2. 赛多美(北京)农业科技有限公司, 北京 100027
2. Cytozyme (Beijing) Agricultural Technology Co., Ltd., Beijing 100027, China
伴随着集约化养殖的不断发展,仔猪早期断奶是近年来养猪过程中的重要环节,可以极大地提高养猪场的生产效率。早期断奶仔猪的胃肠道未完全发育、免疫系统不完善、抗病能力弱,同时受到饲粮组成、饲养环境和心理等因素的影响,会使断奶仔猪产生一系列的断奶应激,导致仔猪发育迟缓、发生腹泻,严重时甚至导致死亡[1-2]。为解决这一难题,养殖生产中主要通过在饲粮中添加抗生素来缓解仔猪早期断奶应激。然而,饲粮中添加抗生素会产生耐药性和药物残留[3-4]。我国已于2020年7月禁止在饲粮中添加抗生素。高剂量氧化锌可以有效降低仔猪腹泻和促进仔猪生长,长期以来一直在断奶仔猪饲粮中使用,特别是“禁抗”以来发挥了很好的“替抗”作用。然而,高剂量氧化锌的添加也带来一系列负面影响,比如增加了机体对抗生素的抗药性,并且大量锌元素的排出对环境造成了很大污染。欧盟将于2022年6月份开始禁止在断奶仔猪饲粮中添加高剂量氧化锌,规定饲粮中锌的添加量不能超过150 mg/kg。因此,为保障畜禽养殖业的生产效益和可持续发展,寻找和开发绿色、高效“替抗”的饲料添加剂已刻不容缓。
近年来,后生元及其有效成分益生作用的研究逐渐受到关注,后生元是继益生菌出现后定义的一个新概念,是一种功能性生物活性化合物。后生元是益生菌经加工处理后的益生菌成分统称,包括菌体与益生菌代谢产物。2019年,国际益生菌和益生元协会把后生元定义为“对宿主起有益作用的灭活菌和/或菌成分”[5]。后生元因具有超长的保质期、安全的剂量参数以及含有多种具有抗菌、抗氧化、免疫调节、抗增殖、降胆固醇等生物学作用而受到人们的关注[6]。Guo等[7]研究发现,来自乳酸乳球菌产生的后生元(胞外多糖)可以增加抗氧化酶[过氧化氢酶(catalase,CAT)、超氧化物歧化酶(superoxide dismutase,SOD)和谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)等]活性,表现出抗氧化活性,同时降低小鼠血浆和肝脏中的脂质过氧化物[丙二醛(malondialdehyde,MDA)等]含量。Izuddin等[8]研究发现,饲粮中添加后生元增加了羔羊的增重、采食量、营养物质摄入量和营养物质消化率,提高了血清总蛋白、尿素氮和葡萄糖含量。
目前,后生元在断奶仔猪饲粮中应用效果的研究较少。因此,本试验选用后生元与高剂量氧化锌进行对比,探讨后生元对断奶仔猪生长性能、腹泻率、抗氧化能力及粪便微生物菌群的影响,以及饲粮中添加后生元对于高剂量氧化锌的替代效果,为后生元在动物生产中的深入研究和开发应用提供数据参考。
1 材料与方法 1.1 试验材料本试验所用后生元为乳酸菌来源后生元产品,由美国某生物科技有限公司提供,该产品为嗜酸乳杆菌(Lactobacillus acidophilus)经过多级发酵而成,含有多种代谢产物,主要包括有机酸、肽聚糖、胞外多糖、磷壁酸、蛋白质和肽等。本产品主要载体为膨润土,粗蛋白质含量为5%。
1.2 试验设计试验选取75头23日龄“杜×长×大”三元杂交断奶阉公猪,初始体重为(6.18±0.21) kg,按体重完全随机区组分为3个组,每组5个重复(栏),每个重复5头猪。阴性对照组(negative control group,NC组)饲喂不添加抗生素和氧化锌的基础饲粮,阳性对照组(positive control group,PC组)饲喂基础饲粮+氧化锌[保育前期(第1~14天)添加2 kg/t,保育后期(第15~42天)不添加],后生元组(postbiotics group,PB组)饲喂基础饲粮+后生元(保育前期添加4 kg/t,保育后期添加2 kg/t)。试验期42 d,分2阶段(保育前期和保育后期)饲喂。仔猪基础饲粮配制参照NRC(2012)保育阶段猪营养需要,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(饲喂基础) Table 1 Composition and nutrient levels of basal diets (as feed basis) |
试验于中国农业科学院饲料研究所廊坊试验基地进行。试验采用全封闭式猪舍,试验仔猪分栏饲养于1.4 m×1.7 m的漏缝式地板圈内,饲喂粉料,自由采食和饮水。猪舍初始室温为28 ℃,每周降低1 ℃,最终温度达到25 ℃。每日检查猪舍照明和通风情况,并定期清洁猪圈。室内使用日光和人造光相结合,使用速度控制风扇通风。每栏配有2个乳头式饮水器和不锈钢可调式料槽。消毒程序及疫苗接种按猪场常规方法进行。
1.4 试验指标测定及方法 1.4.1 生长性能在试验开始当天、第14天、第28天和第42天,分别称量仔猪栏重并记录数据,计算平均日增重(average daily gain,ADG)、平均日采食量(average daily feed intake,ADFI)和重料比(gain to feed ratio,G/F)。仔猪死淘时记录死淘仔猪体重和相对应栏剩余料重,用于校正生长性能数据。计算公式如下:

|
试验期记录第1~14天腹泻情况,每天09:00逐头检测仔猪腹泻情况,观察每头仔猪肛门红肿情况及各栏内稀粪是否存在。采用4分粪便评分系统对每头仔猪每日进行视觉评估监测:0分=坚硬,成形的粪便;1分=柔软,湿润的粪便,成形;2分=软便,不成形;3分=水样便。液体形式粪便(2~3分)将被视为腹泻。腹泻率(%)为腹泻仔猪数除以断奶仔猪总数的百分比,计算公式如下:

