动物肠道是机体最大的消化、免疫器官,对维持机体内环境稳态至关重要。肠道黏膜上皮屏障不仅可以抵抗微生物进入,而且可以让营养物质更好地消化吸收,同时也是机体发挥防御保护作用的重要组成部分之一,主要由机械屏障、化学屏障、免疫屏障和微生物屏障组成,其完整性对维持动物健康具有重要作用。当肠道黏膜上皮屏障功能受到损伤时,极易导致机体肠应激综合征、肝脏炎症、慢性炎症、炎症性肠病等多种自身免疫性疾病和炎症性疾病发生[1-4],从而影响动物健康。因此,寻找一种可有效修复肠道黏膜损伤的措施对动物健康具有重要意义。
槲皮素(quercetin)是一种天然的黄酮醇,具有抗炎、抗氧化、抗癌和抗菌等多种生物活性[5-7],被视为是一种绿色、安全、高效的饲料添加剂,具有较好的应用前景。近年来,学者们发现,槲皮素在促进动物生长、提高生产性能、改善畜禽产品品质、维系肠道健康等方面取得了良好效果[8-11]。大量研究也已表明,槲皮素可增强细胞间紧密连接(tight junctions,TJs),调节肠道免疫反应,维系肠道微生态稳定,进而保护肠道黏膜屏障功能。本文就槲皮素对肠道黏膜屏障功能的影响及其调控机制进行综述,以期为其在畜禽生产中的应用和治疗肠道黏膜屏障损伤等方面提供参考。
1 槲皮素在体内的吸收与代谢机体摄取槲皮素后,可通过被动扩散或有机阴离子转运多肽(organic anion transporting polypeptide,OATPs)吸收进入小肠上皮细胞[12]。之后在肠上皮细胞磺基转移酶(sulfotransferases,SULTs)、尿苷二磷酸葡萄糖醛酸基转移酶(uridine-5′-diphosphate glucuronosyl transferases,UGTs)和儿茶酚氧位甲基转移酶(catechol-O-methyl-transferases,COMTs)的作用下,经历Ⅱ期代谢,形成葡萄糖醛酸化、硫酸化或甲基化的槲皮素[13]。这些槲皮素及槲皮素的衍生物可以经过肠上皮上的多药耐药蛋白(multidrug resistance protein,MRP)和乳腺癌耐药蛋白(breast cancer resistance protein,BCRP)转运到肠腔或血液中[14-16]。未被吸收的槲皮素及其衍生物(葡萄糖醛酸化或硫酸化的槲皮素)在后肠微生物的作用下,通过环裂解,产生酚酸和芳香化合物,随后被吸收、转化或排出体外[17-19]。其中,3-羟基苯乙酸和3, 4-二羟基苯乙酸是最主要的酚酸代谢产物,它们分别通过单羧酸转运体(monocarboxylic acid transporter,MCT)和细胞旁途径转运进入结肠细胞中[20],最后,这些酚酸代谢物随着血液循环系统到达全身各处组织或细胞中[20-21]。而被肠道细胞吸收的槲皮素及其衍生物进入血液,通过肝门静脉到达肝脏,经过被动扩散和/或肝窦膜上的OATPs转运进入肝细胞,在Ⅱ期代谢酶的作用下,生成包括葡萄糖醛酸化、硫酸化和甲基化的槲皮素,一部分分泌到胆汁中,随粪便排出;另一部分通过结肠上的MRP进入循环系统,在肾近端小管细胞的基底膜上的有机阴离子转运体(organic anion transporter,OATs)协同作用下进入肾细胞,经MRP2和BCRP从尿液中排出[22-25]。槲皮素在体内的吸收代谢如图 1所示。
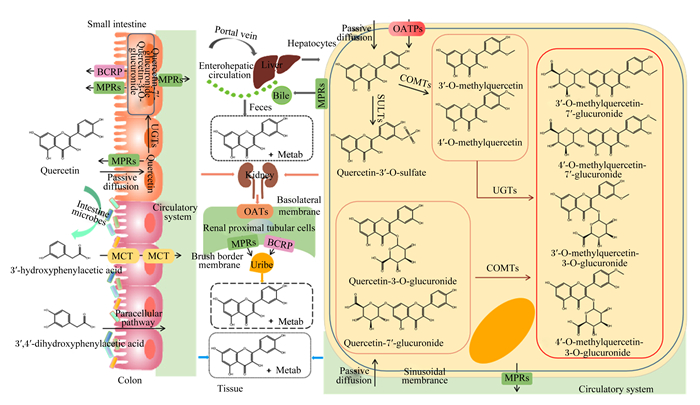
|
Small intestine:小肠;Colon:结肠;Tissue:组织;Liver:肝脏;Hepatocytes:肝细胞;Bile:胆汁;Feces:粪便;Kidney:肾脏;Renal proximal tubular cells:肾近端小管细胞;Uribe:尿液;MPRs:多药耐药蛋白multidrug resistance proteins;BCRP:乳腺癌耐药蛋白breast cancer resistance protein;OATPs:有机阴离子转运多肽organic anion transporting polypeptide;MCT:单羧酸转运体monocarboxylic acid transporter;OATs:有机阴离子转运体organic anion transporter;Intestine microbes:肠道微生物;Passive diffusion:被动扩散;Paracellular pathway:细胞旁途径;Circulatory system:循环系统;Portal vein:门静脉;Enterohepatic circulation:肠肝循环;Sinusoidal membrance:窦状膜;Basolateral membrane:基底膜;Brush border membrane:刷状缘膜;SULTs:磺基转移酶sulfotransferases;COMTs:儿茶酚氧位甲基转移酶catechol-O-methyl-transferases;UGTs:尿苷-5′-二磷酸葡萄糖醛酸转移酶uridine-5′-diphosphate glucuronosyl transferases;Quercetn:槲皮素;Quercetin-3′-O-sulfate:槲皮素-3′-O-硫酸酯;3′-O-methylquercetin:3′-O-甲基槲皮素;4′-O-methylquercetin:4′-O-甲基槲皮素;Quercetin-3-O-glucuronide:槲皮素-3-O-葡萄糖醛酸苷;Quercetin-7′-glucuronide:槲皮素-7′-葡萄糖醛酸苷;3′-O-methylquercetin-7′-glucuronide:3′-O-甲基槲皮素-7′-葡萄糖醛酸苷;4′-O-methylquercetin-7′-glucuronide:4′-O-甲基槲皮素-7′-葡萄糖醛酸苷;3′-O-methylquercetin-3-O-glucuronide:3′-O-甲基槲皮素-3-O-葡萄糖醛酸苷;4′-O-methylquercetin-3-O-glucuronide:4′-O-甲基槲皮素-3-O-葡萄糖醛酸苷;3′-hydroxyphenylacetic acid:3′-羟基-苯乙酸;3′, 4′-dihydroxyphenylacetic acid:3′,4′-二羟基-苯乙酸;Metab:代谢。 图 1 槲皮素在体内的吸收代谢 Fig. 1 Absorption and metabolism of quercetin in vivo[25] |
保持完整的肠道黏膜屏障对维持动物正常的肠道生理功能至关重要,任何一个屏障受到损伤都会导致肠道功能紊乱。研究显示,槲皮素可通过调控肠道黏膜屏障,降低肠道炎症反应、提高肠道免疫力、维系肠道微生态稳定等,进而维持肠道黏膜屏障功能的完整性。
2.1 槲皮素对肠黏膜机械屏障的影响肠道机械屏障主要由黏液层、肠上皮细胞及细胞间TJs组成,它是抵御有害物质侵袭的第1道防线,也是维持肠道黏膜屏障结构和功能的基础[26]。
2.1.1 槲皮素对肠上皮黏液层的影响黏液层由肠上皮细胞所分泌的黏蛋白(mucin,MUC)形成,外层栖息着大量微生物,而内层覆盖着MUC。黏液层的存在既可阻止毒素渗入肠道,也可防止致病菌的入侵[27]。研究发现,槲皮素可通过促进肉鸡回肠中MUC2基因表达[28];刺激Caco-2细胞与人体肠道杯状细胞LS174T细胞的MUC2和MUC5AC分泌并提高其mRNA表达水平[29];增强Caco-2/HT29-MTX细胞中MUC17基因转录[30],进而维护肠黏膜的完整性,保护肠道或肠道细胞免受病原体和细菌侵扰。
2.1.2 槲皮素对肠上皮细胞的影响肠上皮由单层柱状上皮细胞组成,这些细胞构成一个选择性的渗透屏障,可以选择性吸收营养物质、电解质和水,限制有毒大分子进入[31]。在细胞模型中的研究发现,槲皮素可通过促进猪小肠上皮细胞(IPEC-J2细胞)增殖,同时提高其超氧化物歧化酶活性,抑制过氧化氢(H2O2)诱导的细胞凋亡,进而阻碍氧化应激对IPEC-J2细胞屏障功能的破坏,增强其机械屏障强度[32]。在动物模型中的研究表明,槲皮素可通过提高肉鸡回肠抗氧化酶基因的表达,同时改善回肠绒毛高度及绒毛高度/隐窝深度,进而增强肠黏膜机械屏障功能[33]。
2.1.3 槲皮素对肠上皮TJs蛋白的影响肠上皮TJs蛋白是肠道机械屏障的重要组成部分,也是维持机械屏障功能最基本的结构,主要由闭合蛋白(Claudin)、闭锁蛋白(Occludin)、连接黏附分子(junctional adhesion molecule,JAM)、闭合小环蛋白(zonaul occludens,ZO)等胞质分子和细胞骨架共同构成,在维持肠道黏膜屏障功能完整性以及调节细胞通透性方面发挥重要作用[34]。诸多研究表明,槲皮素可通过激活TJs蛋白、增加TJs蛋白表达和增强其在肠上皮细胞膜上的定位与分布来维护机械屏障的完整性,继而保持良好的肠道机械屏障功能。
