2. 广东海洋大学水产学院, 湛江 524088;
3. 广州飞禧特生物科技有限公司, 广州 510640
 )幼鱼生长性能、血浆生化指标、肠道功能和抗氧化能力的影响。试验选取初始质量为(22.02±0.02) g的杂交鳢450尾, 随机分为3组, 每组3个重复, 每个重复50尾。3个组分别投喂添加0(对照)、0.60% Arg、0.03% NCG的3种试验饲料, 饲喂8周。结果表明: 与对照组相比, 饲料中添加0.60% Arg显著提高了杂交鳢增重率、特定生长率(P<0.05), 添加0.03% NCG显著提高了杂交鳢存活率(P<0.05), 添加0.60% Arg或0.03% NCG显著提高了杂交鳢的蛋白质沉积率、全鱼粗蛋白质含量(P<0.05), 显著降低了饲料系数(P<0.05)。各组间杂交鳢的全鱼水分、粗脂肪、粗灰分含量没有显著差异(P>0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢血浆总蛋白、甘油三酯、葡萄糖、尿素氮、总胆固醇含量及血浆谷草转氨酶、谷丙转氨酶及肠道Na+/K+ATP酶活性无显著影响(P>0.05)。与对照组相比, 饲料中添加0.03% NCG显著提高了杂交鳢肠道淀粉酶活性(P<0.05), 饲料中添加0.60% Arg显著提高了杂交鳢肠道蛋白酶、脂肪酶和γ-谷氨酰基转移酶活性(P<0.05)。与对照组相比, 饲料中添加0.60% Arg显著增强了杂交鳢血浆总抗氧化能力(P<0.05), 添加0.03% NCG显著增强了杂交鳢血浆过氧化氢酶和过氧化物酶活性(P<0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢血浆超氧化物歧化酶、谷胱甘肽过氧化物酶活性及丙二醛含量无显著影响(P>0.05)。综上所述, 饲料中添加Arg能够通过提高肠道消化酶活性, 改善杂交鳢生长性能, 而添加NCG可能通过促进抗氧化能力提高杂交鳢成活率。
)幼鱼生长性能、血浆生化指标、肠道功能和抗氧化能力的影响。试验选取初始质量为(22.02±0.02) g的杂交鳢450尾, 随机分为3组, 每组3个重复, 每个重复50尾。3个组分别投喂添加0(对照)、0.60% Arg、0.03% NCG的3种试验饲料, 饲喂8周。结果表明: 与对照组相比, 饲料中添加0.60% Arg显著提高了杂交鳢增重率、特定生长率(P<0.05), 添加0.03% NCG显著提高了杂交鳢存活率(P<0.05), 添加0.60% Arg或0.03% NCG显著提高了杂交鳢的蛋白质沉积率、全鱼粗蛋白质含量(P<0.05), 显著降低了饲料系数(P<0.05)。各组间杂交鳢的全鱼水分、粗脂肪、粗灰分含量没有显著差异(P>0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢血浆总蛋白、甘油三酯、葡萄糖、尿素氮、总胆固醇含量及血浆谷草转氨酶、谷丙转氨酶及肠道Na+/K+ATP酶活性无显著影响(P>0.05)。与对照组相比, 饲料中添加0.03% NCG显著提高了杂交鳢肠道淀粉酶活性(P<0.05), 饲料中添加0.60% Arg显著提高了杂交鳢肠道蛋白酶、脂肪酶和γ-谷氨酰基转移酶活性(P<0.05)。与对照组相比, 饲料中添加0.60% Arg显著增强了杂交鳢血浆总抗氧化能力(P<0.05), 添加0.03% NCG显著增强了杂交鳢血浆过氧化氢酶和过氧化物酶活性(P<0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢血浆超氧化物歧化酶、谷胱甘肽过氧化物酶活性及丙二醛含量无显著影响(P>0.05)。综上所述, 饲料中添加Arg能够通过提高肠道消化酶活性, 改善杂交鳢生长性能, 而添加NCG可能通过促进抗氧化能力提高杂交鳢成活率。 )
)
2. College of Fisheries, Guangdong Ocean University, Zhanjiang 524088, China;
3. Guangzhou Fishtech Biotechnology Co., Ltd., Guangzhou 510640, China
 ). Four hundred and fifty hybrid snakehead with an initial weight of (22.02±0.02) g were randomly divided into 3 groups with 3 replicates per group and 50 fish per replicate. Hybrid snakeheads were fed 3 experimental diets supplemented with 0 (control), 0.60% Arg and 0.03% NCG for 8 weeks, respectively. The results showed that compared with the control group, the addition of 0.60% Arg to the diet significantly increased the weight gain rate (WGR) and specific growth rate (SGR) of the hybrid snakeheads (P < 0.05), and the addition of 0.03% NCG significantly increased the survival rate (SR) of the hybrid snakeheads (P < 0.05), adding 0.60% Arg or 0.03% NCG significantly increased protein deposition rate (PDR) and