|
试验第14天和第42天,分别从每栏中选取1头体重接近平均体重的仔猪,通过前腔静脉进行静脉采血,静置30 min后3 000 r/min离心10 min,分离血浆并置于-20 ℃冰箱保存待测。抗氧化指标包括:MDA、SOD、CAT和GSH-Px;免疫指标包括:免疫球蛋白A(immunoglobulin A,IgA)、免疫球蛋白G(immunoglobulin G,IgG)。上述测定指标所需试剂盒均购自南京建成生物工程研究所,具体操作步骤依照试剂盒说明书进行。
1.4.4 粪便微生物指标试验第14天,从每栏中随机选取3头仔猪,通过人工刺激肛门内外括约肌法和直肠擦拭法,采集新鲜的粪便样本(大约20 g),同一栏粪便混匀装入冻存管并放入-80 ℃冰箱冻存,对粪便样本进行DNA提取和16S rRNA PCR扩增。粪便样品送到上海美吉生物医药科技有限公司,通过高通量测序技术对粪便进行16S rRNA基因测序,构建PE文库,利用Illumina测序(序列相似度:0.97,物种分类数据库:silva138/16s_bacteria,分类置信度:0.7),根据相似度水平的不同对所有序列进行操作分类单元(operational taxonomic unit,OTU)划分,用97%相似度水平的OTU进行统计分析。
1.5 统计分析使用SAS 9.3统计软件分析所有数据,采用随机区组设计进行广义线性模型(GLM)方差分析,使用Tukey HSD法进行多重比较。此外,采用卡方检验分析仔猪腹泻率。利用非参数检验分析粪便样品之间微生物的相对丰度,揭示样品的物种构成。结果以平均值和均值标准误(SEM)表示,P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果 2.1 饲粮中添加后生元对断奶仔猪生长性能及腹泻率的影响由表 2可知,PC组的第28天体重显著低于NC组和PB组(P < 0.05),PC组的第15~28天ADG和ADFI极显著低于NC组和PB组(P < 0.01),PC组的第1~42天ADFI显著低于PB组(P < 0.05),PC组和PB组的第1~14天腹泻率极显著低于NC组(P < 0.01)。
|
|
表 2 饲粮中添加后生元对断奶仔猪生长性能及腹泻率的影响 Table 2 Effects of dietary postbiotics on growth performance and diarrhea incidence of weaned piglets |
由表 3可知,第14天,PC组和PB组的血浆CAT、SOD和GSH-Px活性极显著高于NC组(P < 0.01);PC组和PB组的血浆MDA含量极显著低于NC组(P < 0.01),PB组的血浆MDA含量极显著低于PC组(P < 0.01)。第42天,PC组和PB组的血浆SOD和GSH-Px活性极显著高于NC组(P < 0.01),PB组的血浆MDA含量显著低于NC组(P < 0.05)。
|
|
表 3 饲粮中添加后生元对断奶仔猪血浆抗氧化指标的影响 Table 3 Effects of dietary postbiotics on plasma antioxidant indices of weaned piglets |
由表 4可知,第14天,PC组和PB组的血浆IgA含量极显著高于NC组(P < 0.01)。第42天,PB组的血浆IgA含量极显著高于NC组(P < 0.01),PC组和PB组的血浆IgG含量极显著高于NC组(P < 0.01)。
|
|
表 4 饲粮中添加后生元对断奶仔猪血浆免疫指标的影响 Table 4 Effects of dietary postbiotics on plasma immune indices of weaned piglets |
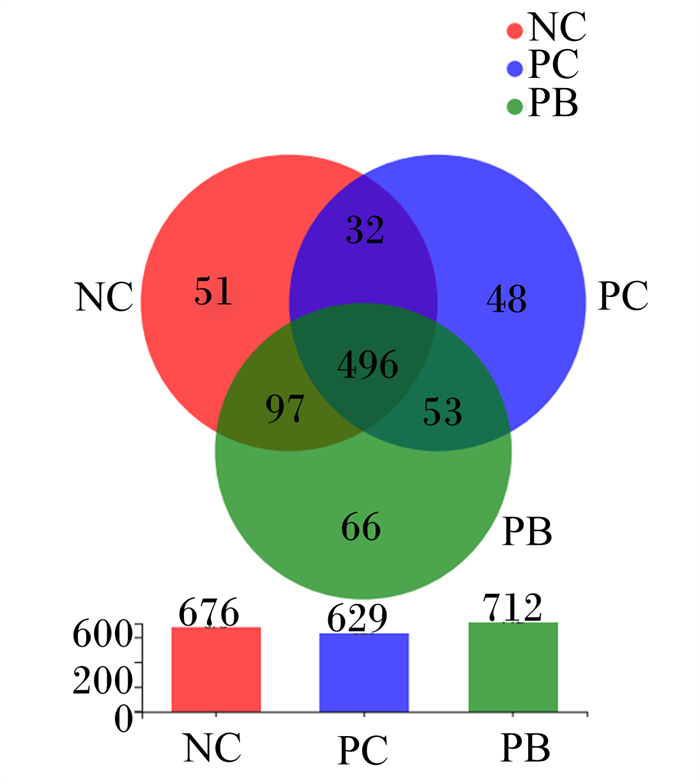
根据第14天粪便样品中的OTU绘制了Venn图,可用来统计不同样品中独有与共有的OTU数目,可以比较直观地体现出样品OTU数目组成的相似性和重叠性。由图 1可知,NC组独有51个OTU,PC组独有48个OTU,PB组独有66个OTU,3组共同拥有496个OTU。
|
NC:阴性对照组negative control group;PC:阳性对照组positive control group;PB:后生元组postbiotics group。下图同the same as below。 图 1 OTU Venn图 Fig. 1 OTU Venn diagram |
根据物种注释结果,分别在门水平和属水平上进行了分析,由表 5可知,PC组和PB组的Shannon指数极显著高于NC组(P < 0.01),PC组和PB组的Simpson指数显著低于NC组(P < 0.05)。各组之间Ace指数和Chao1指数差异不显著(P>0.05)。
|
|
表 5 饲粮中添加后生元对断奶仔猪粪便微生物α多样性的影响 Table 5 Effects of dietary postbiotics on fecal microbiome α diversity of weaned piglets |
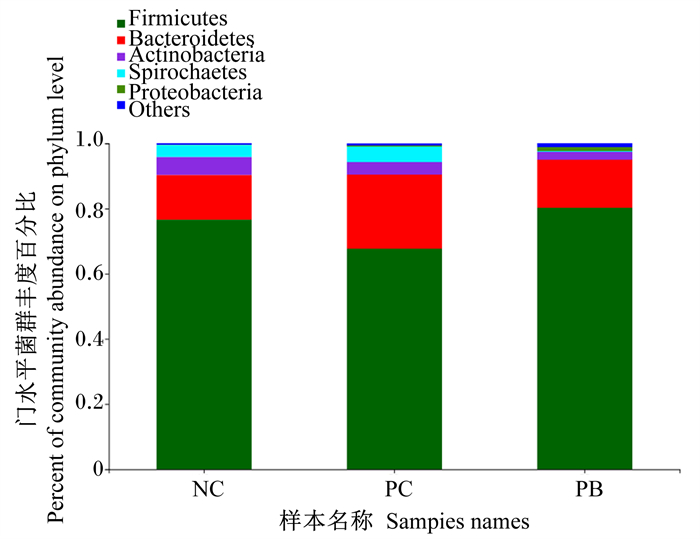
