2. 国家动物消化道营养国际联合研究中心, 南京 210095
2. National Center for International Research on Animal Gut Nutrition, Nanjing 210095, China
在现代养猪生产中通常对仔猪实施早期断奶,而早期断奶会引起仔猪强烈的应激反应,仔猪易出现肠道形态损伤、生理功能失调以及肠道微生物多样性减少等断奶应激现象,影响仔猪肠道健康[1-3]。生产中通常在饲粮中添加抗生素来应对断奶应激,改善肠道健康。但长时间滥用抗生素会产生动物体内病原微生物耐药性增强、动物免疫力下降等问题,因此亟需寻找一种新型饲料添加剂来代替抗生素缓解仔猪断奶应激,并在此基础上维持仔猪肠道微生态稳定,保护肠道健康。
植物提取物含有大量生物活性物质,如生物碱、多酚、甾醇、黄酮类化合物等,因其绿色安全、高效环保的特性受到广泛关注[4]。迷迭香酸(rosmarinic acid,RA)是一种广泛存在于自然界多种植物中的水溶性多酚类羟基酸化合物,常见于唇形科的物种,如迷迭香、紫苏、冬凌草等。体外研究表明RA有着高效的抑菌效应,通过破坏金黄色葡萄球菌、沙门氏菌等细菌的细胞膜,引起细菌胞内还原糖、蛋白质、核酸等物质外渗,起到抑制细菌生长的作用[5-6]。而目前对于RA体内试验的研究主要集中在鼠模型中。RA及主要含RA成分的紫苏提取物在不同程度上抑制核因子-κB(NF-κB)、信号转导子与转录激活子3(STAT3)基因和蛋白表达,降低促炎细胞因子白细胞介素(IL)-1β、IL-6等含量,进而改善葡聚糖硫酸钠(DSS)诱导的结肠炎ICR小鼠模型相关结肠炎病变来发挥抗炎作用[7-8]。此外,在三硝基苯磺酸(TNBS)诱导的溃疡性结肠炎大鼠模型中,RA提高了结肠上皮闭锁小带蛋白-1(ZO-1)基因表达,通过维持正常的肠道屏障功能来阻碍结肠炎症的发展[9]。综上所述,RA在体外试验和鼠模型的体内试验中均表现出抑制金黄色葡萄球菌、沙门氏菌等条件性致病菌增殖的作用,且有助于维持肠黏膜屏障功能,进而缓解结肠炎症。因此,我们推测RA可能在改善断奶仔猪结肠屏障功能、调节结肠菌群组成以及缓解炎症反应方面具有较好的效果。
产肠毒素大肠杆菌K88(enterotoxigenic Escherichia coli K88,ETEC K88)能够释放肠毒素,造成肠道微生态失衡,是导致仔猪腹泻的重要原因之一。且在仔猪肠道中,ETEC K88能够激活Toll样受体(TLR)/NF-κB信号通路,增加促炎细胞因子肿瘤坏死因子-α(TNF-α)、IL-6的产生来促进炎症反应的发生[10],因此被广泛用于构建肠道损伤模型。本研究以断奶仔猪为研究对象,通过给断奶仔猪灌胃ETEC K88重悬液进行攻毒,建立肠道损伤模型,研究RA对ETEC K88攻毒断奶仔猪结肠屏障功能、菌群组成及炎症反应的影响,为其在缓解仔猪断奶应激、改善肠道健康等方面的研究提供理论参考。
1 材料与方法 1.1 试验材料试验用迷迭香酸(95%)购自于南京某生物制品有限公司,攻毒用菌种ETEC K88(CVCC224)为南京农业大学动物医学院惠赠。将甘油保存的ETEC K88菌种置于LB(Luria-Bertani)液体培养基(无琼脂)中,于摇床中200 r/min、37 ℃振荡培养12 h复苏,取菌液于LB固体培养基(添加2%琼脂)平板划线接种,置于恒温培养箱中37 ℃培养24 h,挑选特征明显的单个菌落进行培养,采用平板菌落计数法计算每毫升菌液中的菌落形成单位(CFU),使用生理盐水重悬菌液,调整浓度为109 CFU/mL。
1.2 试验动物与设计试验选用18头遗传相近、初始体重为(6.91±1.02) kg的健康21日龄“杜×长×大”三元杂交断奶仔猪,经过3 d的预饲后,将仔猪随机分为3组,每组6头。饲养试验为期21 d,对照组(CON组)和ETEC K88攻毒组(K88组)仔猪饲喂基础饲粮(无抗生素),迷迭香酸组(RA组)仔猪饲喂含RA的试验饲粮(在基础饲粮饲粮基础上添加500 mg/kg RA)。试验第19~20天,K88组、RA组仔猪每天灌喂4 mL浓度为109 CFU/mL的ETEC K88重悬液攻毒[11],对照组仔猪则每天灌喂同等剂量的无菌生理盐水。基础饲粮为玉米-豆粕型,参照NRC(2012)配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
饲养试验于南京农业大学实验动物中心完成,严格执行实验动物饲养管理规程,试验仔猪常规免疫程序均按相应规定完成。试验期间,所有仔猪自由采食和饮水。本次动物试验依照南京农业大学实验动物中心管理制度执行,试验方案经该机构动物福利与伦理委员会批准。
1.4 样品采集与指标测定于试验第22天采用颈静脉放血法对所有仔猪进行屠宰,剖开腹腔采集结肠中段组织于4%多聚甲醛溶液中固定,用于检测结肠形态结构。采集结肠食糜与黏膜于2 mL无菌离心管中,放入液氮中冷冻。
1.4.1 生长性能和腹泻情况在正式试验开始和结束时分别称取仔猪空腹体重,每日收集并记录饲粮的供给量和剩余量,计算平均日采食量(g/d)、平均日增重(g/d)和料重比(平均日采食量/平均日增重)。参考Pierce等[12]的方法,每天观察仔猪的腹泻头数并记录腹泻评分,计算腹泻率和腹泻指数,计算公式如下:

|
将前述固定好的结肠中段组织进行石蜡包埋、苏木精-伊红(HE)染色,于虚拟显微镜下观察形态结构,使用Image-Pro Plus软件测量隐窝深度以及黏膜厚度,并检查是否存在炎性细胞浸润等病变特征。
1.4.3 结肠菌群的16S rRNA高通量测序参考Wang等[13]的研究,按照十六烷基三乙基溴化铵(CTAB)法提取仔猪结肠食糜中细菌总DNA,使用细菌通用引物319F(5’-ACTCCTACGGGAGGCAGCAG-3’)和806R(5’-GGACTACHVGGGTETCTAAT-3’)通过PCR扩增16S rRNA基因的V3~V4区域,扩增后使用DNA凝胶提取试剂盒进行纯化,在Illumina平台利用等摩尔和双末端测序法进行测序[14]。在得到的有效数据中,将相似度≥97%的序列聚类成1个操作分类单元(OTU),然后对OTU进行归一化处理,使用基迪奥公司的OmicShare Tools分析结肠菌群α多样性、β多样性以及门、属水平上的组成。
1.4.4 结肠食糜中短链脂肪酸含量的测定使用赛默飞全自动气相色谱仪(TRACE-1300/1310)测定结肠食糜中短链脂肪酸含量。称取结肠食糜0.1 g于2 mL离心管中,加入1 mL双蒸水混合均匀,充分离心后取1 mL上清液,加入0.2 mL 25%偏磷酸巴豆酸溶液,吸取0.8 mL上清液按照1 ∶ 1比例加入乙醚萃取,充分混匀离心,通过0.22 μm微孔滤膜后注入进样瓶内衬管中用于上机待测。色谱柱为美国Sigma公司产品,30 m×0.32 mm×0.25 μm;气相色谱仪参数:柱温箱温度150 ℃,平衡时间3 min,进样口温度220 ℃,氢火焰离子化检测器温度210 ℃,载气为氮气。
