characteristics and fecal noxious gas content in finishing pigs[J]. Asian-Australasian Journal of Animal Sciences, 2006, 19(4): 587-592. DOI:10.5713/ajas.2006.587 |
| [6] |
高擎燏, 李平华, 黄瑞华, 等. 猪源乳酸菌的抗逆性及益生性研究[J]. 畜牧与兽医, 2015, 47(10): 41-46. GAO Q Y, LI P H, HUANG R H, et al. Study on stress resistance and beneficial nature of porcine lactic acid bacteria[J]. Animal Husbandry & Veterinary Medicine, 2015, 47(10): 41-46 (in Chinese). |
| [7] |
HUANG C H, QIAO S Y, LI D F, et al. Effects of lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs[J]. Asian-Australasian Journal of Animal Sciences, 2004, 17(3): 401-409. DOI:10.5713/ajas.2004.401 |
| [8] |
HERES L, ENGEL B, URLINGS H A P, et al. Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella[J]. Veterinary Microbiology, 2004, 99(3/4): 259-267. |
| [9] |
栾海宏. 复合型功能性寡糖对仔猪生长性能、肠道微生物菌群和免疫功能的影响[J]. 饲料研究, 2021, 44(5): 29-32. LUAN H H. Effect of compound functional oligosaccharides on growth performance, intestinal microflora and immune function of piglets[J]. Feed Research, 2021, 44(5): 29-32 (in Chinese). |
| [10] |
MISSOTTEN J A M, MICHIELS J, GORIS J, et al. Screening of two probiotic products for use in fermented liquid feed[J]. Livestock Science, 2007, 108(1/3): 232-235. |
| [11] |
RATELIFFE B, COLE C B, FULLER R, et al. The effect of yoghurt and milk fermented with a porcine intestinal strain of Lactobacillus reuteri on the performance and gastrointestinal flora of pigs weaned at two days of age[J]. Food Microbiology, 1986, 3(3): 203-211. DOI:10.1016/0740-0020(86)90001-8 |
| [12] |
DAI Z L, ZHANG J, WU G Y, et al. Utilization of amino acids by bacteria from the pig small intestine[J]. Amino Acids, 2010, 39(5): 1201-1215. DOI:10.1007/s00726-010-0556-9 |
| [13] |
POLLMANN D. 13-probiotics in pig diets[M]//HARESIGN W, COLE D J A. Recent advances in animal nutrition. Amsterdam: Elsevier Ltd., 1986: 193-205.
|
| [14] |
徐秀景, 谢长文, 刘敏, 等. 乳酸菌发酵饲料对猪生长性能、肉品质和血液抗氧化指标的影响[J]. 中国饲料, 2018(10): 67-71. XU X J, XIE C W, LIU M, et al. Effects of Lactobacillus fermented feed on growth performance, meat quality and blood antioxidant capacity of pigs[J]. China Feed, 2018(10): 67-71 (in Chinese). |
| [15] |
张常明. 乳酸菌对断奶仔猪生长性能和免疫功能影响的研究[D]. 硕士学位论文. 广州: 华南农业大学, 2005. ZHANG C M. Effects of lactic acid bacteria on growth performance and immune function of weaned piglets[D]. Master's Thesis. Guangzhou: South China Agricultural University, 2005. (in Chinese) |
| [16] |
陈鲜鑫, 王金全, 刘震坤, 等. 玉米乳杆菌发酵液体饲料对断奶仔猪生产性能、养分消化率(全收粪法)和血清生化免疫指标的影响[J]. 饲料研究, 2017(5): 5-9, 39. CHEN X X, WANG J Q, LIU Z K, et al. Effects of Lactobacillus fermented liquid feed on performance, nutrient digestibility(total fecal collection method)and serum biochemical immune indices of weaned piglets[J]. Feed Research, 2017(5): 5-9, 39 (in Chinese). |
| [17] |
COLLINGTON G K, PARKER D S, ARMSTRONG D G. The influence of inclusion of either an antibiotic or a probiotic in the diet on the development of digestive enzyme activity in the pig[J]. British Journal of Nutrition, 1990, 64(1): 59-70. DOI:10.1079/BJN19900009 |
| [18] |