由图 2可知,在门水平上,粪便微生物中优势菌门主要是厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidota)、放线菌门(Actinobacteriota)、螺旋菌门(Spirochaetota)、变形菌门(Proteobacteria)。其中,NC组、PC组和PB组的粪便厚壁菌门相对丰度分别为76.61%、67.79%和80.35%,拟杆菌门相对丰度分别为13.72%、22.55%和14.77%。
|
Firmicutes:厚壁菌门;Bacteroidetes:拟杆菌门;Actinobacteria:放线菌门;Spirochaetes:螺旋菌门;Proteobacteria:变形菌门;Others:其他。 图 2 门水平粪便微生物组成分析 Fig. 2 Analysis of microbiome composition in feces at phylum level |
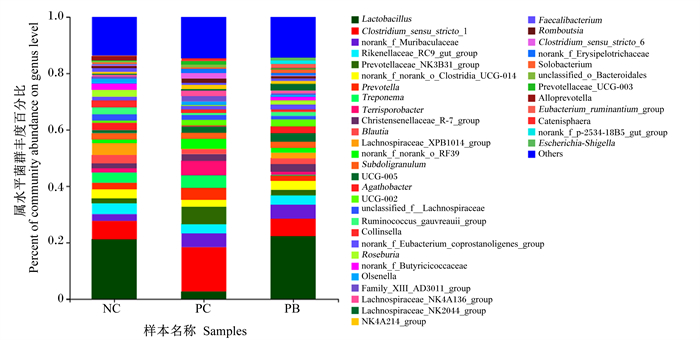
由图 3可知,在属水平上,粪便微生物中优势菌属主要是乳杆菌属(Lactobacillus)、狭义梭菌属1(Clostridium_sensu_stricto_1)、orank_f_Muribaculaceae、理研菌科RC9肠群(Rikenellaceae_RC9_gut_group)、普雷沃菌科NK3B31群(Prevotellaceae NK3B31_group)、普雷沃菌属(Prevotella)、密螺旋菌属(Treponema)、肠杆菌属(Terrisporobacter)、克里斯滕森菌科R-7群(Christensenellaceae_R-7_group)、布劳特氏菌属(Blautia)等。其中,NC组、PC组和PB组的粪便乳杆菌属相对丰度分别为21.13%、2.69%和22.27%,粪便狭义梭菌属1相对丰度分别为6.54%、15.71%和6.37%。
|
Lactobacillus:乳杆菌属;Clostridium_sensu_stricto_1:狭义梭菌属1;Rikenellaceae_RC9_gut_group:理研菌科RC9肠群;Prevotellaceae_ NK3B31_group:普雷沃菌科NK3B31群;Prevotella:普雷沃菌属;Treponema:密螺旋菌属;Terrisporobacter:肠杆菌属;Blautia:布劳特氏菌属;Subdoligranulum:罕见小球菌属;Agathobacter:无加杆菌属;Roseburia:罗斯氏菌属;Faecalibacterium:粪杆菌属;Romboutsia:罗姆布茨菌属;Clostridium_sensu_stricto_6:狭义的梭菌属6;Ruminococcus:瘤胃球菌属;Escherichia-Shigella:埃希氏菌-志贺氏菌属;Streptococcus:链球菌属;Butyricicoccus:丁酸菌属;Desulfovibrio:脱硫弧菌属;Catenibacterium:链状杆菌属;Oribacterium:东方杆菌属;Syntrophococcus:互营球菌属;Oscillospira:颤螺旋菌属;Anaerovibrio:厌氧弧菌属;Christensenellaceae_R-7_group:克里斯滕森菌科R-7群;Lachnospiraceae_XPB1014_group:毛螺旋菌科_XPB1014群;unclassified_f_Lachnospiraceae:未分类-毛螺旋菌科;Lachnospiraceae_NK4A136_group:毛螺旋菌科NK4A136群;Lachnospiraceae_AC2044_group:毛螺旋菌科AC2044群;NK4A214_group:NK4A214群;Eubacterium_ruminantium_group:优杆菌属反刍动物群;Eubacterium_hallii_group:优杆菌属哈利群;Eubacterium_siraeum_group:优杆菌属西雷姆群;Ruminococcus_torques_group:瘤胃球菌属扭矩群;Lachnospiraceae_NK3A20_group:毛螺旋菌科NK3A20群;Others:其他。 图 3 属水平粪便微生物组成分析 Fig. 3 Analysis of microbiome composition in feces at genus level |
由表 6可知,NC组和PB组的粪便乳杆菌属相对丰度极显著高于PC组(P<0.01),PC组的粪便狭义梭菌属1相对丰度极显著高于NC组和PC组(P<0.01)。
|
|
表 6 饲粮中添加后生元对断奶仔猪粪便乳杆菌属和狭义梭菌属1的相对丰度的影响 Table 6 Effects of dietary postbiotics on relative abundances of Lactobacillus and Clostridium_sensu_stricto_1 in feces of weaned piglets |