TJs蛋白的磷酸化、分布和表达水平在调节TJs屏障功能中起着关键作用,它们受到细胞内蛋白激酶(protein kinase,PK)C、PKA和PKG信号转导通路的严格调控,同时也受到Rho相关蛋白激酶(Rho associated kinase,ROCK)、肌球蛋白轻链激酶(myosin light-chain kinase,MLCK)/肌球蛋白轻链(myosin light-chain,MLC)、丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)以及磷脂酰肌醇-3-激酶(phosphatidylinositol 3-kinase,PI3K)/PKB等信号通路的调控[35-36]。
MLCK/MLC途径是调控肠道TJs蛋白最常见的途径之一,其活化可使TJs蛋白重新分布,从而导致肠道黏膜屏障功能损伤[37-38]。刘丽娜等[39]发现,槲皮素可显著抑制肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)诱导的Caco-2细胞损伤,促进Claudin-1和Occludin的磷酸化以及增加Occludin的蛋白表达量。进一步研究证明,槲皮素可能通过降低TNF-α含量,并抑制其介导的MLCK信号通路的激活,来增强Occludin和Claudin-1蛋白表达量,修复受损的肠道机械屏障,进而减少结肠炎症,最终增强肠道屏障功能[40-42]。此外,槲皮素还可通过抑制Ras基因同源家族蛋白A(Ras homolog gene family member A,RhoA)/ROCK/MLC和钙离子(Ca2+)/c-Jun氨基末端激酶(c-Jun N-terminal kinases,JNK)/非受体型酪氨酸蛋白激酶(Src)信号通路的激活,进而增加IEC-6细胞中ZO-1、Occludin和Claudin-1的mRNA或蛋白表达量,最终增强肠机械屏障的完整性,维护肠道黏膜屏障功能[43-45]。另有研究表明,槲皮素可通过抑制PKCδ和PI3K信号转导,促进TJs蛋白(ZO-2、Occludin和Claudin-1)的磷酸化,上调Claudin-4基因的转录,并增加Claudin-4的蛋白表达量,同时使Claudin-4在质膜上的免疫荧光定位加强,进而维护肠上皮细胞TJs结构,最终增强Caco-2细胞的机械屏障功能[46-49]。此外,槲皮素还可通过抑制Toll样受体(Toll-like receptor,TLR)4/髓样分化因子88(myeloid differentiation factor88,MyD88)/p38MAPK信号通路的激活和内质网应激,进而增加Occludin、Claudin-1和ZO-1的蛋白表达量,从而改善急性胰腺炎模型大鼠中肠道黏膜屏障完整性的破坏,保护肠道屏障功能[50]。槲皮素对细胞模型和动物模型肠道TJs蛋白表达的影响及其调控机制分别见表 1和图 2。
|
|
表 1 槲皮素对肠道TJs蛋白表达量的影响 Table 1 Effects of quercetin on expression level of TJs protein |
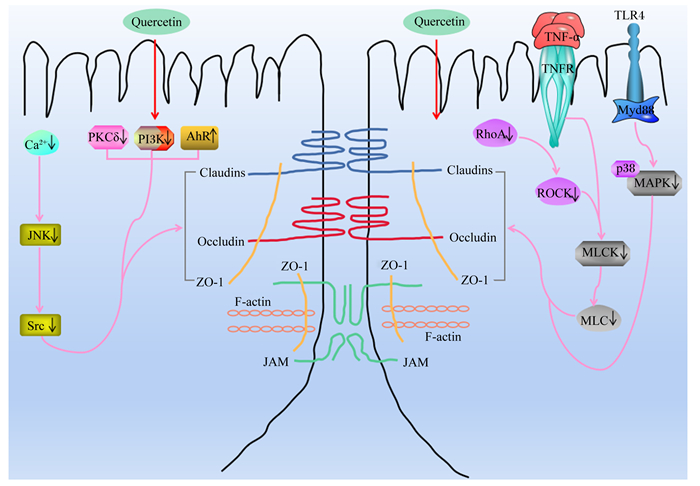
|
Claudins:闭合蛋白;Occludin:闭锁蛋白;ZO-1:闭合小环蛋白-1 zonaul occluden-1;JAM:连接黏附分子junctional adhesion molecule;F-actin:纤维形肌动蛋白fibrous actin;Ca2+:钙离子calcium ion;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinases;Src:非受体型酪氨酸蛋白激酶non-receptor protein-tyrosine kinase;RhoA:Ras基因同源家族蛋白A Ras homolog gene family member A;ROCK:Rho相关蛋白激酶Rho associated kinase;MLCK:肌球蛋白轻链激酶myosin light-chain kinase;MLC:肌球蛋白轻链myosin light-chain;TNFR:肿瘤坏死因子受体tumor necrosis factor receptor;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;TLR4:Toll样受体4 Toll-like receptor 4;MyD88:髓样分化因子88 myeloid differentiation factor 88;p38MAPK:p38丝裂原活化蛋白激酶p38 mitogen-activated protein kinase;PKCδ:蛋白激酶δ protein kinase δ;PI3K:磷酯酰肌醇-3-激酶phosphoinositide 3-kinase;AhR:芳香烃受体aryl hydrocarbon receptor;Quercein:槲皮素。 图 2 槲皮素调节TJs的作用机制 Fig. 2 Mechanism of quercetin regulating TJs |
综上所述,槲皮素一方面通过促进MUC基因的表达和分泌;另一方面通过促进肠上皮细胞的增殖、分化与更新并改善其形态结构,同时增加TJs蛋白的基因和蛋白表达量,增强TJs蛋白在膜上的定位与分布来增强肠道机械屏障的完整性,进而维持肠道机械屏障功能。
2.2 槲皮素对肠黏膜化学屏障的影响肠道的化学屏障由胃酸、胆汁、消化酶、溶菌酶、糖蛋白、抗菌肽等由胃肠道分泌的化学物质以及肠道微生物产生的抑菌物质组成,在维系肠道微生态平衡和肠道健康中发挥重要作用[55]。研究发现,槲皮素可通过调节胆汁合成和分泌、肠碱性磷酸酶(intestinal alkaline phosphatase,IAP)的蛋白表达量和抑菌物质的生成,从而影响肠黏膜稳态。
胆汁是肠黏膜化学屏障的重要组成部分,其中的胆汁酸是一种良好的肠道功能促进剂,不仅可调节肠道炎症反应,而且还能有效降低肠道pH,抑制潜在致病菌的增殖或其毒力相关基因的表达,促进肠道内环境平衡,进而维系肠道健康[56]。张敏[57]研究表明,槲皮素可通过增强大鼠肝X受体α(liver X receptor α,LXRα)的基因转录并提高其蛋白表达量,从而提高胆固醇7α羟化酶(cholesterol 7α-hydroxylase,CYP7A1)的活性,最终促进肝脏胆汁的合成和分泌。短链脂肪酸作为肠上皮细胞和肠道微生物的重要能量来源,对维持肠上皮细胞更新换代发挥着关键作用,同时还可改善小肠形态、维护肠道黏膜屏障功能等,在机体生理和免疫方面发挥多种调节作用,被认为是具有抗炎和调节肠道免疫耐受功能的有益代谢产物[58-60]。据报道,槲皮素可刺激肠道菌群对乙酸、丙酸、丁酸等短链脂肪酸的生成[61],增加断奶仔猪空肠和结肠中的丙酸和丁酸含量[62],继而对肠道黏膜屏障产生调控作用,维护正常肠道黏膜屏障功能,保持肠道健康。此外,槲皮素还可增加非酒精性脂肪肝小鼠模型IAP的蛋白表达[63],而IAP可通过催化活性/毒性脂质A部分的去磷酸化来解毒细菌内毒素脂多糖(lipopolysaccharide,LPS),防止局部炎症以及活性LPS转移到体循环中,从而降低由LPS引起的肠道和机体炎症的风险[64]。抗菌肽是由潘氏细胞分泌的,主要包括防御素、cathelicidins和histatins,在调节机体免疫反应和肠道内环境稳定中起着重要作用[65]。肖风林[66]发现,槲皮素可通过提高肉鸡回肠中β-防御素的mRNA表达水平来发挥免疫和抗炎功效,从而改善肠黏膜免疫功能。
上述研究表明,槲皮素可通过促进胆汁的合成与分泌、增强抗菌肽的基因转录、增加短链脂肪酸的产生等来改善肠黏膜化学屏障功能,最终起到了缓解肠道炎症和保护肠黏膜的作用。
2.3 槲皮素对肠黏膜生物屏障的影响肠道中的生物屏障是由肠道常驻细菌形成的,各个菌群之间相互依存、相互制约,共同维持肠道微生态环境的平衡。在正常情况下,肠道黏膜表面会附着大量厌氧细菌,如双歧杆菌、乳酸杆菌等益生菌,它们可以通过黏附与肠上皮紧密结合,形成一层膜屏障,阻碍致病菌在肠道中的定植[67],同时促进肠黏膜细胞增殖,改善机体免疫[68]。而一旦肠道菌群失衡,致病菌就可大量增殖,从而抑制肠上皮细胞的蛋白质合成,损伤肠绒毛结构,破坏肠道黏膜屏障,并导致各类疾病的发生,如肠道炎症、腹泻等[27, 69]。