1.4.5 结肠黏膜基因表达的测定使用TRIzol试剂盒(Invitrogen,美国)提取结肠黏膜中的总RNA,然后用反转录试剂盒(Vazyme,中国)将1 μL浓度为1 μg/mL的RNA反转录成cDNA。利用QuantStudio 7荧光定量PCR仪(ABI,美国)及QuantStudio®实时荧光定量PCR系统(Applied Biosystems®,美国)进行实时荧光定量PCR(RT-qPCR)。使用相对定量法测定结肠黏膜中肠道屏障相关基因ZO-1、闭锁蛋白(Occludin)、封闭蛋白-1(Claudin-1)与TLR/髓样分化因子88(MyD88)/NF-κB信号通路相关分子基因TLR-2、TLR-4、TLR-5、NF-κB、MyD88的表达量。试验所使用的引物序列见表 2,以β-肌动蛋白(β-actin)作为内参基因,采用2-△△Ct法计算目的基因的mRNA相对表达量[15]。
1.4.6 结肠黏膜中炎性细胞因子含量的检测取0.1 g结肠黏膜于1 mL离心管中,加入900 μL预冷生理盐水,匀浆后制备10%组织匀浆液,使用BCA蛋白试剂盒(Biosharp,兰杰柯科技有限公司)检测结肠黏膜中总蛋白含量。使用酶联免疫吸附测定(ELISA)试剂盒[生工生物工程(上海)股份有限公司]检测结肠黏膜中炎性细胞因子IL-1β、IL-6、IL-8、IL-10、TNF-α的含量。
1.5 统计分析使用Excel 2016对试验数据进行初步整理,采用SPSS 25.0统计软件进行统计,采用单因素方差分析分别比较K88组与CON组、RA组与K88组的差异显著性,P < 0.05为差异显著,P < 0.01为差异极显著,0.05≤P < 0.10为差异有显著趋势。数据采用平均值±标准误(mean±SE)的形式呈现。
2 结果与分析 2.1 饲粮添加RA对ETEC K88攻毒断奶仔猪生长性能和腹泻的影响如表 3所示,CON组、K88组、RA组仔猪的ADG、ADFI以及F/G无显著差异(P>0.05)。与CON组相比,K88组仔猪腹泻指数显著增加(P < 0.05),并且腹泻率有增加的趋势(P=0.057);而与K88组相比,RA组仔猪腹泻率极显著降低(P < 0.01)。
|
|
表 3 RA对ETEC K88攻毒断奶仔猪生长性能和腹泻的影响 Table 3 Effects of RA on growth performance and diarrhea of weaned piglets challenged by ETEC K88 (n=6) |
如图 1和表 4所示,CON组仔猪结肠组织形态结构正常,无明显病变;与CON组相比,K88组仔猪结肠黏膜层增厚(P < 0.05),隐窝加深(P < 0.01),出现炎性细胞浸润;与K88组相比,RA组仔猪结肠形态结构正常,黏膜层变薄(P < 0.01),隐窝变浅(P < 0.01),无明显病变。
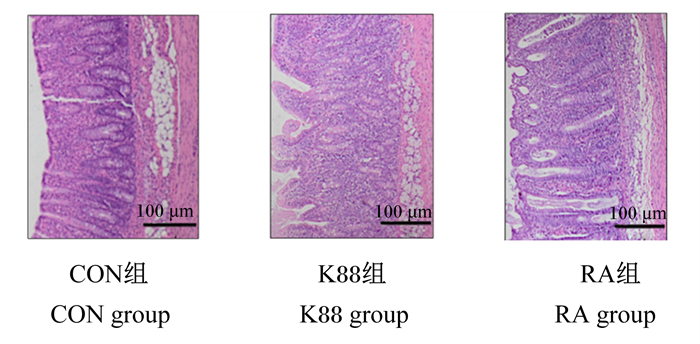
|
图 1 各组断奶仔猪结肠组织切片 Fig. 1 Colonic tissue sections of weaned piglets in each group |
|
|
表 4 RA对ETEC K88攻毒断奶仔猪结肠形态结构的影响 Table 4 Effects of RA on colonic morphology of weaned piglets challenged by ETEC K88 (n=6) |
由表 5可知,与CON相比,K88组仔猪结肠菌群的Chao1和ACE指数显著降低(P < 0.05);而与K88组相比,RA组仔猪结肠菌群的Chao1和ACE指数均有所升高,但差异不显著(P>0.05)。
|
|
表 5 RA对ETEC K88攻毒断奶仔猪结肠菌群α多样性的影响 Table 5 Effects of RA on α diversity of colonic microbiota of weaned piglets challenged by ETEC K88 |
由图 2可知,基于Bray-Curtis距离,在OTU水平上对仔猪结肠菌群进行主坐标分析(PCoA),结果显示K88组与CON组(R=0.474,P=0.002)、K88组与RA组仔猪结肠菌群显著分开(R=0.431,P=0.003)。
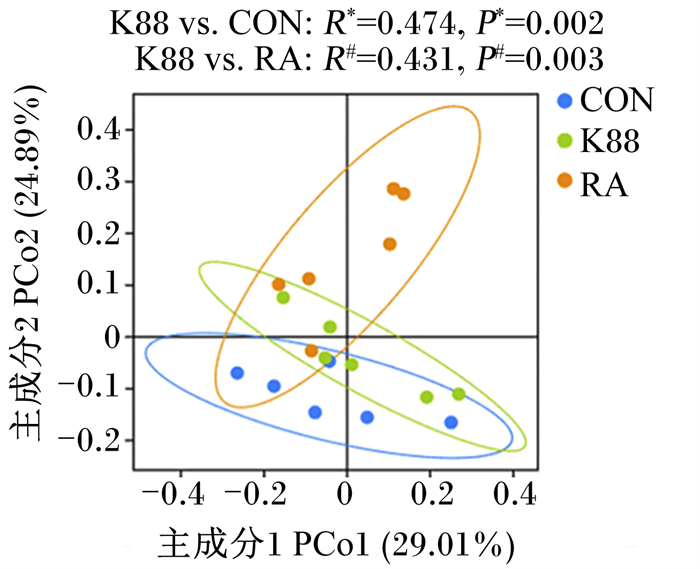
|
CON:CON组;K88:K88组;RA:RA组。下图同。 CON: CON group; K88: K88 group; RA: RA group. The same as below. 图 2 断奶仔猪结肠菌群PCoA图 Fig. 2 PCoA diagram of colonic microbiota of weaned piglets |
由图 3-A可知,断奶仔猪结肠菌群中厚壁菌门(Fimicutes)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、变形菌门(Proteobacteria)为优势菌门。对结肠菌群门水平相对丰度进行统计(图 3-B)可知,与CON组相比,K88组仔猪结肠菌群中变形菌门的相对丰度极显著增加(P < 0.01),厚壁菌门的相对丰度有降低趋势(P=0.054),拟杆菌门的相对丰度有增加趋势(P=0.051);而与K88组相比,RA组仔猪结肠菌群中拟杆菌门的相对丰度极显著降低(P < 0.01),变形菌门的相对丰度显著降低(P < 0.05),放线菌门的相对丰度显著增加(P < 0.05),厚壁菌门的相对丰度有增加趋势(P=0.072)。
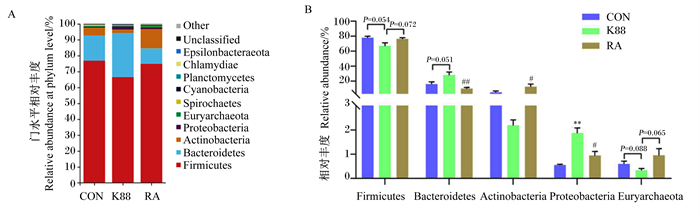
|
Firmicutes:厚壁菌门;Bacteroidetes:拟杆菌门;Actinobacteria:放线菌门;Proteobacteria:变形菌门;Euryarchaeota:广古菌门;Spirochaetes:螺旋体门;Cyanobacteria:蓝细菌门;Planctomycetes:浮霉菌门;Chlamydiae:衣原体门;Unclassified:未分类;Other:其他。 K88组数据柱标注“*”和“* *”分别表示与CON组相比差异显著(P < 0.05)和极显著(P < 0.01);RA组数据柱标注“#”和“##”分别表示与K88组相比差异显著(P < 0.05)和极显著(P < 0.01)。下图同。 Data columns of K88 group with "*" and "* *" indicated significant difference (P < 0.05) and extremely significant difference (P < 0.01) compared with CON group, respectively; data columns of RA group with "#" and "##" indicated significant difference (P < 0.05) and extremely significant difference (P < 0.01) compared with K88 group, respectively. The same as below. 图 3 断奶仔猪结肠菌群门水平物种组成(A)和相对丰度变化(B) Fig. 3 Species composition (A) and relative abundance change (B) of colonic microbiota at phylum level in weaned piglets (n=6) |