本研究证实了饲粮中添加氧化锌可通过降低断奶仔猪腹泻率促进保育前期生长的效果[9-11],从而建立了一个很好的动物模型来确定饲粮中添加后生元对断奶仔猪生长性能和腹泻率的作用。尽管如此,Pieper等[12]研究表明,氧化锌对于肠道有益菌和有害菌均有抑制作用,对断奶仔猪后期生长产生不良的影响。本研究中,PC组仔猪在保育后期的生长性能较差,这可能是保育前期氧化锌抑制了肠道内微生物活性,在保育后期无氧化锌的作用时使肠道菌群失衡,从而抑制了肠道对营养物质的吸收和后期的生长。早期研究发现,饲粮中添加后生元可以降低仔猪的腹泻率,对于生长有一定的促进作用。Thu等[13]研究表明,断奶仔猪饲粮中添加植物乳杆菌液体代谢物组合可以降低断奶仔猪腹泻率,提高断奶仔猪的生长性能。Sukegawa等[14]研究表明,断奶仔猪饲粮中添加热灭活的粪肠球菌IHARA可以促进断奶仔猪小肠绒毛的生长,提高机体对营养物质的吸收效率,降低腹泻率,进而提高断奶仔猪的生长性能。Fu等[15]研究表明,断奶仔猪饲粮中添加酵母水解物能够促进断奶仔猪肠道屏障功能,提高生长性能。Loh等[16]研究表明,饲粮中添加0.5%的植物乳杆菌代谢物组合可以改善断奶仔猪的肠道健康环境和生长性能,提高蛋白质消化率;但添加0.1%的植物乳杆菌代谢物组合无明显的作用效果,说明后生元对于断奶仔猪的作用效果与添加量密切相关。与上述研究结果相似,本试验中,饲粮中添加后生元可降低断奶仔猪腹泻率,可能与其提高仔猪机体健康和肠道完整性有关。本研究团队前期研究发现,饲粮中添加没食子酸可通过抑制肠道炎症反应,提高肠道完整性,从而降低仔猪断奶后腹泻率[17]。但本研究发现,饲粮中添加后生元对断奶仔猪生长性能虽有所促进,但无显著影响,这可能与添加的后生元种类和添加量、仔猪自身条件以及饲养管理有关,需要后续相关试验来进行验证。
3.2 饲粮中添加后生元对断奶仔猪血浆抗氧化和免疫指标的影响氧化应激是活性氧的产生和清除之间的失衡,会造成动物体内不可修复的氧化损伤,进而影响整个机体的功能[18-19]。研究发现,仔猪断奶会破坏体内的氧化平衡,破坏自由基的正常代谢,降低机体抗氧化能力[20]。MDA直接反映脂质氧化损伤的程度[21],而CAT、SOD和GSH-Px的活性强弱反映了机体抗氧化能力的强弱[22-26]。Xu等[27]研究发现,植物乳杆菌胞外多糖具有显著的抗氧化活性,能够对1, 1-二苯基-2-三硝基苯肼(DPPH)和2, 2’-联氨-双-3-乙基苯并噻唑啉-6-磺酸(ABTS)自由基起到清除活性作用,并且可以保护DNA免受损伤。Zhang等[28]研究表明,植物乳杆菌C88多糖可以抑制MDA的形成,提高总抗氧化能力(T-AOC)和SOD活性,并呈剂量依赖性。Czech等[29]研究发现,在怀孕母猪饲粮中添加酵母甘露寡糖,可以提高后代仔猪机体CAT和SOD活性。血浆IgA、IgG在动物机体参与免疫应答反应中发挥着主要作用[30-31]。WANG等[32]研究发现,植物乳杆菌胞外多糖可以显著提高大鼠肠道IgA含量。Xiong等[33]研究表明,饲粮中添加酵母水解物可以增加断奶仔猪血浆IgG含量。本试验中,饲粮中添加氧化锌和后生元降低了断奶仔猪血浆MDA含量,提高了血浆CAT、SOD、GSH-Px活性和IgA和IgG含量,说明饲粮中添加氧化锌和后生元可以提高断奶仔猪抗氧化能力,并增强免疫功能,从而提高仔猪断奶后的抗病力并抑制仔猪腹泻。此外,饲粮中添加后生元可以显著降低断奶仔猪第14天和第42天血浆MDA含量,说明机体的脂质过氧化和自由基生成水平降低。而添加氧化锌仅降低了第14天血浆MDA含量,可能说明饲粮中添加后生元对于调节机体脂质过氧化和自由基生成的效果相比氧化锌更好。因此,在抗氧化能力和免疫功能方面,后生元具有替代氧化锌的效果。
3.3 饲粮中添加后生元对断奶仔猪粪便微生物菌群的影响肠道菌群与生物体的健康和疾病的发生发展密切相关,肠道微生物的存在为机体创造了良好的微生态环境,肠道菌群与机体相互依赖,相互制约[34]。肠道微生物的多样性影响着机体健康,微生物多样性越丰富越有利于机体的健康[35]。Da Silva等[36]研究发现,饲粮中添加2 500 mg/kg普通氧化锌可以降低盲肠菌群的相对丰度,Simpson指数有下降的趋势。杜泓明[37]研究表明,益生菌培养物可以提高断奶仔猪肠道内菌群多样性。本试验结果与上述研究结果一致,饲粮中添加氧化锌降低了菌群OTU数目和特有OTU数目,而PB组总OTU数目和特有OTU数目有所增加。Shannon指数和Simpson指数可以反映粪便菌群的多样性,分别与物种多样性呈正相关和负相关[38]。本试验中,与NC组相比,PC组和PB组Shannon指数提高,Simpson指数降低。综上所述,饲粮中添加后生元在一定程度上可以提高断奶仔猪粪便微生物菌群数量以及多样性,添加氧化锌降低了断奶仔猪粪便微生物菌群数量。
微生物群落组成结构受饲粮组成和环境因素影响较大。相关研究表明,猪肠道微生物中,在门水平上以厚壁菌门相对丰度最高,依次是拟杆菌门、变形杆菌门、放线菌门和螺旋体门等[39]。本研究结果与上述一致,在门水平上主要是厚壁菌门、拟杆菌门、放线菌门、螺旋菌门、变形菌门,其中厚壁菌门的相对丰度最高,占菌落的60%~80%;与NC组相比,PC组的粪便厚壁菌门相对丰度降低,粪便拟杆菌门相对丰度升高,说明饲粮中添加氧化锌可能一定程度上破坏了粪便微生物的平衡;PB组与NC组差异不明显,说明后生元能够维持粪便微生物在门水平上的平衡。吴飞等[40]研究表明,饲粮中添加0.6%后生元(乳酸杆菌菌体成分及其代谢产物)可提高回肠食糜中乳杆菌属相对丰度,降低梭菌属(Clostridium)相对丰度。本试验在属水平上结果显示,PB组与NC组粪便微生物在属水平上的相对丰度差异不明显,但饲粮中添加氧化锌降低了乳杆菌属相对丰度,并提并高了狭义梭菌属1相对丰度。Starke等[41]研究发现,饲粮中添加高剂量氧化锌降低了仔猪肠道内乳酸杆菌的数量。狭义梭菌属是肠道有害菌属,可产生毒素对机体产生危害[42]。本试验提高的狭义梭菌属1相对丰度说明了氧化锌对于微生物的影响较大,PC组后期的生长性能显著降低可能与狭义梭菌属相对丰度提高有关联,也有可能是因为氧化锌的后置性,导致有害菌增殖破坏肠道微生物菌群平衡所致。此外,饲粮中添加后生元对粪便微生物在属水平的相对丰度无显著影响,说明饲粮中添加后生元能够维持肠道微生物的正常水平。