诸多研究表明,槲皮素可通过选择性促进肠道有益菌的增殖,抑制有害菌在肠道的定植,进而优化肠道中的微生态环境,最终维系动物肠道乃至整个机体的健康。Etxeberria等[70]研究发现,槲皮素可通过降低粪便中厚壁菌门/拟杆菌门(Firmicutes/Bacteroidetes)比例,降低肥胖相关微生物韦荣球菌科(Erysipelotrichaceae)、芽孢杆菌属(Bacillus)和圆柱状真杆菌(Eubacterium cylindroides)的相对丰度,进而抑制高脂蔗糖饮食诱导的大鼠肠道微生物区系失调,维系肠道菌群平衡。Shi等[71]在抗生素治疗的小鼠模型中发现,槲皮素可通过提高肠道乳酸杆菌属(Lactobacillus)、阿克曼菌属(Akkermensia)等有益菌菌属的相对丰度,并提高肠道微生物多样性,同时增加丁酸代谢产物含量,进而增加结肠绒毛高度和结肠黏膜厚度,最终增强肠道黏膜屏障功能的稳定性以及维持肠道微生物稳态。在肉鸡和蛋鸡中的研究表明,饲粮中添加200~600 mg/kg槲皮素可通过增加盲肠中有益菌双歧杆菌属(Bifidobacterium)和Lactobacillus数量,降低有害菌大肠杆菌(Escherichia coli)数量,并提升Lactobacillus的相对丰度,进而维持肠道微生物平衡和肠道健康[10, 28, 72-73, 74-75]。此外,槲皮素还可能通过增强β-防御素的mRNA表达水平或抑制TLR信号通路的激活来调节小鼠和肉鸡肠道微生物组成,继而维护肠道菌群稳态平衡[63, 66]。另有研究发现,槲皮素还具有独特的生物活性,它可通过减少细菌黏附、抑制群体感应、破坏或改变质膜、抑制外排泵、阻碍核酸合成等途径,来抑制粪肠球菌(Enterococcus faecalis)、金黄色葡萄球菌(Staphylococcus aureus)、变形链球菌(Streptococcus mutans)、Escherichia coli、绿脓杆菌(Pseudomonas aeruginosa)等致病菌的生物膜形成,影响细菌的生长繁殖和新陈代谢,最终抑制其增殖[76-83]。
综上所述,槲皮素在维系肠道微生态平衡方面具有良好的效果,可通过调节肠道菌群组成,提高肠道有益菌的相对丰度或数量,抑制潜在致病菌的增殖,同时增加肠道微生物的多样性,促进有机酸的分泌,进而维持肠道微生物的稳态,增强肠道生物屏障,减少肠道炎症的发生,最终维系动物肠道乃至整个机体的健康。槲皮素对肠道微生物的影响如表 2所示。
|
|
表 2 槲皮素对肠道微生物的影响 Table 2 Effects of quercetin on intestinal microorganisms |
肠黏膜免疫屏障主要由肠道相关淋巴组织(gut-associated lymphoid tissues,GALT)、免疫细胞和免疫因子构成[84]。肠黏膜免疫屏障可在肠道抗原的刺激下产生免疫球蛋白(immunoglobulin,Ig)、干扰素(interferon,IFN)、白细胞介素(interleukin,IL)等因子来调控机体局部和全身免疫反应,进而调节肠黏膜免疫功能,保护机体免受损伤。
分泌型免疫球蛋白A(secretory immunoglobulin A,SIgA)作为肠黏膜表面的第1道免疫防线,不仅可与细菌上的特异性抗原结合形成抗原抗体复合物,具有刺激肠黏液分泌、抑制病原微生物在黏膜上的黏附等作用,而且还具有免疫调节、免疫排斥和促进抗菌因子产生等功能[85-88]。肠黏膜免疫是由IgA介导的,它可使抗原在细胞内被中和,同时将其产物返回肠腔,防止上皮细胞因细胞裂解而受损[89]。IgG在免疫应答中可活化补体,清除病原体[90]。研究发现,槲皮素可提高大鼠血清中SIgA含量,增加蛋鸡血清IgA、IgM和IgG以及生长猪血清中IgG含量[91-93]。血清Ig含量可反映动物机体系统免疫能力的高低,而肠道免疫系统是机体免疫系统的一部分。因此,上述研究血清Ig含量的升高表明,槲皮素可能增强了肠道免疫系统的防御功能。
TLR是天然免疫细胞和其他细胞表面的识别受体,可识别LPS、病原微生物双链DNA、单链RNA、脂蛋白等刺激,在调节肠道先天性和适应性免疫中有着重要作用[94]。TLR介导的核因子-κB(nuclear factor-κB,NF-κB)、MAPK、PI3K/PKB等信号转导途径,可调节肠黏膜炎症因子的生成,对机体肠道黏膜屏障的调控具有重要影响[95-97]。研究发现,槲皮素可通过抑制TLR4/NF-κB信号通路的活化,来抑制TNF-α、IL-1β、IL-6等促炎因子的生成,进而改善由LPS诱导的小鼠巨噬细胞和大鼠肠上皮细胞的炎症反应[98-99]。Zou等[11]研究也发现,槲皮素可能通过抑制活性氧(reactive oxygen species,ROS)和MAPK、NF-κB和PKB等信号通路的激活,继而限制空肠中TNF-α、IL-6和IL-1β以及单核细胞趋化蛋白-1(monocyte chemotactic protein-1,MCP-1)的基因转录,从而改善生长猪肠道炎症反应。另有研究表明,槲皮素还通过激活芳香烃受体(ary1 hydrocarbon receptor,AhR)途径,来增加Claudin-1的蛋白表达量,同时下调中性粒细胞和巨噬细胞活性以及维系辅助性T细胞17(T helper cell 17,Th17)/调节性T细胞(regulatory T cells,Treg)之间的平衡,来恢复先天性和适应性肠道免疫系统的动态平衡,进而恢复上皮完整性,缓解小鼠肠黏膜损伤[100]。此外,槲皮素还可通过血红素氧化酶-1(heme oxygenase-1,HO-1)依赖途径调节巨噬细胞促炎和抗炎之间的平衡,进而降低结肠中TNF-α、IL-6和IFN-γ含量,最终抑制小鼠试验性结肠炎[101]。除上述通路外,槲皮素还可通过抑制Caco-2细胞中含核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)炎症小体的活化,来减少IL-1β和IL-8的释放,进而发挥抗炎功效[102]。
由此可见,槲皮素具有较好的抗炎和免疫调节活性,可有效调控肠黏膜免疫反应,进而改善肠道免疫屏障并提高机体免疫力。
3 小结综上所述,槲皮素可通过调节肠道微生物的组成、黏液层厚度、肠上皮细胞及细胞间的TJs蛋白、胆汁和有机酸以及抗菌肽的生成、免疫相关因子和免疫细胞来保护和改善肠道黏膜屏障功能(图 3)。

|
Intestinal mucosal mechanical barrier:肠黏膜机械屏障;Intestinal mucosal chemical barrier:肠黏膜化学屏障;Intestinal mucosal biological barrier:肠黏膜生物屏障;Intestinal mucosal immune barrier:肠黏膜免疫屏障;Intestinal mucosa:肠黏膜;Epithelial cell:上皮细胞;Lamina propria:固有层;Intestinal villi:肠绒毛;Goblet cell:杯状细胞;Mucus:黏液;MUCs:黏蛋白mucin;Ca2+:钙离子calcium ion;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinases;Src:非受体型酪氨酸蛋白激酶non-receptor protein-tyrosine kinase;RhoA:Ras基因同源家族蛋白A Ras homolog gene family member A;ROCK:Rho相关蛋白激酶Rho associated kinase;MLCK:肌球蛋白轻链激酶myosin light-chain kinase;MLC:肌球蛋白轻链myosin light-chain;TLR4:Toll样受体4 Toll-like receptor 4;MAPK:丝裂原活化蛋白激酶mitogen-activated protein kinase;PKCδ:蛋白激酶δ protein kinase C δ;PI3K:磷酯酰肌醇-3-激酶phosphoinositide 3-kinase;NF-κB:核因子-κB nuclear factor-κB;AhR:芳香烃受体aryl hydrocarbon receptor;TJ:紧密连接蛋白tight junction protein;Defensin:防御素;Hepatocytes:肝细胞;LXRα:肝X受体α liver X receptor α;CYP7A1:胆固醇7α羟化酶cholesterol 