whole body crude protein content (P < 0.05), and significantly decreased feed conversion ratio (FCR) (P < 0.05). There were no significant differences in water, crude fat and ash contents of whole fish among all groups (P>0.05). Diet supplemented with 0.60% Arg or 0.03% NCG had no significant effects on plasma contents of total protein, triglyceridin, glucose, urea nitrogen, total cholesterol, and the plasma activities of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and intestinal Na+/K+ATPase (P>0.05). Compared with the control group, the addition of 0.03% NCG to the diet significantly increased the intestinal amylase activity of the hybrid snakeheads (P < 0.05), and the addition of 0.60% Arg to the diet significantly increased the intestinal protease, lipase and γ-glutamyl transferase activities of the hybrid snakeheads (P < 0.05). Compared with control group, 0.60% Arg supplementation significantly increased plasma total antioxidant capacity (P < 0.05), and 0.03% NCG supplementation significantly increased plasma catalase and peroxidase activities (P < 0.05). Diet supplemented with 0.60% Arg or 0.03% NCG had no significant effects on plasma superoxide dismutase (SOD) and glutathione peroxidase (GSH) activities and malondialdehyde (MDA) content (P>0.05). In conclusion, dietary Arg can improve the growth performance of hybrid snakeheads by improving the activities of intestinal digestive enzymes, while dietary NCG may improve the survival rate of hybrid snakeheads by promoting antioxidant ability. [Chinese Journal of Animal Nutrition, 2022, 34(8): 5304-5312]
). Four hundred and fifty hybrid snakehead with an initial weight of (22.02±0.02) g were randomly divided into 3 groups with 3 replicates per group and 50 fish per replicate. Hybrid snakeheads were fed 3 experimental diets supplemented with 0 (control), 0.60% Arg and 0.03% NCG for 8 weeks, respectively. The results showed that compared with the control group, the addition of 0.60% Arg to the diet significantly increased the weight gain rate (WGR) and specific growth rate (SGR) of the hybrid snakeheads (P < 0.05), and the addition of 0.03% NCG significantly increased the survival rate (SR) of the hybrid snakeheads (P < 0.05), adding 0.60% Arg or 0.03% NCG significantly increased protein deposition rate (PDR) and whole body crude protein content (P < 0.05), and significantly decreased feed conversion ratio (FCR) (P < 0.05). There were no significant differences in water, crude fat and ash contents of whole fish among