4 结论① 饲粮中添加后生元能够降低保育前期断奶仔猪腹泻率,提高其抗氧化能力和免疫功能,具有替代高剂量氧化锌的作用。
② 饲粮中添加后生元可丰富断奶仔猪粪便中菌群多样性,维持有益菌的数量,从而保障仔猪稳定生长。
| [1] |
HU C H, SONG J, YOU Z T, et al. Zinc oxide-montmorillonite hybrid influences diarrhea, intestinal mucosal integrity, and digestive enzyme activity in weaned pigs[J]. Biological Trace Element Research, 2012, 149(2): 190-196. DOI:10.1007/s12011-012-9422-9 |
| [2] |
WIJTTEN P J A, VAN DER MEULEN J, VERSTEGEN M W A. Intestinal barrier function and absorption in pigs after weaning: a review[J]. British Journal of Nutrition, 2011, 105(7): 967-981. DOI:10.1017/S0007114510005660 |
| [3] |
VAHJEN W, PIETRUSZYÚSKA D, STARKE I C, et al. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs[J]. Gut Pathogens, 2015, 7(1): 23. DOI:10.1186/s13099-015-0071-3 |
| [4] |
MENZ J, OLSSON O, KÜMMERER K. Antibiotic residues in livestock manure: does the EU risk assessment sufficiently protect against microbial toxicity and selection of resistant bacteria in the environment?[J]. Journal of Hazardous Materials, 2019, 379: 120807. DOI:10.1016/j.jhazmat.2019.120807 |
| [5] |
SALMINEN S, COLLADO M C, ENDO A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics[J]. Nature Reviews.Gastroenterology & Hepatology, 2021, 18(9): 649-667. |
| [6] |
AGUILAR-TOALÁ J E, GARCIA-VARELA R, GARCIA H S, et al. Postbiotics: an evolving term within the functional foods field[J]. Trends in Food Science & Technology, 2018, 75: 105-114. |
| [7] |
GUO Y X, PAN D D, LI H, et al. Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp. Lactis[J]. Food Chemistry, 2013, 138(1): 84-89. DOI:10.1016/j.foodchem.2012.10.029 |
| [8] |
IZUDDIN W I, LOH T C, SAMSUDIN A A, et al. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs[J]. BMC Veterinary Research, 2019, 15(1): 315. DOI:10.1186/s12917-019-2064-9 |
| [9] |
YU T, ZHU C, CHEN S C, et al. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets[J]. Frontiers in Microbiology, 2017, 8: 825. DOI:10.3389/fmicb.2017.00825 |
| [10] |
OU D Y, LI D F, CAO Y H, et al. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs[J]. Journal of Nutritional Biochemistry, 2007, 18(12): 820-826. DOI:10.1016/j.jnutbio.2006.12.022 |
| [11] |