断奶仔猪结肠菌群中相对丰度排名前40的菌属如图 4-A所示,其中乳杆菌属(Lactobacillus)、巨球型菌属(Megasphaera)、考拉杆菌属(Phascolarctobacterium)、产粪甾醇真细菌群(Eubacterium_coprostanoligenes_group)为优势菌属。由图 4-B可知,与CON组相比,K88组仔猪结肠菌群中乳杆菌属的相对丰度显著降低(P < 0.05),克里斯滕森菌科R-7群(Christensenellaceae_R-7_group)的相对丰度极显著降低(P < 0.05),脱硫弧菌属(Desulffovibrio)、埃希氏菌-志贺氏菌属(Escherichia-Shigella)的相对丰度显著增加(P < 0.05);而与K88组相比,RA组仔猪结肠菌群中克里斯滕森菌科R-7群极显著增加(P < 0.01),布劳特氏菌属(Blautia)、粪肠球菌属3(Coprococcus_3)、多尔氏菌属(Dorea)、双歧杆菌属(Bifidobacterium)的相对丰度显著增加(P < 0.05),脱硫弧菌属、埃希氏菌-志贺氏菌属的相对丰度显著降低(P < 0.05)。
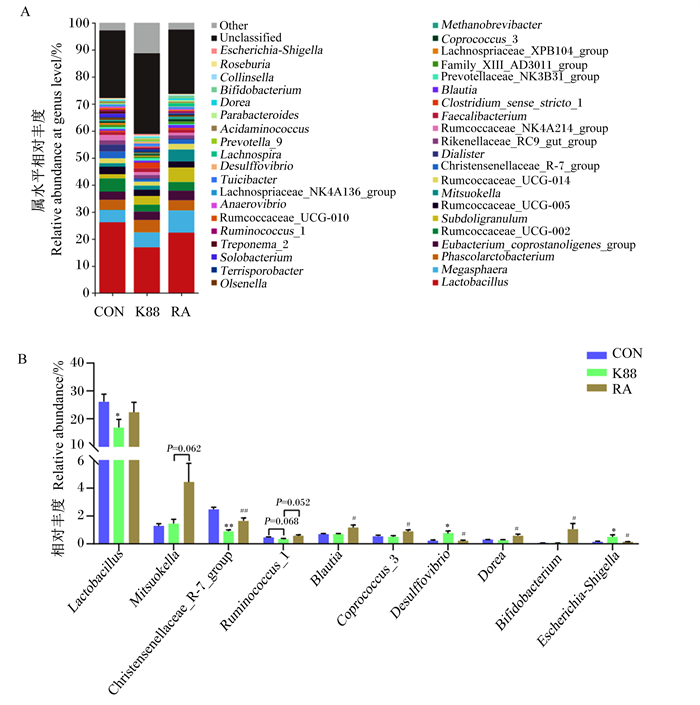
|
Lactobacillus: 乳杆菌属; Megasphaera: 巨球型菌属; Phascolarctobacterium: 考拉杆菌属; Eubacterium_coprostanoligenes_group: 产粪甾醇真细菌群; Rumcoccaceae_UCG-002:瘤胃球菌科UCG-002;Subdoligranulum: 罕见小球菌属; Rumcoccaceae_UCG-005:瘤胃球菌科UCG-005;Mitsuokella: 光冈菌属; Rumcoccaceae_UCG-014:瘤胃球菌科UCG-014;Christensenellaceae_R-7_group: 克里斯滕森菌科R-7群; Dialister: 小杆菌属; Rikenellaceae_RC9_gut_group: 理研菌科RC9肠道群; Rumcoccaceae_NK4A214_group: 瘤胃球菌科NK4A214群; Faecalibacterium: 栖粪杆菌属; Clostridium_sense_stricto_1:狭义梭菌属1;Blautia: 布劳特氏菌属; Prevotellaceae_NK3B31_group: 普雷沃氏菌科NK3B31群; Lachnospriaceae_XPB104_group: 毛螺菌科XPB104群; Coprococcus_3: 粪肠球菌属3;Methanobrevibacter: 甲烷短杆菌属; Olsenella: 颤螺旋菌属; Terrisporobacter: 土孢杆菌属; Solobacterium: 索罗菌属; Treponema_2:密螺旋体属2;Ruminococcus_1:瘤胃球菌属1;Rumcoccaceae_UCG-010:瘤胃球菌科UCG-010;Anaerovibrio: 厌氧弧菌属: Lachnospriaceae_NK4A136_group: 毛螺菌科NK4A136群; Desulffovibrio: 脱硫弧菌属; Lachnospira: 毛螺菌属; Prevotella_9:普雷沃氏菌属9;Acidaminococcus: 氨基酸球菌属; Parabacteroides: 副拟杆菌属; Dorea: 多尔氏菌属; Bifidobacterium: 双歧杆菌属; Collinsella: 柯林斯氏菌属; Roseburia: 罗氏菌属; Escherichia-Shigella: 埃希氏菌-志贺氏菌属; Unclassified: 未分类; Other: 其他。 图 4 断奶仔猪结肠菌群属水平物种组成(A)和相对丰度变化(B) Fig. 4 Species composition (A) and relative abundance change (B) of colonic microbiota at genus level in weaned piglets (n=6) |
由表 6可知,与CON组相比,K88组断奶仔猪结肠食糜中乙酸和总短链脂肪酸含量极显著降低(P < 0.01),异戊酸含量显著降低(P < 0.05);而与K88组相比,RA组断奶仔猪结肠食糜中乙酸、丁酸和总短链脂肪酸含量均显著增加(P < 0.05)。
|
|
表 6 RA对ETEC K88攻毒断奶仔猪结肠食糜中短链脂肪酸含量的影响 Table 6 Effects of RA on SCFA concentrations in colonic chyme of weaned piglets challenged by ETEC K88 (n=6) |
如图 5所示,与CON组相比,K88组仔猪结肠黏膜中Occludin、ZO-1的mRNA相对表达量极显著降低(P < 0.01),Claudin-1的mRNA相对表达量显著降低(P < 0.05);而与K88组相比,RA组仔猪结肠黏膜中Occludin、ZO-1的mRNA相对表达量极显著增加(P < 0.01),且Claudin-1的mRNA相对表达量有增加的趋势(P=0.056)。
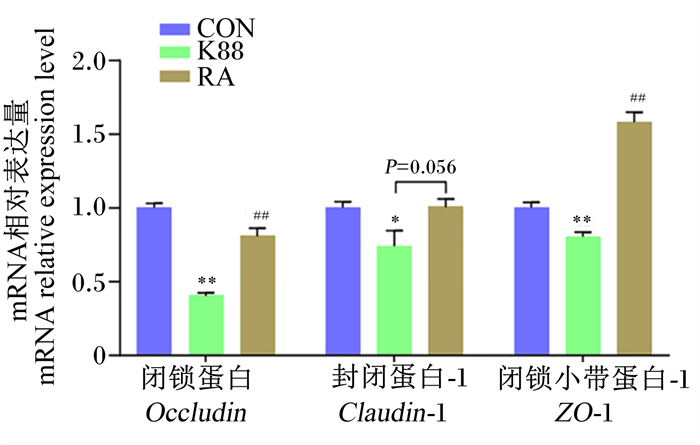
|
图 5 RA对ETEC K88攻毒断奶仔猪结肠黏膜中紧密连接蛋白基因表达的影响 Fig. 5 Effects of RA on expression of tight junction proteins genes in colonic mucosa of weaned piglets challenged by ETEC K88 (n=6) |
由表 7可知,与CON组相比,K88组仔猪结肠黏膜中IL-10含量极显著下降(P < 0.01),TNF-α含量显著升高(P < 0.05);而与K88组相比,RA组仔猪结肠黏膜中IL-1β、TNF-α含量极显著降低(P < 0.01),IL-10含量极显著增加(P < 0.01)。