7α-hydroxylase;SCFAs:短链脂肪酸short chain fatty acids;Bile:胆汁;IAP:肠碱性磷酸酶intestinal alkaline phosphatase;TLRs:Toll样受体Toll-like receptors;Intestinal microbiota:肠道微生物群;Mø:巨噬细胞macrophages;HO-1:血红素氧合酶-1 heme oxygenase-1;ROS:活性氧reactive oxygen species;PKB:蛋白激酶B protein kinase B;NLRP3:核苷酸结合寡聚化结构域样受体蛋白3 nucleotide-binding oligomerization domain-like receptor protein 3;Pro-inflammatory cytokines production:促炎细胞因子产生;B cell:B细胞;SIgA:分泌型免疫球蛋白secretory immunoglobulin A;IgA:免疫球蛋白A immunoglobulin A;IgG:免疫球蛋白G immunoglobulin G;IgM:免疫球蛋白M immunoglobulin M;Native T cell:初始T细胞;Th17:辅助性T细胞17 T helper cell 17;Treg:调节性T细胞regulatory T cells;Quercetin:槲皮素。 图 3 槲皮素调节肠道黏膜屏障的途径 Fig. 3 Regulatory pathway of quercetin on intestinal mucosal barrier |
在畜禽生产中,由肠道黏膜损伤引起的动物肠道疾病常常危害到动物机体健康,寻找适宜的药物或者添加剂成为畜禽健康养殖研究的重点。目前,槲皮素已被证明可以较好地保护肠道黏膜屏障功能。然而,槲皮素水溶性差、体内代谢快、半衰期短,导致其生物利用度低。本文通过综述槲皮素在人和动物或细胞模型中的应用,阐明了槲皮素对肠道黏膜屏障可能的调控机制,为其在畜牧业中的应用提供了参考。但今后,还需加大其研究工作,进一步深入研究槲皮素在不同动物、不同生理状况下的吸收与代谢及对肠道黏膜屏障具体的调控机制和信号转导通路;研究槲皮素的低生物利用度与肠道微生物之间的互作关系;研究肠道微生物及其代谢产物对相关信号通路的调控机制,提高槲皮素的生物利用度,确定槲皮素在不同动物不同生长阶段的适宜添加剂量等,以期为槲皮素作为畜禽饲料添加剂的开发应用和维护动物肠道健康提供科学依据。
| [1] |
SÁNCHEZ DE MEDINA F, ROMERO-CALVO I, MASCARAQUE C, et al. Intestinal inflammation and mucosal barrier function[J]. Inflammatory Bowel Diseases, 2014, 20(12): 2394-2404. DOI:10.1097/MIB.0000000000000204 |
| [2] |
OSHIMA T, MIWA H. Gastrointestinal mucosal barrier function and diseases[J]. Journal of Gastroenterology, 2016, 51(8): 768-778. DOI:10.1007/s00535-016-1207-z |
| [3] |
NATIVIDAD J M M, VERDU E F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications[J]. Pharmacological Research, 2013, 69(1): 42-51. DOI:10.1016/j.phrs.2012.10.007 |
| [4] |
TURNER J R. Intestinal mucosal barrier function in health and disease[J]. Nature Reviews Immunology, 2009, 9(11): 799-809. DOI:10.1038/nri2653 |
| [5] |
LESJAK M, BEARA I, SIMIN N, et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives[J]. Journal of Functional Foods, 2018, 40: 68-75. DOI:10.1016/j.jff.2017.10.047 |
| [6] |
KUMAR V D, VERMA P R P, SINGH S K. Morphological and in vitro antibacterial efficacy of quercetin loaded nanoparticles against food-borne microorganisms[J]. LWT-Food Science and Technology, 2016, 66: 638-650. DOI:10.1016/j.lwt.2015.11.004 |
| [7] |
NAM J S, SHARMA A R, NGUYEN L T, et al. Application of bioactive quercetin in oncotherapy: from nutrition to nanomedicine[J]. Molecules, 2016, 21(1): 108. DOI:10.3390/molecules21010108 |
| [8] |
ZOU Y, XIANG Q H, WANG J, et al. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress[J]. Livestock Science, 2016, 192: 33-38. DOI:10.1016/j.livsci.2016.08.005 |
| [9] |
ZHANG S, KIM I H. Effect of quercetin (flavonoid) supplementation on growth performance, meat stability, and immunological response in broiler chickens[J]. Livestock Science, 2020, 242: 104286. DOI:10.1016/j.livsci.2020.104286 |
| [10] |
LIU H N, LIU Y, HU L L, et al. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens[J]. Poultry Science, 2014, 93(2): 347-353. DOI:10.3382/ps.2013-03225 |
| [11] |
ZOU Y, WEI H K, XIANG Q H, et al. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation[J]. Journal of Veterinary Medical Science, 2016, 78(9): 1487-1494. DOI:10.1292/jvms.16-0090 |
| [12] |
NAIT CHABANE M, AL AHMAD A, PELUSO J, et al. Quercetin and naringenin transport across human intestinal Caco-2 cells[J]. Journal of Pharmacy and Pharmacology, 2009, 61(11): 1473-1483. DOI:10.1211/jpp.61.11.0006 |
| [13] |
GRAF B A, AMEHO C, DOLNIKOWSKI G G, et al. Rat gastrointestinal tissues metabolize quercetin[J]. The Journal of Nutrition, 2006, 136(1): 39-44. DOI:10.1093/jn/136.1.39 |
| [14] |