all groups (P>0.05). Diet supplemented with 0.60% Arg or 0.03% NCG had no significant effects on plasma contents of total protein, triglyceridin, glucose, urea nitrogen, total cholesterol, and the plasma activities of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and intestinal Na+/K+ATPase (P>0.05). Compared with the control group, the addition of 0.03% NCG to the diet significantly increased the intestinal amylase activity of the hybrid snakeheads (P < 0.05), and the addition of 0.60% Arg to the diet significantly increased the intestinal protease, lipase and γ-glutamyl transferase activities of the hybrid snakeheads (P < 0.05). Compared with control group, 0.60% Arg supplementation significantly increased plasma total antioxidant capacity (P < 0.05), and 0.03% NCG supplementation significantly increased plasma catalase and peroxidase activities (P < 0.05). Diet supplemented with 0.60% Arg or 0.03% NCG had no significant effects on plasma superoxide dismutase (SOD) and glutathione peroxidase (GSH) activities and malondialdehyde (MDA) content (P>0.05). In conclusion, dietary Arg can improve the growth performance of hybrid snakeheads by improving the activities of intestinal digestive enzymes, while dietary NCG may improve the survival rate of hybrid snakeheads by promoting antioxidant ability. [Chinese Journal of Animal Nutrition, 2022, 34(8): 5304-5312]精氨酸(Arg)作为鱼类必需氨基酸,参与机体蛋白质、肌酸等合成,对促进水生动物生长、提高机体抗氧化能力等方面极为重要[1]。研究结果表明,Arg可提高养殖动物饲料效率和蛋白质沉积率[2];降低血清谷草转氨酶、谷丙转氨酶活性,维持正常氨基酸代谢能力[3];提高肠道谷胱甘肽过氧化物酶活性,增强机体抗氧能力[4];降低肠道丙二醛含量,减少肠道氧化损伤[5]。N-氨甲酰谷氨酸(NCG)作为N-乙甲酰谷氨酸(NAG)类似物,可激活氨甲酰磷酸合酶-1(CPS-1),促进内源性Arg合成[6-7]。同时作为一种安全、代谢稳定的营养物质,可在治疗疾病有关的方面发挥有益作用[8]。然而,饲料中直接添加Arg在体内易被精氨酸酶降解,并且会影响机体对其他氨基酸的吸收效率。因此,利用NCG内源性合成Arg是目前解决Arg使用问题的可行方法,饲料中直接添加NCG,不仅代谢稳定性高,而且吸收效率强,相对于饲料补充Arg大幅降低了饲料成本。
杂交鳢子一代较斑鳢(Channa maculata)、乌鳢(Channa Argus)在生长性能、抗病能力方面有显著优势。目前在水产养殖中,易驯食人工配合饲料,养殖周期短,珠三角养殖产量占比较高[9]。近年来,随着养殖产量不断攀升,营养性、微生物性疾病不断增多。本实验室开展了杂交鳢对Arg需要量的养殖试验,试验结果表明,杂交鳢Arg适宜水平为2.91%~2.98%[10]。目前,由于不同鱼种饲料添加Arg存在其他氨基酸吸收拮抗、投入成本太高等因素,因此内源性合成Arg途径成为高效经济的方法,但是关于NCG在水产动物饲料中的应用研究较少。NCG研究主要集中为内源性激活Arg,也有研究证明,饲料添加NCG可提高大菱鲆(Scophthalmus maximus)的生长性能[11],改善镜鲤(Cyprinus carpio)的机体免疫活性[12]。NCG在鱼类机体内可内源性合成Arg,生成的Arg与NCG比值为(10~20) ∶ 1[13]。本实验室开展了Arg或NCG在黄颡鱼(Pelteobagrus fulvidraco)中的应用试验,发现黄颡鱼饲料中NCG的适宜添加水平是Arg适宜添加水平的1/20[14]。在黄颡鱼[14]、大菱鲆[11]、花鲈(Lateolabrax maculatus)[15]、罗非鱼(Oreochromis mossambicus)[16]、镜鲤[12]等水产动物中的研究表明,NCG在饲料中的适宜添加水平为0.03%左右。前期开展试验获得的杂交鳢饲料Arg适宜添加水平为0.6%[10],本试验NCG添加水平设计主要依据以上研究文献,按照杂交鳢饲料Arg适宜添加水平的1/20即0.03%添加。本试验通过在饲料中添加Arg或NCG,研究其对杂交鳢幼鱼生长性能、体成分、肠道功能、血浆生化指标及抗氧化能力的影响,为NCG在杂交鳢配合饲料中的应用提供理论依据。