|
|
表 7 RA对ETEC K88攻毒断奶仔猪结肠黏膜炎症因子水平的影响 Table 7 Effects of RA on inflammatory cytokine contents in colonic mucosa of weaned piglets challenged by ETEC K88 (n=6) |
如图 6所示,与CON组相比,K88组仔猪结肠黏膜中TLR-2、TLR-4、TLR-5、NF-κB、的mRNA相对表达量极显著增加(P < 0.01);而与K88组相比,RA组仔猪结肠黏膜中TLR-2、TLR-4、NF-κB的mRNA相对表达量极显著降低(P < 0.01),TLR-5的mRNA相对表达量显著降低(P < 0.05)。
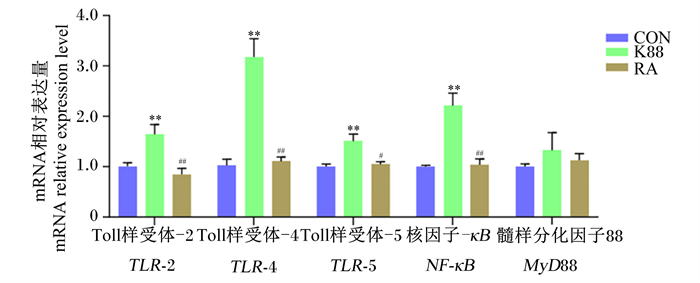
|
图 6 RA对ETEC K88攻毒断奶仔猪结肠黏膜TLR/MyD88/NF-κB信号通路相关分子基因表达的影响 Fig. 6 Effects of RA on expression of TLR/MyD88/NF-κB signaling pathway related molecular genes in colonic mucosa of weaned piglets challenged by ETEC K88 (n=6) |
在猪的整个生长发育过程中,断奶是最大的应激之一,断奶应激产生的仔猪消化不良、腹泻等现象必然伴随着肠道微生态失衡[3, 19]。在本试验中,RA没有对断奶仔猪的生长性能产生显著影响,这可能是由于RA主要被微生物代谢成次级产物,再被有效吸收和利用,此过程RA次级代谢产物在肠道细胞中的运输效率和吸收效率都很低[20-21]。稳定的肠道微生态对于维持仔猪肠道健康具有重要意义,由于RA能够抑制金黄色葡萄球菌、沙门氏菌和大肠杆菌等多种腹泻型致病菌生长,因此可能具有调控肠道微生态平衡的潜力[5-6, 22]。在本研究中,ETEC K88攻毒显著增加了断奶仔猪的腹泻指数,表明本研究成功建立了ETEC K88攻毒模型,而饲粮添加RA极显著降低了ETEC K88攻毒断奶仔猪的腹泻率,表明RA可以改善仔猪腹泻情况,有利于仔猪肠道健康,这可能与RA改善仔猪肠道微生态有关。因此,本研究着重探究了饲粮添加RA对ETEC K88攻毒断奶仔猪结肠菌群组成、屏障功能及炎症反应的影响,并且探讨它们之间可能存在的联系。
肠道形态结构与肠道健康密切相关,肠道的绒毛高度、隐窝深度、黏膜厚度能够直观地反映肠道健康状况[23]。绒毛高度降低、隐窝加深导致肠上皮细胞向肠绒毛顶端迁移的距离缩短、速度加快,没有充足的时间进行分化和成熟,从而影响断奶仔猪肠道消化吸收功能,极易引发腹泻,降低仔猪生长性能[24]。研究表明,在DSS诱导的结肠炎小鼠中通常出现结肠隐窝加深、黏膜增厚的现象[25]。在本研究中,与CON组相比,ETEC K88攻毒使仔猪结肠隐窝加深,黏膜变厚,出现炎性细胞浸润等现象,表明ETEC K88攻毒破坏了仔猪结肠形态结构,可能引发仔猪肠道炎症,不利于仔猪消化吸收营养物质。而与K88组相比,RA组仔猪结肠隐窝变浅,黏膜变薄,无明显病变,这表明饲粮添加RA可以改善ETEC K88攻毒引起的断奶仔猪结肠形态结构受损,有利于维持仔猪正常的肠道消化吸收功能。
肠道菌群能够黏附在肠上皮紧密连接结构、肠黏液层中形成一层覆盖肠道的生物膜,通过菌膜占位性保护阻止外来致病菌入侵和定植[26]。一旦肠道形态结构受损,病原菌侵袭导致肠道微生态失调,极易诱发肠道炎性疾病[27]。因此,我们研究了RA对ETEC K88攻毒断奶仔猪结肠菌群组成的影响。肠道菌群的丰富度可以用Chao1和ACE指数来衡量,Chao1和ACE指数越大,肠道菌群丰富度越高,有助于维持肠道微生态稳定,促进肠道健康[28]。本研究结果显示,与CON组相比,K88组仔猪结肠菌群的Chao1和ACE指数显著降低,而饲粮添加RA改善了这一现象。此外,仔猪结肠菌群β多样性的PCoA结果表明,3组仔猪结肠菌群聚类分离,这表明ETEC K88攻毒以及饲粮添加RA均能改变断奶仔猪结肠菌群组成。
进一步分析结肠菌群门水平组成变化发现,与CON组相比,ETEC K88攻毒降低了仔猪厚壁菌门的相对丰度,而饲粮添加RA增加了厚壁菌门的相对丰度,并且显著增加了放线菌门的相对丰度,显著或极显著降低了拟杆菌门、变形菌门的相对丰度。在属水平上,与CON组相比,ETEC K88攻毒显著或极显降低了仔猪结肠菌群中乳杆菌属、克里斯滕森菌科R-7群的相对丰度,而饲粮添加RA极显著增加了克里斯滕森菌科R-7群的相对丰度。厚壁菌门中的多数成员有益于肠道健康。Gao等[29]研究表明,乳杆菌属相对丰度的增加可能有助于增强低聚半乳糖对脂多糖(LPS)刺激哺乳仔猪结肠的保护作用;Guzmán-Castañeda等[30]研究发现,克里斯滕森菌属细菌可作为益生菌,改善心血管疾病中血脂异常、血压升高现象和2型糖尿病中糖代谢受损、胰岛素紊乱现象。同时,与K88组相比,饲粮粮添加RA还显著增加了仔猪结肠菌群中布劳特氏菌属、粪肠球菌属3、双歧杆菌属的相对丰度。其中,粪肠球菌属细菌能改善仔猪脂质代谢功能障碍,通过调控新陈代谢来影响宿主健康[31];双歧杆菌属细菌能够提高肠易激综合征患者肠道免疫力,改善肠道免疫缺陷引起的病变[32]。此外,与CON组相比,ETEC K88攻毒显著增加了仔猪结肠菌群中埃希氏菌-志贺氏菌属、脱硫弧菌属的相对丰度,而饲粮添加RA显著改善了这一现象。属于变形菌门的埃希氏菌-志贺氏菌属细菌是一种病原菌,常见于动物肠道中,具有潜在的致病性,能够引起仔猪腹泻[33];脱硫弧菌属细菌与溃疡性结肠炎有关,在溃疡性结肠炎患者结肠中相对丰度显著增加[34]。在Yang等[35]的试验中,ETEC K88攻毒使仔猪腹泻率增加,小肠形态遭到破坏,并引起炎症反应,降低仔猪生长性能。综上所述,饲粮中添加RA调节了断奶仔猪的结肠菌群组成,通过提高乳杆菌属、双歧杆菌属等益生菌的相对丰度,减少潜在致病菌埃希氏菌-志贺氏菌属、脱硫弧菌属的定植,在一定程度上有利于维持断奶仔猪肠道微生态平衡,促进肠道健康。
肠道菌群可代谢碳水化合物产生大量短链脂肪酸,其中丁酸可直接为肠黏膜上皮细胞提供能量,有利于维持黏膜屏障的完整性,还能抑制巨噬细胞和中性粒细胞增殖,防止结肠炎症的发生[36]。本研究结果显示,与CON组相比,ETEC K88攻毒极显著降低了仔猪结肠食糜中乙酸的含量,显著降低了异戊酸的含量;而与K88组相比,饲粮添加RA显著增加了仔猪结肠食糜中乙酸、丁酸的含量。前人在体外发酵试验中研究结果表明,添加植物乳杆菌与低聚果糖提高了发酵液中乳杆菌属和双歧杆菌属的相对丰度,产生了更多的乙酸和丁酸[37]。此外,在注射LPS的仔猪饲粮中添加粪肠球菌,能够增加结肠食糜中丁酸等短链脂肪酸的含量,改善仔猪的生长性能[38]。在本研究中,与K88组相比,RA组仔猪结肠食糜中乳杆菌属、双歧杆菌属、粪肠球菌属3等短链脂肪酸产生菌的相对丰度增加,进而提高了短链脂肪酸的含量,表明RA可以通过调节结肠菌群组成来影响细菌代谢产物短链脂肪酸的含量。