SHELTON N W, TOKACH M D, NELSSEN J L, et al. Effects of copper sulfate, tri-basic copper chloride, and zinc oxide on weanling pig performance[J]. Journal of Animal Science, 2011, 89(8): 2440-2451. DOI:10.2527/jas.2010-3432 |
| [12] |
PIEPER R, VAHJEN W, NEUMANN K, et al. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2012, 96(5): 825-833. DOI:10.1111/j.1439-0396.2011.01231.x |
| [13] |
THU T V, LOH T C, FOO H L, et al. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets[J]. Tropical Animal Health and Production, 2011, 43(1): 69-75. DOI:10.1007/s11250-010-9655-6 |
| [14] |
SUKEGAWA S, IHARA Y, YUGE K, et al. Effects of oral administration of heat-killed Enterococcus faecium strain NHRD IHARA in post-weaning piglets[J]. Animal Science Journal, 2014, 85(4): 454-460. DOI:10.1111/asj.12163 |
| [15] |
FU R Q, LIANG C, CHEN D W, et al. Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets[J]. Journal of Animal Physiology and Animal Nutrition, 2021, 105(5): 898-907. DOI:10.1111/jpn.13529 |
| [16] |
LOH T C, THU T V, FOO H L, et al. Effects of different levels of metabolite combination produced by Lactobacillus plantarum on growth performance, diarrhoea, gut environment and digestibility of postweaning piglets[J]. Journal of Applied Animal Research, 2013, 41(2): 200-207. DOI:10.1080/09712119.2012.741046 |
| [17] |
CAI L, LI Y P, WEI Z X, et al. Effects of dietary gallic acid on growth performance, diarrhea incidence, intestinal morphology, plasma antioxidant indices, and immune response in weaned piglets[J]. Animal Feed Science and Technology, 2020, 261: 114391. DOI:10.1016/j.anifeedsci.2020.114391 |
| [18] |
LI P F, PIAO X S, RU Y J, et al. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health[J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(11): 1617-1626. DOI:10.5713/ajas.2012.12292 |
| [19] |
VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radicals and antioxidants in normal physiological functions and human disease[J]. The International Journal of Biochemistry & Cell Biology, 2007, 39(1): 44-84. |
| [20] |
YIN J, WU M M, XIAO H, et al. Development of an antioxidant system after early weaning in piglets[J]. Journal of Animal Science, 2014, 92(2): 612-619. DOI:10.2527/jas.2013-6986 |
| [21] |
PIRINCCIOGLU A G, GÖKALP D, PIRINCCIOGLU M, et al. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia[J]. Clinical Biochemistry, 2010, 43(15): 1220-1224. DOI:10.1016/j.clinbiochem.2010.07.022 |