1 材料与方法 1.1 试验饲料基础饲料的主要原料为鱼粉、面粉、豆粕、玉米蛋白粉,在基础饲料中分别添加0(对照)、0.60% Arg(L-Arg,纯度≥98%)、0.03% NCG(纯度≥98%)配制成3种试验饲料。饲料Arg含量采用GB/T 18246—2019的常规酸水解法测定。饲料营养水平及维生素预混料、矿物质预混料添加水平按照乌鳢营养需求量添加[17]。试验饲料组成及营养水平见表 1。
|
|
表 1 试验饲料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of experimental diets (DM basis) |
根据饲料配方将原料粉碎,要求过60目筛,而后进行称量,称量后将其逐级混匀,加入磷脂油、豆油、鱼油、水进行再次混匀,混匀后,将原料置于膨化机内膨化制成膨化颗粒饲料(华强膨化机械T52型膨化机),而后将膨化饲料置于55 ℃温度烘干。
1.2 试验设计及养殖管理将杂交鳢鱼苗(锦龙渔业有限公司)运输至广东省农业科学院动物科学研究所白云实验基地。鱼苗运回后在暂养网箱暂养1周,暂养网箱规格为2.5 m×2.5 m×1.5 m,暂养期每天饱食投喂2次基础饲料,暂养结束后饥饿24 h开始正式试验。随机挑选初始体重为(22.02±0.02) g、体格健壮的杂交鳢鱼苗450尾,随机分为3组,分别为对照组、0.60% Arg组、0.03% NCG组,每组3个重复(网箱),每个网箱50尾鱼,分别投喂3种试验饲料。
试验网箱规格为1.5 m×1.5 m×1.5 m,水体有效体积为293 L,饲喂8周。每天08:00、16:00定时表观饱食投喂2次,并根据水温、摄食和生长等因素调整投喂量,并记录摄食及死亡情况。每日对养殖环境进行检测,要求溶氧含量大约为8 mg/L,酸碱度大约为8.0,水温为25~32 ℃,氨氮含量小于0.1 mg/L。
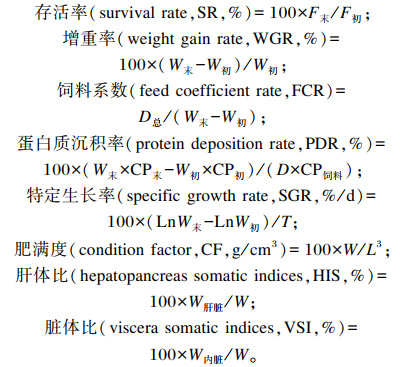
1.3 样品采集及测定指标 1.3.1 生长性能和形态学指标生长性能和形态学指标计算公式如下:
|
式中:F初为初始尾数;F末为终末尾数;W为体重;L为体长;W初为初始鱼体重;W末为终末鱼体重;CP初为初始鱼体蛋白质含量;CP末为终末鱼体蛋白质含量;CP饲料为饲料蛋白质含量;D总为摄入饲料总重;D为饲料摄入量;T为养殖时间;W肝脏为肝脏重;W内脏为内脏重。
1.3.2 饲料营养水平及鱼体营养成分测定养殖试验结束后进行样品采集,将试验鱼禁食24 h,记录每个试验网箱称重计数结果。随后从每个网箱中随机选取3尾鱼检测鱼体营养成分。饲料及鱼体粗蛋白质、粗脂肪、粗灰分、水分含量分别采用GB/T 6432—2018、GB/T 6433—2006、GB/T 6438—2007、GB/T 6435—2014的方法进行测定。
1.3.3 血浆制备及生化指标测定从每个网箱中随机选取8尾鱼抽取血液制备血浆,首先采用浓度为120 mg/L MS-222溶液麻醉,尾静脉采血法采集血液,肝素钠抗凝管收集血液,将采集好的血液采用离心机4 000 r/min进行离心,离心时间10 min,制备血浆。
血浆总蛋白(total protein,TP)含量采用双缩脲法测定,谷草转氨酶(aspartate transaminase,AST)、谷丙转氨酶(alanine transaminase,ALT)活性采用速率法测定, 尿素氮(urea nitrogen,UN)含量采用酶偶联速率法测定,总胆固醇(total cholesterol,TCHO)、甘油三酯(triglyceride,TG)、葡萄糖(glucose,GLU)含量采用酶活性法测定。血浆生化指标采用的测定仪器均为全自动化分析仪(贝克曼ProCX4,德国)。
1.3.4 肠道消化酶、功能性指标及血浆抗氧化指标测定从每个网箱中随机选取3尾鱼解剖后取肠道组织,进行肠道消化酶、功能性指标检测。采用南京建成生物工程研究所试剂盒测定肠道蛋白酶、脂肪酶(lipase,LPS)、淀粉酶(amylase,AMS)、Na+/K+ATP酶(sodium-potassium ATPase,Na+/K+ATPase)、γ-谷氨酰基转移酶(γ-glutamyltransferase,γ-GT),血浆超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)、过氧化氢酶(catalase,CAT)、过氧化物酶(peroxidase,POD)活性,总抗氧化能力(total antioxidant capacity,T-AOC)及丙二醛(malondialdehyde,MDA)含量,测定方法、步骤、计算公式等见试剂盒说明书。
1.4 数据统计与分析试验数据分析结果均以平均值±标准差(mean±SD)表示,数据采用SPSS 25.0统计软件进行单因素方差分析,而后采用Duncan氏法进行多重比较,差异显著水平为P < 0.05。
2 结果与分析 2.1 饲料添加Arg和NCG对杂交鳢生长性能及形态学指标的影响由表 3可知,与对照组和0.03% NCG组相比,饲料中添加0.60% Arg显著提高了杂交鳢WGR及SGR(P<0.05)。对照组和0.03% NCG组间杂交鳢WGR及SGR没有显著差异(P>0.05)。与对照组相比,饲料中添加0.60% Arg和0.03% NCG显著降低了杂交鳢FCR(P<0.05)。与对照组相比,饲料中添加0.60% Arg和0.03% NCG显著提高了杂交鳢的PDR及SR(P<0.05),0.60% Arg和0.03% NCG组间PDR和SR显著差异(P<0.05)。与对照组相比,各试验组CF、HSI、VSI无显著差异(P>0.05)。
|
|