肠道屏障功能是机体抵御外源病原菌的重要防线之一,对肠道健康有重要作用[26]。研究发现,双歧杆菌和乳酸杆菌产生的乙酸能够阻止肠腔内抗原穿过肠上皮,应对TNF-α介导的肠道屏障功能失调,特异性恢复肠道紧密连接结构,增强肠上皮屏障功能[39]。饲粮中添加RA通过调节仔猪结肠菌群组成增加了结肠食糜中短链脂肪酸的含量,可能有利于提高仔猪肠道屏障功能,促进肠道健康。本研究中,与CON组相比,ETEC K88攻毒显著或极显著降低了结肠黏膜中ZO-1、Occludin、Claudin-1的mRNA相对表达量,而饲粮添加RA极显著提高了仔猪结肠黏膜中ZO-1、Occludin的mRNA相对表达量,这表明RA有利于维持仔猪结肠上皮屏障功能。
肠道菌群失调直接或间接引起的肠道屏障功能障碍导致肠道通透性增加,加剧肠道炎症[40]。在本研究中,与CON组相比,K88组仔猪结肠形态受损,出现炎性细胞浸润,而RA组仔猪结肠形态结构逐渐恢复至正常水平,说明饲粮中添加RA能够有效缓解ETEC K88攻毒引起的仔猪结肠炎症。进一步分析结肠黏膜中炎性细胞因子含量可知,ETEC K88攻毒显著提高了TNF-α的含量,极显著降低了IL-10的含量,而饲粮添加RA能够改善这一现象,并且极显著降低IL-1β的含量。研究发现,在DSS诱导的结肠炎小鼠模型中,RA能够降低结肠黏膜中IL-1β、IL-6、IL-22的含量,缓解结肠炎相关病症的发展,改善结肠炎[7]。并且丁酸能够下调动物结肠中TNF-α、IL-1β、IL-6和IL-8的表达,上调IL-10和转化生长因子-β(TGF-β)的表达[41],说明RA可能通过提高丁酸的含量增加仔猪结肠应对炎症的能力。TLR/MyD88/NF-κB信号通路是肠道炎症反应中一条重要的信号通路,TLR识别病原微生物后,将信号通过MyD88分子传递给NF-κB,调控炎性细胞因子的生成[42]。在本研究中,与K88组相比,RA组仔猪结肠黏膜中TLR-2、TLR-4、TLR-5、NF-κB的mRNA相对表达量显著或极显著降低,说明RA通过调节TLR/MyD88/NF-κB信号通路相关分子基因的表达缓解了ETEC K88攻毒引起的仔猪结肠炎症反应。陈仪[43]对溃疡性结肠炎模型大鼠的研究发现,甘草泻心汤能够显著抑制大鼠结肠黏膜中TLR-4、MyD88、NF-κB p65的mRNA转录和蛋白表达,降低TNF-α的含量,提高IL-10的含量,具有改善结肠炎症的作用。因此,饲粮中添加RA通过抑制TLR/MyD88/NF-κB信号通路相关分子的激活来减轻ETEC K88攻毒引起的仔猪肠道炎症,保护肠道健康。此外,肠道形态遭到破坏以及ZO-1、Claudin等紧密连接蛋白基因表达下调使肠道屏障功能减弱,产肠毒素大肠杆菌(ETEC)等机会致病菌容易透过肠黏膜引发肠道炎症[44],而给仔猪口服乳酸杆菌能够增加仔猪肠道紧密连接蛋白表达以及抑炎细胞因子的含量,改善肠道形态与肠道屏障功能,提高仔猪应对肠道炎症的能力并缓解腹泻[45]。因此,饲粮添加RA缓解ETEC K88攻毒断奶仔猪结肠炎症反应,降低腹泻率,可能与RA改善了断奶仔猪结肠形态,调节了结肠菌群组成,提高了结肠中丁酸等短链脂肪酸含量,进而增强了仔猪结肠屏障功能有关。
4 结论综上所述,饲粮添加RA可以改善ETEC K88攻毒断奶仔猪结肠形态结构,调节结肠菌群组成,提高细菌代谢产物短链脂肪酸的含量,改善结肠屏障功能,缓解ETEC K88攻毒造成的结肠炎症反应,有利于改善腹泻情况,维持断奶仔猪肠道健康。
| [1] |
WU T, LI K, YI D, et al. Dietary supplementation with trihexanoin enhances intestinal function of weaned piglets[J]. International Journal of Molecular Sciences, 2018, 19(10): 3277. DOI:10.3390/ijms19103277 |
| [2] |
TERCIOLO C, DAPOIGNY M, ANDRE F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption[J]. Clinical and Experimental Gastroenterology, 2019, 12: 67-82. DOI:10.2147/CEG.S181590 |
| [3] |
CHEN S, WU X, XIA Y Y, et al. Effects of dietary gamma-aminobutyric acid supplementation on amino acid profile, intestinal immunity, and microbiota in ETEC-challenged piglets[J]. Food & Function, 2020, 11(10): 9067-9074. |
| [4] |
王梦璇, 董丽, 袁浩洋, 等. 植物提取物在断奶仔猪饲粮中应用的最新研究进展[J]. 中国饲料, 2021(13): 1-6. WANG M X, DONG L, YUAN H Y, et al. Recent progress in the application of plant extracts in the diet of weaned piglets[J]. China Feed, 2021(13): 1-6 (in Chinese). |
| [5] |
汪蕾, 刘洋, 孙杨赢, 等. 迷迭香酸协同ε-聚赖氨酸对金黄色葡萄球菌的抑菌机理初探[J]. 食品工业科技, 2020, 41(14): 192-196, 227. WANG L, LIU Y, SUN Y Y, et al. Primary exploration on antibacterial mechanism of the combination of rosmarinic acid and ε-polylysine against Staphylococcus aureus[J]. Science and Technology of Food Industry, 2020, 41(14): 192-196, 227 (in Chinese). |
| [6] |