| [22] |
MAO X B, LV M, YU B, et al. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets[J]. Journal of Animal Science and Biotechnology, 2014, 5(1): 49. DOI:10.1186/2049-1891-5-49 |
| [23] |
CHEN J S, LONG L N, JIANG Q, et al. Effects of dietary supplementation of Lycium barbarum polysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(4): 1106-1115. DOI:10.1111/jpn.13247 |
| [24] |
YANG Y, KARAKHANOVA S, WERNER J, et al. Reactive oxygen species in cancer biology and anticancer therapy[J]. Current Medicinal Chemistry, 2013, 20(30): 3677-3692. DOI:10.2174/0929867311320999165 |
| [25] |
ZHENG P, YU B, LLY M, et al. Effects of oxidative stress induced by diquat on arginine metabolism of postweaning pigs[J]. Asian-Australasian Journal of Animal Sciences, 2010, 23(1): 98-105. |
| [26] |
DESAGHER S, GLOWINSKI J, PREMONT J. Astrocytes protect neurons from hydrogen peroxide toxicity[J]. Journal of Neuroscience, 1996, 16(8): 2553-2562. DOI:10.1523/JNEUROSCI.16-08-02553.1996 |
| [27] |
XU Y M, CUI Y L, WANG X, et al. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041[J]. International Journal of Biological Macromolecules, 2019, 128: 480-492. DOI:10.1016/j.ijbiomac.2019.01.117 |
| [28] |
ZHANG L, LIU C H, LI D, et al. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88[J]. International Journal of Biological Macromolecules, 2013, 54: 270-275. DOI:10.1016/j.ijbiomac.2012.12.037 |
| [29] |
CZECH A, MOKRZYCKA A, GRELA E R, et al. Influence of mannanoligosaccharides additive to sows diets on blood parameters of sows and their piglets[J]. Bulletin of the Veterinary Institute in Puławy, 2009, 53(1): 89-95. |
| [30] |
HAN Y S, TANG C H, ZHAO Q Y, et al. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets[J]. Livestock Science, 2018, 216: 210-218. DOI:10.1016/j.livsci.2018.08.013 |
| [31] |
SUN P, WANG J Q, ZHANG H T. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves[J]. Journal of Dairy Science, 2010, 93(12): 5851-5855. DOI:10.3168/jds.2010-3263 |
| [32] |
WANG J, WU T, FANG X B, et al. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu[J]. International Journal of Biological Macromolecules, 2018, 115: 985-993. DOI:10.1016/j.ijbiomac.2018.04.099 |
| [33] |