表 2 饲料添加Arg和NCG对杂交鳢生长性能及形态学指标的影响 Table 2 Effects of dietary Arg or NCG on growth performance and morphological indices of hybrid snakehead |
|
|
表 3 饲料添加Arg和NCG对杂交鳢鱼体成分的影响 Table 3 Effects of dietary Arg or NCG on body composition of hybrid snakehead |
由表 4可知,与对照组相比,饲料中添加0.60% Arg和0.03% NCG显著增加了杂交鳢全鱼CP含量(P<0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢全鱼水分、EE、Ash含量无显著影响(P>0.05)。
|
|
表 4 饲料添加Arg和NCG对杂交鳢血浆生化指标的影响 Table 4 Effects of dietary Arg or NCG on plasma biochemical indexes of hybrid snakehead |
由表 5可知,饲料中添加0.60% Arg或0.03% NCG对杂交鳢血浆TP、TG、GLU、UN、TC含量及AST、ALT活性均无显著影响(P>0.05)。
|
|
表 5 饲料添加Arg和NCG对杂交鳢肠道消化酶活性及功能性指标的影响 Table 5 Effects of dietary Arg or NCG on digestive enzyme activities and function indices of hybrid snakehead |
由表 6可知,与对照组相比,饲料中添加0.60% Arg显著增加了杂交鳢肠道蛋白酶、LPS、AMS和γ-GT活性(P<0.05)。与对照组和0.60% Arg组相比,饲料中添加0.03% NCG显著提高了杂交鳢肠道AMS活性(P<0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢肠道Na+/K+ATPase酶活性无显著影响(P>0.05)。
|
|
表 6 饲料添加Arg和NCG对杂交鳢血浆抗氧化指标的影响 Table 6 Effects of dietary Arg or NCG on plasma antioxidant indices of hybrid snakehead |
由表7可知,与对照组相比,饲料中添加0.60% Arg显著提高了杂交鳢血浆T-AOC(P<0.05)。与对照组和0.60% Arg组相比,饲料中添加0.03% NCG显著提高了杂交鳢血浆POD活性(P<0.05)。0.60% Arg组杂交鳢血浆POD活性与对照组之间没有显著差异(P>0.05)。与0.60% Arg组相比,饲料中添加0.03% NCG显著提高了杂交鳢血浆CAT活性(P<0.05)。饲料中添加0.60% Arg或0.03% NCG对杂交鳢SOD、GSH-Px活性及MDA含量无显著影响(P>0.05)。
3 讨论由于水生动物鸟氨酸转羧化酶(OTC)、氨甲酰磷酸合成酶(CPS) Ⅲ的活性偏低,因此,必需外源补充Arg以满足鱼类生长和代谢所必需[1]。Arg可激活雷帕霉素靶蛋白(TOR)信号通路调节蛋白质合成,参与鸟氨酸循环,促进蛋白质在体内沉积,提高机体生长性能[18]。水生动物缺乏Arg会导致生长迟缓、免疫神经受损、营养障碍性疾病等[19]。在本试验条件下,饲料添加0.03% NCG对杂交鳢WGR及SGR没有显著影响,但添加0.60% Arg使饲料Arg含量达到2.92%,可显著提高杂交鳢WGR及SGR,可能由于杂交鳢对NCG合成的内源Arg利用效率低于饲料添加的Arg。但饲料中添加Arg和NCG可降低FCR,提高PDR。这表明外源添加Arg或NCG都可提高机体蛋白质沉积,提高生长效率。在本试验条件下,与对照组相比,饲料中添加Arg和NCG可提高PDR,并且提高杂交鳢鱼体CP含量,但对全鱼水分、EE、CF含量没有显著影响,说明适量添加Arg可有效促进鱼体蛋白质沉积,促进氨基酸吸收利用,提高肌肉品质。NCG作为NAG类似物,由于NAG易降解,NCG不易降解,并且与直接添加Arg相比,可降低饲料成本,提高代谢相对稳定性[13]。目前,在水产动物中关于Arg和NCG的应用研究效果较缺乏,并且在研究中还要考虑到添加量、试验动物种类以及实际生产成本等因素。
血浆生化指标直观反映鱼体健康水平、代谢能力及机体内环境稳态,也可反映机体病理变化[20]。在本研究中,杂交鳢血浆TP、TG、GLU、UN、TC含量及AST、ALT活性各组间均无显著差异,表明外源添加Arg或内源合成途径并未对杂交鳢机体产生不良影响。
肠道作为运输、消化食物的主要场所,是一个复杂的多功能器官,同时也是抵御病原微生物入侵的防线,对鱼类生长及健康非常重要[21]。本试验中,与对照组及0.60% Arg组相比较,饲料添加0.03% NCG显著增加了杂交鳢肠道AMS活性,饲料添加Arg可显著提高仿刺参蛋白酶活性[22],显著提高建鲤蛋白酶及LPS活性[23],与本试验研究结果相同,但NCG在水产动物消化方面研究相对较少。本试验结果表明,在饲料中添加Arg可显著促进营养物质交换,提高杂交鳢肠道酶活性及能量转化效率,但Arg代谢物多胺可作用于细胞增殖分化,同时保护肠道的完整性[24],Arg在促进营养物质消化吸收方面的作用不仅取决于消化酶的活性,还可能与其代谢产物如一氧化氮或多胺有关。研究显示,饲料补充NCG可促进罗非鱼脂肪沉积[16],显著提高黄颡鱼肠道LPS活性[25],显著提高大菱鲆PDR[11],显著提高花鲈PDR,减少肝腹脂沉积[15]。本试验结果表明,添加Arg提高肠道蛋白酶活性,作用于机体蛋白质合成,促进机体生长,但NCG只显著提高了肠道AMS活性,与上述研究结果不一致,原因可能由于内源性合成Arg利用效率低,或与添加量及动物种类相关联。