蔡晓军, 孙杨赢, 潘道东, 等. 迷迭香主要组分对沙门氏菌的抑制机理[J]. 中国食品学报, 2019, 19(3): 134-140. CAI X J, SUN Y Y, PAN D D, et al. Antibacterial mechanism of rosemarys' main component against Salmonella[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(3): 134-140 (in Chinese). |
| [7] |
JIN B R, CHUNG K S, CHEON S Y, et al. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation[J]. Scientific Reports, 2017, 7: 46252. DOI:10.1038/srep46252 |
| [8] |
URUSHIMA H, NISHIMURA J, MIZUSHIMA T, et al. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2015, 308(1): G32-G41. DOI:10.1152/ajpgi.00294.2014 |
| [9] |
DE OLIVEIRA FORMIGA R, ALVES JÚNIOR E B, VASCONCELOS R C, et al. p-cymene and rosmarinic acid ameliorate TNBS-induced intestinal inflammation upkeeping ZO-1 and MUC-2:role of antioxidant system and immunomodulation[J]. International Journal of Molecular Sciences, 2020, 21(16): 5870. DOI:10.3390/ijms21165870 |
| [10] |
LI H H, LIU X J, SHANG Z Y, et al. Clostridium butyricum helps to alleviate inflammation in weaned piglets challenged with enterotoxigenic Escherichia coli K88[J]. Frontiers in Veterinary Science, 2021, 8: 683863. DOI:10.3389/fvets.2021.683863 |
| [11] |
ZHANG M Y, HOU G J, HU P, et al. Nano chitosan-zinc complex improves the growth performance and antioxidant capacity of the small intestine in weaned piglets[J]. British Journal of Nutrition, 2021, 126(6): 801-812. DOI:10.1017/S0007114520004766 |
| [12] |
PIERCE K M, CALLAN J J, MCCARTHY P, et al. Performance of weanling pigs offered low or high lactose diets supplemented with avilamycin or inulin[J]. Animal Science, 2005, 80(3): 313-318. DOI:10.1079/ASC40900313 |
| [13] |
WANG J, TIAN S Y, YU H, et al. Response of colonic mucosa-associated microbiota composition, mucosal immune homeostasis, and barrier function to early life galactooligosaccharides intervention in suckling piglets[J]. Journal of Agricultural and Food Chemistry, 2019, 67(2): 578-588. DOI:10.1021/acs.jafc.8b05679 |
| [14] |
SHI C, ZHU Y Z, NIU Q Y, et al. The changes of colonic bacterial composition and bacterial metabolism induced by an early food introduction in a neonatal porcine model[J]. Current Microbiology, 2018, 75(6): 745-751. DOI:10.1007/s00284-018-1442-z |
| [15] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [16] |
冯丹, 张民扬, 田时祎, 等. 壳聚糖螯合锌对大肠杆菌攻毒断奶大鼠回肠菌群结构及代谢产物的影响[J]. 畜牧与兽医, 2020, 52(11): 48-54. FENG D, ZHANG M Y, TIAN S Y, et al. Effects of chitosan-chelated zinc on ileal microbial community and metabolites in weaned rats challenged with Escherichia coli[J]. Animal Husbandry & Veterinary Medicine, 2020, 52(11): 48-54 (in Chinese). |
| [17] |
陈婷婷. 饲粮添加可溶性和不溶性纤维对断奶仔猪生长性能及肠道健康的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2018. CHEN T T. Effects of dietary soluble fibre and insoluble fibre supplementation on growth performance and intestinal health of weaned piglets[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2018. (in Chinese) |
| [18] |
石海仁. C型产气荚膜梭菌对仔猪小肠TLR4/MyD88/NF-κB信号通路关键分子的影响[D]. 硕士学位论文. 兰州: 甘肃农业大学, 2018. SHI H R. Effect of Clostridium perfringens type C on TLR4/MyD88/NF-κB signaling pathway in piglet small intestines[D]. Master's Thesis. Lanzhou: Gansu Agricultural University, 2018. (in Chinese) |