XIONG X, YANG H S, LI B, et al. Dietary supplementation with yeast product improves intestinal function, and serum and ileal amino acid contents in weaned piglets[J]. Livestock Science, 2015, 171: 20-27. DOI:10.1016/j.livsci.2014.10.012 |
| [34] |
LIU C, ZHAO L P, SHEN Y Q. A systematic review of advances in intestinal microflora of fish[J]. Fish Physiology and Biochemistry, 2021, 47(6): 2041-2053. DOI:10.1007/s10695-021-01027-3 |
| [35] |
MARCHESI J R, ADAMS D H, FAVA F, et al. The gut microbiota and host health: a new clinical frontier[J]. Gut, 2016, 65(2): 330-339. DOI:10.1136/gutjnl-2015-309990 |
| [36] |
DA SILVA C A, BENTIN L A T, DIAS C P, et al. Impact of zinc oxide, benzoic acid and probiotics on the performance and cecal microbiota of piglets[J]. Animal Microbiome, 2021, 3(1): 86. DOI:10.1186/s42523-021-00151-y |
| [37] |
杜泓明. 不同益生菌培养物对断奶仔猪生长性能、免疫功能及盲肠微生物区系的影响[D]. 硕士学位论文. 长春: 吉林农业大学, 2017. DU H M. Effect of different probiotic cultures on growth performance, immune function and cecum microflora diversity of weaned piglets[D]. Master's Thesis. Changchun: Jilin Agricultural University, 2017. (in Chinese) |
| [38] |
ZHAO X, FU H Y, QIU S N, et al. Effects of early protein restriction on the growth performance and gut development of pigs fed diets with or without antibiotic[J]. Animal, 2020, 14(7): 1392-1401. DOI:10.1017/S1751731119002921 |
| [39] |
KIM H B, BOREWICZ K, WHITE B A, et al. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs[J]. Veterinary Microbiology, 2011, 153(1/2): 124-133. |
| [40] |
吴飞, 刘虎, 马树良, 等. 无抗日粮中添加后生元对断奶仔猪生长性能及肠道菌群结构的影响[J]. 中国畜牧杂志, 2021, 57(S1): 253-256. WU F, LIU H, MA S L, et al. Effect of postbiotics supplementation in the non-antibiotic diet on growth performance and intestinal microbial community structure of weaned piglets[J]. Chinese Journal of Animal Science, 2021, 57(S1): 253-256 (in Chinese). |
| [41] |
STARKE I C, PIEPER R, NEUMANN K, et al. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets[J]. FEMS Microbiology Ecology, 2014, 87(2): 416-427. DOI:10.1111/1574-6941.12233 |
| [42] |
王诗琦, 陈雪娇, 梁丽萍, 等. 饲粮添加酶解蛋白肽对断奶仔猪生长性能、血清免疫指标和肠道菌群的影响[J]. 动物营养学报, 2022, 34(2): 839-851. WANG S Q, CHEN X J, LIANG L P, et al. Effects of dietary enzymatic protein peptide on growth performance, serum immune indices and intestinal flora of weaned piglets[J]. Chinese Journal of Animal Nutrition, 2022, 34(2): 839-851 (in Chinese). |