SESINK A L A, ARTS I C W, DE BOER V C J, et al. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides[J]. Molecular Pharmacology, 2005, 67(6): 1999-2006. DOI:10.1124/mol.104.009753 |
| [15] |
WALGREN R A, KARNAKY K J, J r, LINDENMAYER G E, et al. Efflux of dietary flavonoid quercetin 4'-beta-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2[J]. The Journal of Pharmacology and Experimental Therapeutics, 2000, 294(3): 830-836. |
| [16] |
HOFFMANN U, KROEMER H K. The ABC transporters MDR1 and MRP2:multiple functions in disposition of xenobiotics and drug resistance[J]. Drug Metabolism Reviews, 2004, 36(3/4): 669-701. |
| [17] |
KAWABATA K, MUKAI R, ISHISAKA A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability[J]. Food & Function, 2015, 6(5): 1399-1417. |
| [18] |
WILLIAMSON G, CLIFFORD M N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols[J]. Biochemical Pharmacology, 2017, 139: 24-39. DOI:10.1016/j.bcp.2017.03.012 |
| [19] |
NAJMANOVÁ I, POUROVÁ J, VOPRŠALOVÁ M, et al. Flavonoid metabolite 3-(3-hydroxyphenyl) propionic acid formed by human microflora decreases arterial blood pressure in rats[J]. Molecular Nutrition & Food Research, 2016, 60(5): 981-991. |
| [20] |
KONISHI Y. Transepithelial transport of microbial metabolites of quercetin in intestinal Caco-2 cell monolayers[J]. Journal of Agricultural and Food Chemistry, 2005, 53(3): 601-607. DOI:10.1021/jf048662l |
| [21] |
GROSS M, PFEIFFER M, MARTINI M, et al. The quantitation of metabolites of quercetin flavonols in human urine[J]. Cancer Epidemiology, Biomarkers & Prevention, 1996, 5(9): 711-720. |
| [22] |
WONG C C, BOTTING N P, ORFILA C, et al. Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6)[J]. Biochemical Pharmacology, 2011, 81(7): 942-949. DOI:10.1016/j.bcp.2011.01.004 |
| [23] |
LIU Y, LIU Y, DAI Y, et al. Enteric disposition and recycling of flavonoids and ginkgo flavonoids[J]. Journal of Alternative and Complementary Medicine, 2003, 9(5): 631-640. DOI:10.1089/107555303322524481 |
| [24] |
O'LEARY K A, DAY A J, NEEDS P W, et al. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism[J]. Biochemical Pharmacology, 2003, 65(3): 479-491. DOI:10.1016/S0006-2952(02)01510-1 |
| [25] |
HAI Y, ZHANG Y X, LIANG Y Z, et al. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives: absorption, metabolism and function of quercetin[J]. Food Frontiers, 2020, 1(4): 420-434. DOI:10.1002/fft2.50 |
| [26] |
PENG H, SHEN Y, ZHANG Q, et al. Qihuang decoction promotes the recovery of intestinal immune barrier dysfunction after gastrectomy in rats[J]. American Journal of Translational Research, 2018, 10(3): 827-836. |
| [27] |
HAO W D, HAO C Y, WU C R, et al. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers[J]. Chemosphere, 2022, 288: 132556. DOI:10.1016/j.chemosphere.2021.132556 |
| [28] |
DONG Y Y, LEI J Q, ZHANG B K. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil[J]. Poultry Science, 2020, 99(10): 4892-4903. DOI:10.1016/j.psj.2020.06.028 |
| [29] |
DAMIANO S, SASSO A, DE FELICE B, et al. Quercetin increases MUC2 and MUC5AC gene expression and secretion in intestinal goblet cell-like LS174T via PLC/PKCα/ERK1-2 pathway[J]. Frontiers in Physiology, 2018, 9: 357. DOI:10.3389/fphys.2018.00357 |
| [30] |
VOLSTATOVA T, MARCHICA A, HRONCOVA Z, et al. Effects of chlorogenic acid, epicatechin gallate, and quercetin on mucin expression and secretion in the Caco-2/HT29-MTX cell model[J]. Food Science & Nutrition, 2019, 7(2): 492-498. |
| [31] |
LUISSINT A C, PARKOS C A, NUSRAT A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair[J]. Gastroenterology, 2016, 151(4): 616-632. DOI:10.1053/j.gastro.2016.07.008 |
| [32] |
CHEN Z G, YUAN Q L, XU G R, et al. Effects of quercetin on proliferation and H2O2-induced apoptosis of intestinal porcine enterocyte cells[J]. Molecules, 2018, 23(8): 2012. DOI:10.3390/molecules23082012 |