| [19] |
MIYOSHI J, RAO M C, CHANG E B. Navigating the human gut microbiome: pathway to success from lessons learned[J]. Gastroenterology, 2020, 159(6): 2019-2024. DOI:10.1053/j.gastro.2020.09.002 |
| [20] |
KONISHI Y, KOBAYASHI S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers[J]. Bioscience Biotechnology and Biochemistry, 2005, 69(3): 583-591. DOI:10.1271/bbb.69.583 |
| [21] |
BABA S, OSAKABE N, NATSUME M, et al. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid[J]. Life Sciences, 2004, 75(2): 165-178. DOI:10.1016/j.lfs.2003.11.028 |
| [22] |
孙峋, 汪靖超, 李洪涛, 等. 迷迭香酸的抗菌机理研究[J]. 青岛大学学报(自然科学版), 2005, 18(4): 41-45. SUN X, WANG J C, LI H T, et al. A study on the antibacterial mechanism of rosmarinic acid[J]. Journal of Qingdao University (Natural Science Edition), 2005, 18(4): 41-45 (in Chinese). DOI:10.3969/j.issn.1006-1037.2005.04.010 |
| [23] |
HAN H, ZHANG S F, ZHONG R Q, et al. Effects of chlortetracycline on growth performance and intestinal functions in weaned piglets[J]. Journal of Applied Microbiology, 2022, 132(3): 1760-1767. DOI:10.1111/jam.15364 |
| [24] |
DING H, ZHAO X C, AZAD M A K, et al. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets[J]. Food & Function, 2021, 12(13): 5837-5849. |
| [25] |
BELTZER A, KAULISCH T, BLUHMKI T, et al. Evaluation of quantitative imaging biomarkers in the DSS colitis model[J]. Molecular Imaging and Biology, 2016, 18(5): 697-704. DOI:10.1007/s11307-016-0937-x |
| [26] |
CHELAKKOT C, GHIM J, RYU S H. Mechanisms regulating intestinal barrier integrity and its pathological implications[J]. Experimental & Molecular Medicine, 2018, 50(8): 1-9. |
| [27] |
PURCHIARONI F, TORTORA A, GABRIELLI M, et al. The role of intestinal microbiota and the immune system[J]. European Review for Medical and Pharmacological Sciences, 2013, 17(3): 323-333. |
| [28] |
VALDES A M, WALTER J, SEGAL E, et al. Role of the gut microbiota in nutrition and health[J]. BMJ, 2018, 361: k2179. |
| [29] |
GAO R, TIAN S Y, WANG J, et al. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 92. DOI:10.1186/s40104-021-00612-z |
| [30] |
GUZMÁN-CASTAÑEDA S J, ORTEGA-VEGA E L, DE LA CUESTA-ZULUAGA J, et al. Gut microbiota composition explains more variance in the host cardiometabolic risk than genetic ancestry[J]. Gut Microbes, 2020, 11(2): 191-204. DOI:10.1080/19490976.2019.1634416 |
| [31] |
HUANG S M, WU Z H, LI T T, et al. Perturbation of the lipid metabolism and intestinal inflammation in growing pigs with low birth weight is associated with the alterations of gut microbiota[J]. Science of the Total Environment, 2020, 719: 137382. DOI:10.1016/j.scitotenv.2020.137382 |
| [32] |
STAUDACHER H M, ROSSI M, KAMINSKI T, et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal bifidobacteria abundance in irritable bowel syndrome[J]. Neurogastroenterology and Motility, 2022, 34(4): e14241. |