在水生动物中,机体抗氧化防御系统通过清除体内外氧自由基,减少内外环境因素造成的氧化应激,保护机体免受氧自由基损伤,其中抗氧化酶的保护机制主要与清除ROS相关,抗氧化防御系统由SOD、GSH-Px、T-AOC等组成,调节机体氧化平衡[26-27]。在本试验条件下,与对照组相比,饲料添加0.60% Arg显著增强了杂交鳢血浆T-AOC,T-AOC是机体抗氧化能力的综合指标,直接参与并反映机体受外部刺激及氧自由基代谢的能力。与0.60% Arg组相比,0.03% NCG组显著提高杂交鳢血浆CAT及POD活性,NCG的抗氧化活性效果要优于Arg。
4 结论综上所述,饲料中添加0.60% Arg或0.03% NCG均能显著增强杂交鳢PDR、鱼体CP含量、肠道LPS及γ-GT活性、血浆T-AOC。而饲料中添加0.03% NCG显著提高了杂交鳢血浆CAT、POD活性。在本试验条件下,饲料中添加Arg在生长性能上优于NCG,但对抗氧化能力而言,饲料中添加NCG效果优于Arg。
| [1] |
WANG Q C, XU Z, AI Q H. Arginine metabolism and its functions in growth, nutrient utilization, and immunonutrition of fish[J]. Animal Nutrition, 2021, 7(3): 716-727. DOI:10.1016/j.aninu.2021.03.006 |
| [2] |
CHENG Z Y, GATLIN D M, BUENTELLO A. Dietary supplementation of arginine and/or glutamine influences growth performance, immune responses and intestinal morphology of hybrid striped bass (Morone chrysops×Morone saxatilis)[J]. Aquaculture, 2012, 362/363: 39-43. DOI:10.1016/j.aquaculture.2012.07.015 |
| [3] |
ZHOU F, SHAO Q J, XIAO J X, et al. Effects of dietary arginine and lysine levels on growth performance, nutrient utilization and tissue biochemical profile of Black Sea bream, Acanthopagrus schlegelii, fingerlings[J]. Aquaculture, 2011, 319(1/2): 72-80. |
| [4] |
CHEN Q, ZHAO H, HUANG Y, et al. Effects of dietary arginine levels on growth performance, body composition, serum biochemical indices and resistance ability against ammonia-nitrogen stress in juvenile yellow catfish (Pelteobagrus fulvidraco)[J]. Animal nutrition, 2016, 2(3): 204-210. DOI:10.1016/j.aninu.2016.07.001 |
| [5] |
王连生, 吴俊光, 徐奇友, 等. 饲料中精氨酸水平对杂交鲟幼鱼肠道消化酶活性及形态结构的影响[J]. 大连海洋大学学报, 2017, 32(1): 51-55. WANG L S, WU J G, XU Q Y, et al. Effects of dietary arginine levels on intestinal digestive enzyme activity and morphology in juvenile hybrid sturgeon[J]. Journal of Dalian Fisheries University, 2017, 32(1): 51-55 (in Chinese). |
| [6] |
YAO K, GUAN S, LI T J, et al. Dietary L-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets[J]. British Journal of Nutrition, 2011, 105(5): 703-709. DOI:10.1017/S000711451000365X |
| [7] |
LIU Z Q, GENG M, SHU X G, et al. Dietary NCG supplementation enhances the expression of N-acetylglutamate synthase in intestine of weaning pig[J]. Journal of Food Agriculture and Environment, 2012, 10(1): 408-412. |
| [8] |
FRANK J W, ESCOBAR J, NGUYEN H V, et al. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets[J]. Journal of Nutrition, 2007, 137(2): 315-319. DOI:10.1093/jn/137.2.315 |
| [9] |