| [33] |
ABDEL-LATIF M A, ELBESTAWY A R, EL-FAR A H, et al. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mRNA expression genes in broiler chickens[J]. Animals, 2021, 11(8): 2302. DOI:10.3390/ani11082302 |
| [34] |
LEE S H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases[J]. Intestinal Research, 2015, 13(1): 11-18. DOI:10.5217/ir.2015.13.1.11 |
| [35] |
GONZÁLEZ-MARISCAL L, TAPIA R, CHAMORRO D. Crosstalk of tight junction components with signaling pathways[J]. Biochimica et Biophysica Acta, 2008, 1778(3): 729-756. DOI:10.1016/j.bbamem.2007.08.018 |
| [36] |
HARHAJ N S, ANTONETTI D A. Regulation of tight junctions and loss of barrier function in pathophysiology[J]. The International Journal of Biochemistry & Cell Biology, 2004, 36(7): 1206-1237. |
| [37] |
SCOTT K G E, MEDDINGS J B, KIRK D R, et al. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion[[J]. Gastroenterology, 2022, 123(4): 1179-1190. |
| [38] |
WANG F J, GRAHAM W V, WANG Y M, et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression[J]. The American Journal of Pathology, 2005, 166(2): 409-419. DOI:10.1016/S0002-9440(10)62264-X |
| [39] |
刘丽娜, 孙志广, 蔡雪婷, 等. 槲皮素改善TNF-α诱导的肠上皮Caco-2细胞功能障碍[J]. 中国药科大学学报, 2012, 43(6): 541-545. LIU L N, SUN Z G, CAI X T, et al. Quercetin improves TNF-α induced intestinal barrier dysfunction in Caco-2 cells[J]. Journal of China Pharmaceutical University, 2012, 43(6): 541-545 (in Chinese). |
| [40] |
刘丽娜, 孙志广, 邵铭, 等. 槲皮素增强肠易激综合征模型大鼠肠屏障功能的实验研究[J]. 解剖与临床, 2012, 17(6): 481-486. LIU L N, SUN Z G, SHAO M, et al. Intestinal barrier function enhancement in a rat model of irritable bowel syndrome by quercetin[J]. Anatomy and Clinics, 2012, 17(6): 481-486 (in Chinese). DOI:10.3969/j.issn.1671-7163.2012.06.009 |
| [41] |
ZHANG H L, TANG Z Y, YANG J X, et al. Bi-directional regulation of emodin and quercetin on smooth muscle myosin of gizzard[J]. FEBS Letters, 2006, 580(2): 469-473. DOI:10.1016/j.febslet.2005.12.041 |
| [42] |
BRUEWER M, LUEGERING A, KUCHARZIK T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms[J]. The Journal of Immunology, 2003, 171(11): 6164-6172. DOI:10.4049/jimmunol.171.11.6164 |
| [43] |
FAN J, LI T J, ZHAO X H. Barrier-promoting efficiency of two bioactive flavonols quercetin and myricetin on rat intestinal epithelial (IEC-6) cells via suppressing Rho activation[J]. RSC Advances, 2020, 10(46): 27249-27258. DOI:10.1039/D0RA04162A |
| [44] |
SAMAK G, CHAUDHRY K K, GANGWAR R, et al. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium[J]. Biochemical Journal, 2015, 465(3): 503-515. DOI:10.1042/BJ20140450 |
| [45] |
FAN J, LI B R, ZHANG Q, et al. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation[J]. Food and Chemical Toxicology, 2021, 147: 111896. DOI:10.1016/j.fct.2020.111896 |
| [46] |
SUZUKI T, HARA H. Quercetin enhances intestinal barrier function through the assembly of zonnula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells[J]. The Journal of Nutrition, 2009, 139(5): 965-974. DOI:10.3945/jn.108.100867 |
| [47] |
NODA S, TANABE S, SUZUKI T. Quercetin increases claudin-4 expression through multiple transcription factors in intestinal Caco-2 cells[J]. Journal of Functional Foods, 2014, 10: 112-116. DOI:10.1016/j.jff.2014.06.004 |
| [48] |
AMASHEH M, SCHLICHTER S, AMASHEH S, et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells[J]. The Journal of Nutrition, 2008, 138(6): 1067-1073. DOI:10.1093/jn/138.6.1067 |
| [49] |
SCHLICHTER S. Einfluss des flavonoids quercetin auf die epitheliale barriere funktion der humanen kolonkarzinom-zelllinie Caco-2[D]. Ph. D. Thesis. Potsdam: Universität Potsdam, 2007.