XUAN Z N, KIM J D, HEO K N, et al. Study on the development of a probiotics complex for weaned pigs[J]. Asian-Australasian Journal of Animal Sciences, 2001, 14(10): 1425-1428. DOI:10.5713/ajas.2001.1425 |
| [19] |
KORNEGAY E T, RISLEY C R. Nutrient digestibilities of a corn-soybean meal diet as influenced by Bacillus products fed to finishing swine[J]. Journal of Animal Science, 1996, 74(4): 799-805. DOI:10.2527/1996.744799x |
| [20] |
王志祥, 乔家运, 王自恒, 等. 乳酸杆菌对断奶仔猪生长性能、养分表观消化率和消化酶活性的影响[J]. 西北农林科技大学学报(自然科学版), 2006(4): 23-27. WANG Z X, QIAO J Y, WANG Z H, et al. Effect of Lactobacillus on growth performance, nutrient digestibility and digestive enzyme activities of weaned piglets[J]. Journal of Northwest A & F University(Natural Science Edition), 2006(4): 23-27 (in Chinese). DOI:10.3321/j.issn:1671-9387.2006.04.006 |
| [21] |
崔艳红, 韩庆功, 崔艺佳, 等. 益生菌复合发酵料对断奶仔猪消化环境、血清生化指标和代谢激素水平的影响[J]. 西北农业学报, 2018, 27(1): 16-23. CUI Y H, HAN Q G, CUI Y J, et al. Effects of probiotics composite fermentation feed on gastrointestinal circumstance, serum biochemical and metabolic hormones indexes of weaning piglets[J]. Acta Agriculturae Boreali-occidentalis Sinica, 2018, 27(1): 16-23 (in Chinese). |
| [22] |
杜泓明, 汪晓东, 王晓磊, 等. 不同益生菌培养物对断奶仔猪生长性能、免疫功能及血液生化指标的影响[J]. 中国兽医学报, 2017, 37(7): 1379-1384, 1400. DU H M, WANG X D, WANG X L, et al. Effect of different probiotic cultures on growth performance, immunological function and blood biochemical indexes of weaned piglets[J]. Chinese Journal of Veterinary Science, 2017, 37(7): 1379-1384, 1400 (in Chinese). |
| [23] |
刘辉, 季海峰, 王四新, 等. 复合乳酸菌发酵饲料对生长猪生长性能、粪便菌群、血清免疫和抗氧化指标的影响[J]. 动物营养学报, 2022, 34(2): 783-794. LIU H, JI H F, WANG S X, et al. Effects of compound lactic acid bacteria fermented feed on growth performance, fecal microflora, serum immune and antioxidant indexes of growing pigs[J]. Chinese Journal of Animal Nutrition, 2022, 34(2): 783-794 (in Chinese). |
| [24] |
WANG S L, YAO B Q, GAO H, et al. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs[J]. BMC Veterinary Research, 2019, 15(1): 239. DOI:10.1186/s12917-019-1991-9 |
| [25] |
孙玉亭. 芽孢乳杆菌S1生产工艺优化及其合生素对仔猪生长性能与粪样菌群的影响[D]. 硕士学位论文. 南京: 南京农业大学, 2019. SUN Y T. Production process optimization of Sporolactobacillus S1 and its effect on growth performance and fecal flora of piglets[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2019. (in Chinese) |
| [26] |
CASTILLO M, MARTÍN-ORÚE S M, MANZANILLA E G, et al. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR[J]. Veterinary Microbiology, 2006, 114(1/2): 165-170. |
| [27] |
GIANG H H, VIET T Q, OGLE B, et al. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria[J]. Livestock Science, 2010, 129(1/2/3): 95-103. |
| [28] |
PIEPER R, JANCZYK P, URUBSCHUROV V, et al. Effect of a single oral administration of Lactobacillus plantarum DSMZ 8862/8866 before and at the time point of weaning on intestinal microbial communities in piglets[J]. International Journal of Food Microbiology, 2009, 130(3): 227-232. DOI:10.1016/j.ijfoodmicro.2009.01.026 |
| [29] |