ZHAO P F, LI F J, CHEN X R, et al. Dietary lipid concentrations influence growth, liver oxidative stress, and serum metabolites of juvenile hybrid snakehead (Channa argus ×Channa maculata)[J]. Aquaculture International, 2016, 24(5): 1353-1364. DOI:10.1007/s10499-016-9993-0 |
| [10] |
李培佳, 陈晓瑛, 赵红霞, 等. 精氨酸对杂交鳢生长性能、体组成、血浆生化指标及抗氧化能力的影响[J]. 动物营养学报, 2022, 34(3): 1820-1830. LI P J, CHEN X Y, ZHAO H X, et al. Effects of arginine on growth performance, body composition, plasma biochemical indexes and antioxidant capacity of hybrid snakehead(Channa maculata♀×Channa argus  )[J]. Chinese Journal of Animal Nutrition, 2022, 34(3): 1820-1830 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.03.042 )[J]. Chinese Journal of Animal Nutrition, 2022, 34(3): 1820-1830 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.03.042 |
| [11] |
尚晓迪, 陈春秀, 贾磊, 等. N-氨甲酰谷氨酸对大菱鲆幼鱼生长性能的影响[J]. 饲料研究, 2017(3): 35-38. SHANG X D, CHEN C X, JIA L, et al. Effects of n-carbamoylglutamate on growth performance of juvenile turbot[J]. Feed Research, 2017(3): 35-38 (in Chinese). |
| [12] |
WANG L S, LI J N, WANG C A, et al. Effect of N-carbamoylglutamate supplementation on the growth performance, antioxidant status and immune response of mirror carp (Cyprinus carpio) fed an arginine-deficient diet[J]. Fish & Shellfish Immunology, 2019, 84: 280-289. |
| [13] |
WU X, YIN Y L, LIU Y Q, et al. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows[J]. Animal Reproduction Science, 2012, 132(3/4): 187-192. |
| [14] |
ZHAO H X, QIAO G X, CAO J M, et al. Dietary supplementation of N-carbamylglutamate and effects on growth, intestinal enzyme activities, immunological and antioxidant abilities of juvenile yellow catfish (Pelteobagrus fulvidraco)[J]. Aquaculture Nutrition, 2019, 25(6): 1250-1260. DOI:10.1111/anu.12939 |
| [15] |
黄皓琰. N-氨甲酰谷氨酸在花鲈饲料中的有效性和耐受评价及其调控营养代谢的机制研究[D]. 硕士学位论文. 北京: 中国农业科学院, 2019. HUANG H Y. Efficacy and tolerance evaluation of N-carbamylglutamate injapanese seabass (Lateolabrax japonicus) diet and the related nutrient metabolism regulation mechanism[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2019. (in Chinese) |
| [16] |
程炜轩, 张丽, 许国焕, 等. N-氨甲酰谷氨酸对罗非鱼幼鱼生长、血液氨基酸组成及脂肪沉积的影响[J]. 水生生物学报, 2015, 39(3): 490-497. CHENG W X, ZHANG L, XU G H, et al. Effects of arginine on the regulation of the growth, the blood amino acid composition and the fat deposition in Nile tilapia (Oreochromis niloticus)[J]. Acta Hydrobiologica Sinica, 2015, 39(3): 490-497 (in Chinese). |
| [17] |