|
| [50] |
ZHENG J Y, XU H, HUANG C L, et al. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition[J]. Pancreatology, 2018, 18(7): 742-752. DOI:10.1016/j.pan.2018.08.001 |
| [51] |
CARRASCO-POZO C, MORALES P, GOTTELAND M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression[J]. Journal of Agricultural and Food Chemistry, 2013, 61(22): 5291-5297. DOI:10.1021/jf400150p |
| [52] |
VALENZANO M C, DIGUILIO K, MERCADO J, et al. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients[J]. PLoS One, 2015, 10(7): e0133926. DOI:10.1371/journal.pone.0133926 |
| [53] |
AMASHEH M, LUETTIG J, AMASHEH S, et al. Effects of quercetin studied in colonic HT-29/B6 cells and rat intestine in vitro[J]. Annals of the New York Academy of Sciences, 2012, 1258(1): 100-107. DOI:10.1111/j.1749-6632.2012.06609.x |
| [54] |
SHIGESHIRO M, TANABE S, SUZUKI T. Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis[J]. Journal of Functional Foods, 2013, 5(2): 949-955. DOI:10.1016/j.jff.2013.02.008 |
| [55] |
REN Z H, GUO C Y, YU S M, et al. Progress in mycotoxins affecting intestinal mucosal barrier function[J]. International Journal of Molecular Sciences, 2019, 20(11): 2777. DOI:10.3390/ijms20112777 |
| [56] |
钱桂敏, 齐莉莉, 范哲于. 鹅去氧胆酸对大鼠肠道菌群结构及肠道功能的影响[J]. 核农学报, 2018, 32(11): 2267-2273. QIAN G M, QI L L, FAN Z Y. Effects of chenodeoxycholic acid on the rat intestinal microbiota structure and gut functions[J]. Journal of Nuclear Agricultural Sciences, 2018, 32(11): 2267-2273 (in Chinese). DOI:10.11869/j.issn.100-8551.2018.11.2267 |
| [57] |
张敏. 槲皮素调节胆固醇代谢作用的途径分析[D]. 博士学位论文. 北京: 中国人民解放军军事医学科学院, 2016. ZHANG M. Regulation effects of quercetin on cholesterol metabolism[D]. Ph. D. Thesis. Beijing: Academy of Military Medical Sciences, 2016. (in Chinese) |
| [58] |
LIU C, HUA H Y, ZHU H K, et al. Aloe polysaccharides ameliorate acute colitis in mice via Nrf2/HO-1 signaling pathway and short-chain fatty acids metabolism[J]. International Journal of Biological Macromolecules, 2021, 185: 804-812. DOI:10.1016/j.ijbiomac.2021.07.007 |
| [59] |
MA J Y, PIAO X S, SHANG Q H, et al. Mixed organic acids as an alternative to antibiotics improve serum biochemical parameters and intestinal health of weaned piglets[J]. Animal Nutrition, 2021, 7(3): 737-749. DOI:10.1016/j.aninu.2020.11.018 |
| [60] |
KUMAR M, BABAEI P, JI B Y, et al. Human gut microbiota and healthy aging: recent developments and future prospective[J]. Nutrition and Healthy Aging, 2016, 4(1): 3-16. DOI:10.3233/NHA-150002 |
| [61] |
MAO S Y, ZHU W Y. Effects of six flavonoid compounds addition on short-chain fatty acids production and human fecal microbial community change during in vitro fermentation[J]. African Journal of Microbiology Research, 2011, 5(26): 4484-4491. |
| [62] |
XU B Y, QIN W X, XU Y Z, et al. Dietary quercetin supplementation attenuates diarrhea and intestinal damage by regulating gut microbiota in weanling piglets[J]. Oxidative Medicine and Cellular Longevity, 2021, 2021: 6221012. |
| [63] |
PORRAS D, NISTAL E, MARTÍNEZ-FLÓREZ S, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation[J]. Free Radical Biology & Medicine, 2017, 102: 188-202. |
| [64] |
GHOSH S S, WANG J, YANNIE P J, et al. Intestinal barrier dysfunction, LPS translocation, and disease development[J]. Journal of the Endocrine Society, 2020, 4(2): bvz039. DOI:10.1210/jendso/bvz039 |
| [65] |
JI J, QU H, SHU D M. Crosstalk between bioactive peptide and intestinal barrier in gut homeostasis[J]. Current Protein & Peptide Science, 2015, 16(7): 604-612. |
| [66] |
肖风林. 槲皮素对肉鸡回肠禽β-防御素和菌群作用的Toll样受体信号转导机制[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2020. XIAO F L. Toll-like receptor signal transduction mechanism of quercetin on beta-defensin and microflora in ileum of broilers[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2020. (in Chinese) |
| [67] |
SOKOL H. Probiotics and antibiotics in IBD[J]. Digestive Diseases, 2014, 32(Suppl.1): 10-17. |
| [68] |
ASHIDA H, OGAWA M, KIM M, et al. Bacteria and host interactions in the gut epithelial barrier[J]. Nature Chemical Biology, 2012, 8(1): 36-45. DOI:10.1038/nchembio.741 |
| [69] |
LEE S H, INGALE S L, KIM J S, et al. Effects of dietary supplementation with Bacillus subtilis LS 1-2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig[J]. Animal Feed Science and Technology, 2014, 188: 102-110. DOI:10.1016/j.anifeedsci.2013.12.001 |
| [70] |
ETXEBERRIA U, ARIAS N, BOQUÉ N, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats[J]. The Journal of Nutritional Biochemistry, 2015, 26(6): 651-660. DOI:10.1016/j.jnutbio.2015.01.002 |
| [71] |
SHI T L, BIAN X Y, YAO Z X, et al. Quercetin improves gut dysbiosis in antibiotic-treated mice[J]. Food & Function, 2020, 11(9): 8003-8013. |
| [72] |
王盛楠. 槲皮素对AA肉鸡蛋白质消化利用的作用及机制[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2018. WANG S N. Effects and mechanism of quercetin on protein digestion and utilization in AA broilers[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2018. (in Chinese) |
| [73] |
WANG S G, YAO J Y, ZHOU B, et al. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro[J]. Journal of Food Protection, 2018, 81(1): 68-78. DOI:10.4315/0362-028X.JFP-17-214 |
| [74] |
金芳, 李垚, 刘红南, 等. 槲皮素对39周龄蛋鸡生产性能的影响[J]. 中国畜牧杂志, 2013, 49(5): 62-65. JIN F, LI Y, LIU H N, et al. Effect of quercetin on performance of 39-week-old laying hens[J]. Chinese Journal of Animal Science, 2013, 49(5): 62-65 (in Chinese). DOI:10.3969/j.issn.0258-7033.2013.05.015 |
| [75] |
滕楠. 槲皮素抑菌作用的体内和体外研究[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2015. TENG N. Bacteriostasis of quercetin in vivo and in vitro[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2015. (in Chinese) |
| [76] |