蔡艳, 叶盛, 韩晓云, 等. 乳酸菌对仔猪生长性能及肠道菌群的影响[J]. 江西畜牧兽医杂志, 2018(5): 15-16. CAI Y, YE S, HAN X Y, et al. Effects of lactic acid bacteria on growth performance and intestinal microflora of piglets[J]. Jiangxi Journal of Animal Husbandry & Veterinary Medicine, 2018(5): 15-16 (in Chinese). |
| [30] |
杜泓明. 不同益生菌培养物对断奶仔猪生长性能、免疫功能及盲肠微生物区系的影响[D]. 硕士学位论文. 长春: 吉林农业大学, 2017. DU H M. Effect of different probiotic cultures on growth performance, immune function and cecum microflora diversity of weaned piglets[D]. Master's Thesis. Changchun: Jilin Agricultural University, 2017. (in Chinese) |
| [31] |
刘统. 德氏乳杆菌对哺乳仔猪胃肠道微生物多样性影响研究[D]. 硕士学位论文. 长沙: 湖南农业大学, 2012. LIU T. Effects of L. debrueckii on gastrointestinal microflora diversity of sucking piglets[D]. Master's Thesis. Changsha: Hunan Agricultural University, 2012. (in Chinese) |
| [32] |
DOS SANTOS T F, MELO T A, SANTOS D S, et al. Efficacy of oral administration of lactic acid bacteria isolated from cocoa in a fermented milk preparation: reduction of colitis in an experimental rat model[J]. Genetics and Molecular Research, 2016, 15(3): gmr.15038097. |
| [33] |
JHA R, BERROCOSO J D. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine[J]. Animal, 2015, 9(9): 1441-1452. |
| [34] |
张丽. 新型酵母培养物的制备及其对断奶仔猪生长性能、表观消化率和粪便微生物的影响[D]. 硕士学位论文. 北京: 中国农业科学院, 2016. ZHANG L. The preparation of a novel yeast culture and its effect on growth performance, apparent nutrient digestibility and fecal microbiota of weaned piglets[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2016. (in Chinese) |
| [35] |
PARK S J, KIM J, LEE J S, et al. Characterization of the fecal microbiome in different swine groups by high-throughput sequencing[J]. Anaerobe, 2014, 28: 157-162. |
| [36] |
KIM H B, BOREWICZ K, WHITE B A, et al. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs[J]. Veterinary Microbiology, 2011, 153(1/2): 124-133. |
| [37] |
YU H, ZHOU T, GONG J H, et al. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection[J]. BMC Microbiology, 2010, 10: 182. |
| [38] |
LAN G Q, ABDULLAH N, JALALUDIN S, et al. Purification and characterization of a phytase Mitsuokella jalaludinii, a bovine rumen bacterium[J]. African Journal of Biotechnology, 2011, 10(59): 12766-12776. |
| [39] |
BHATTI M A, FRANK M O. Veillonella parvula meningitis: case report and review of Veillonella infections[J]. Clinical Infectious Diseases, 2000, 31(3): 839-840. |
| [40] |
WANG Y, TAO H X, HUANG H M, et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome[J]. Food & Function, 2021, 12(7): 3142-3158. |
| [41] |
LIU Y Y, LI T, ALIM A, et al. Regulatory effects of stachyose on colonic and hepatic inflammation, gut microbiota dysbiosis, and peripheral CD4+ T cell distribution abnormality in high-fat diet-fed mice[J]. Journal of Agricultural and Food Chemistry, 2019, 67(42): 11665-11674. |
| [42] |
KIM H, PARK T, KWON I, et al. Specific inhibition of Streptococcus bovis by endolysin LyJH307 supplementation shifts the rumen microbiota and metabolic pathways related to carbohydrate metabolism[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 93. |