尹东鹏, 陈秀梅, 刘丹妮, 等. 乌鳢饲料赖氨酸及其它必需氨基酸营养需求量的研究[J]. 饲料工业, 2018, 39(14): 18-23. YIN D P, CHEN X M, LIU D N, et al. Research on nutrient requirement of lysine and other essential amino acid in feed of juvenile Channa argus[J]. Feed Industry, 2018, 39(14): 18-23 (in Chinese). |
| [18] |
YU H X, SUN L, FAN W H, et al. Effects of dietary arginine on growth, anti-oxidative enzymes, biomarkers of immunity, amino acid metabolism and resistance to Vibrio parahaemolyticus challenge in abalone Haliotis discus hannai[J]. Aquaculture, 2022, 549: 737707. DOI:10.1016/j.aquaculture.2021.737707 |
| [19] |
HE Q H, KONG X F, WU G Y, et al. Metabolomic analysis of the response of growing pigs to dietary L-arginine supplementation[J]. Amino Acids, 2009, 37(1): 199-208. |
| [20] |
SILVEIRA-COFFIGNY R, PRIETO-TRUJILLO A, ASCENCIO-VALLE F. Effects of different stressors in haematological variables in cultured Oreochromis aureus S[J]. Comparative Biochemistry and Physiology Part C-Toxicology & Pharmacology, 2004, 139(4): 245-250. |
| [21] |
HAMLIN H J, HERBING I H V, KLING L J. Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny[J]. Journal of Fish Biology, 2000, 57(3): 716-732. |
| [22] |
王际英, 李宝山, 张德瑞, 等. 饲料中添加精氨酸对仿刺参幼参生长、免疫能力及消化酶活力的影响[J]. 水产学报, 2015, 39(3): 410-420. WANG J Y, LI B S, ZHANG D R, et al. Effects of dietary arginine on growth performance, immune responses and digestive enzyme of juvenile sea cucumber Apostichopus japonicus Selenka[J]. Journal of Fisheries of China, 2015, 39(3): 410-420 (in Chinese). |
| [23] |
CHEN G F, FENG L, KUANG S Y, et al. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian)[J]. British Journal of Nutrition, 2012, 108(2): 195-207. |
| [24] |
GILLOTEAUX J, KASHOUTY R, YONO N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: ultrastructural aspects[J]. Tissue and Cell, 2008, 40(1): 7-20. |
| [25] |
赵红霞, 乔国贤, 李培佳, 等. 饲料添加L-精氨酸或N-氨甲酰谷氨酸对黄颡鱼生长、肠道功能、血清生化指标和抗氨氮应激能力的影响[J]. 动物营养学报, 2021, 33(11): 6330-6339. ZHAO H X, QIAO G X, LI P J, et al. Effects of dietary L-arginine or N-carbamylglutamate on growth performance, intestinal function, serum biochemical indexes and anti-ammonia-nitrogen stress ability of yellow catfish (Pelteobagrus fulvidraco)[J]. Chinese Journal of Animal Nutrition, 2021, 33(11): 6330-6339 (in Chinese). |
| [26] |
REN M C, LIAO Y J, XIE J, et al. Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala[J]. Aquaculture, 2013, 414/415: 229-234. |
| [27] |
WINTERBOURN C C. Revisiting the reactions of superoxide with glutathione and other thiols[J]. Archives of Biochemistry and Biophysics, 2016, 595: 68-71. |