KIM Y K, ROY P K, ASHRAFUDOULLA M, et al. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression[J]. Food Control, 2022, 137: 108964. DOI:10.1016/j.foodcont.2022.108964 |
| [77] |
LEE J H, PARK J H, CHO H S, et al. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus[J]. Biofouling, 2013, 29(5): 491-499. DOI:10.1080/08927014.2013.788692 |
| [78] |
LIU B R, CHEN F G, BI C W, et al. Quercitrin, an inhibitor of Sortase A, interferes with the adhesion of Staphylococcal aureus[J]. Molecules, 2015, 20(4): 6533-6543. DOI:10.3390/molecules20046533 |
| [79] |
OUYANG J, SUN F, FENG W, et al. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa[J]. Journal of Applied Microbiology, 2016, 120(4): 966-974. DOI:10.1111/jam.13073 |
| [80] |
SURIYANARAYANAN B, SAROJINI SANTHOSH R. Docking analysis insights quercetin can be a non-antibiotic adjuvant by inhibiting Mmr drug efflux pump in Mycobacterium sp. and its homologue EmrE in Escherichia coli[J]. Journal of Biomolecular Structure and Dynamics, 2015, 33(8): 1819-1834. DOI:10.1080/07391102.2014.974211 |
| [81] |
PLAPER A, GOLOB M, HAFNER I, et al. Characterization of quercetin binding site on DNA gyrase[J]. Biochemical and Biophysical Research Communications, 2003, 306(2): 530-536. DOI:10.1016/S0006-291X(03)01006-4 |
| [82] |
QAYYUM S, SHARMA D, BISHT D, et al. Identification of factors involved in Enterococcus faecalis biofilm under quercetin stress[J]. Microbial Pathogenesis, 2019, 126: 205-211. DOI:10.1016/j.micpath.2018.11.013 |
| [83] |
HASAN S, SINGH K, DANISUDDIN M, et al. Inhibition of major virulence pathways of Streptococcus mutans by quercitrin and deoxynojirimycin: a synergistic approach of infection control[J]. PLoS One, 2014, 9(3): e91736. DOI:10.1371/journal.pone.0091736 |
| [84] |
PETERSON L W, ARTIS D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis[J]. Nature Reviews Immunology, 2014, 14(3): 141-153. DOI:10.1038/nri3608 |
| [85] |
CORTHÉSY B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis?[J]. The Journal of Immunology, 2007, 178(1): 27-32. DOI:10.4049/jimmunol.178.1.27 |
| [86] |
SILVEY K J, HUTCHINGS A B, VAJDY M, et al. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches[J]. Journal of Virology, 2001, 75(22): 10870-10879. DOI:10.1128/JVI.75.22.10870-10879.2001 |
| [87] |
刘冬妍, 刘沛. 肠道分泌型IgA的成分及功能[J]. 世界华人消化杂志, 2004, 12(12): 2845-2848. LIU D Y, LIU P. Composition of intestinal secretory IgA and its function[J]. World Chinese Journal of Digestology, 2004, 12(12): 2845-2848 (in Chinese). DOI:10.3969/j.issn.1009-3079.2004.12.018 |
| [88] |
WOOF J M, KERR M A. The function of immunoglobulin A in immunity[J]. The Journal of Pathology, 2006, 208(2): 270-282. DOI:10.1002/path.1877 |
| [89] |
CERUTTI A. The regulation of IgA class switching[J]. Nature Reviews Immunology, 2008, 8(6): 421-434. DOI:10.1038/nri2322 |
| [90] |
HEIDEBRECHT H J, KULOZIK U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode.Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM[J]. International Dairy Journal, 2019, 93: 1-10. DOI:10.1016/j.idairyj.2019.01.009 |
| [91] |
黄泓轲, 罗健玮, 李晓婷, 等. 槲皮素与5-氨基水杨酸协同治疗大鼠感染后肠易激综合征的疗效研究[J]. 中华医院感染学杂志, 2017, 27(22): 5053-5056. HUANG H K, LUO J W, LI X T, et al. Efficacy of quercetin combined with 5-aminosalicylic acid in collaborative treatment of irritable bowel syndrome rats with infections[J]. Chinese Journal of Nosocomiology, 2017, 27(22): 5053-5056 (in Chinese). |
| [92] |
AMEVOR F K, CUI Z F, NING Z F, et al. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens[J]. Poultry Science, 2021, 100(12): 101481. DOI:10.1016/j.psj.2021.101481 |
| [93] |
PARK J H, SURESHKUMAR S, KIM I H. Influences of dietary flavonoid (quercetin) supplementation on growth performance and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide[J]. Journal of Animal Science and Technology, 2020, 62(5): 605-613. DOI:10.5187/jast.2020.62.5.605 |
| [94] |
LU Y, LI X R, LIU S S, et al. Toll-like receptors and inflammatory bowel disease[J]. Frontiers in Immunology, 2018, 9: 72. DOI:10.3389/fimmu.2018.00072 |
| [95] |
WULLAERT A. Role of NF-κB activation in intestinal immune homeostasis[J]. International Journal of Medical Microbiology, 2010, 300(1): 49-56. DOI:10.1016/j.ijmm.2009.08.007 |
| [96] |
DADDAOUA A, MARTÍNEZ-PLATA E, ORTEGA-GONZÁLEZ M, et al. The nutritional supplement active hexose correlated compound (AHCC) has direct immunomodulatory actions on intestinal epithelial cells and macrophages involving TLR/MyD88 and NF-κB/MAPK activation[J]. Food Chemistry, 2013, 136(3/4): 1288-1295. |
| [97] |
BERRI M, OLIVIER M, HOLBERT S, et al. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production[J]. Algal Research, 2017, 28: 39-47. DOI:10.1016/j.algal.2017.10.008 |
| [98] |
CAI S Q, ZHANG Q, ZHAO X H, et al. The in vitro anti-inflammatory activities of galangin and quercetin towards the LPS-injured rat intestinal epithelial (IEC-6) cells as affected by heat treatment[J]. Molecules, 2021, 26(24): 7495. DOI:10.3390/molecules26247495 |
| [99] |
ENDALE M, PARK S C, KIM S, et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells[J]. Immunobiology, 2013, 218(12): 1452-1467. DOI:10.1016/j.imbio.2013.04.019 |
| [100] |
RIEMSCHNEIDER S, HOFFMANN M, SLANINA U, et al. Indol-3-carbinol and quercetin ameliorate chronic DSS-induced colitis in C57BL/6 mice by AhR-mediated anti-inflammatory mechanisms[J]. International Journal of Environmental Research and Public Health, 2021, 18(5): 2262. DOI:10.3390/ijerph18052262 |
| [101] |
JU S W, GE Y, LI P, et al. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway[J]. Cell Cycle, 2018, 17(1): 53-63. DOI:10.1080/15384101.2017.1387701 |
| [102] |
XUE Y S, DU M, ZHU M J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157 ∶ H7[J]. Free Radical Biology & Medicine, 2017, 108: 760-769. |