| [33] |
BADURA A, LUXNER J, FEIERL G, et al. Prevalence, antibiotic resistance patterns and molecular characterization of Escherichia coli from Austrian sandpits[J]. Environmental Pollution, 2014, 194: 24-30. DOI:10.1016/j.envpol.2014.07.007 |
| [34] |
ROWAN F, DOCHERTY N G, MURPHY M, et al. Desulfovibrio bacterial species are increased in ulcerative colitis[J]. Diseases of the Colon & Rectum, 2010, 53(11): 1530-1536. |
| [35] |
YANG K M, JIANG Z Y, ZHENG C T, et al. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88[J]. Journal of Animal Science, 2014, 92(4): 1496-1503. DOI:10.2527/jas.2013-6619 |
| [36] |
MAURER L H, CAZARIN C B B, QUATRIN A, et al. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: a major role for dietary fiber and fiber-bound polyphenols[J]. Food Research International, 2019, 123: 425-439. |
| [37] |
朱晓峰, 张桢, 崔雷鸿, 等. 猪源乳酸杆菌与益生元组合筛选及其体外发酵特性[J]. 微生物学报, 2021, 61(1): 104-114. ZHU X F, ZHANG Z, CUI L H, et al. Combination screening of Lactobacillus spp. with prebiotics and analysis of its in vitro fermentation characteristics[J]. Acta Microbiological Sinica, 2021, 61(1): 104-114 (in Chinese). |
| [38] |
WANG K L, CHEN G Y, CAO G T, et al. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, intestinal structure, and inflammation in lipopolysaccharide-challenged weaned piglets[J]. Journal of Animal Science, 2019, 97(10): 4140-4151. |
| [39] |
HSIEH C Y, OSAKA T, MORIYAMA E, et al. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum[J]. Physiological Reports, 2015, 3(3): e12327. DOI:10.14814/phy2.12327 |
| [40] |
VAN DER SLUIS M, DE KONING B A E, DE BRUIJN A C J M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection[J]. Gastroenterology, 2006, 131(1): 117-129. |
| [41] |
MOWAT A M, AGACE W W. Regional specialization within the intestinal immune system[J]. Nature Reviews Immunology, 2014, 14(10): 667-685. |
| [42] |
单佳铃, 程虹毓, 文乐, 等. TLR/MyD88/NF-κB信号通路参与不同疾病作用机制研究进展[J]. 中国药理学通报, 2019, 35(4): 451-455. SHAN J L, CHENG H Y, WEN L, et al. Advances in research of TLR/MyD88/NF-κB signaling pathway in different diseases[J]. Chinese Pharmacological Bulletin, 2019, 35(4): 451-455 (in Chinese). |
| [43] |
陈仪. 甘草泻心汤对溃疡性结肠炎大鼠TLR4/MyD88/NF-κB炎症信号通路的调控作用研究[D]. 硕士学位论文. 福州: 福建中医药大学, 2021. CHEN Y. Effect of Gancao Xiexin decoction on TLR4/MyD88/NF-κB inflammatory signaling pathway in ulcerative colitis rats[D]. Master's Thesis. Fuzhou: Fujian University of Traditional Chinese Medicine, 2021. (in Chinese) |
| [44] |
WAN J, ZHANG J, WU G Z, et al. Amelioration of enterotoxigenic Escherichia coli-induced intestinal barrier disruption by low-molecular-weight chitosan in weaned pigs is related to suppressed intestinal inflammation and apoptosis[J]. International Journal of Molecular Sciences, 2019, 20(14): 3485. |
| [45] |
LI Y H, HOU S L, CHEN J S, et al. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets[J]. Journal of Functional Foods, 2019, 63: 103591. |




