烟酸是辅酶烟酰胺腺嘌呤二核苷酸(NAD,辅酶Ⅰ)和烟酰胺腺嘌呤二核苷酸磷酸(NADP,辅酶Ⅱ)的前体,是维生素B复合物中的水溶性维生素[1]。烟酸有2个单体稳定构型,其受体包括:1)G蛋白偶联受体109A(GPR109A),多表达于脂肪组织和免疫细胞,其内源性配体有β-羟基丁酸;2)G蛋白偶联受体109B(GPR109B),其内源性配体有3-羟基辛酸;3)G蛋白偶联受体81,其内源性配体有乳酸;4)G蛋白受体43。烟酸与G蛋白偶联受体A的亲和力最强,其活性基团为羧基基团,在生物体内,烟酸可组成辅酶Ⅰ和辅酶Ⅱ参与脂肪代谢过程并发挥降脂作用[2]。前人研究发现,烟酸可以降低甘油三酯(TG)、极低密度脂蛋白胆固醇(VLDL-C)、总胆固醇(TC)、脂蛋白和低密度脂蛋白胆固醇(LDL-C)水平,降低动脉粥样硬化和非酒精性脂肪肝程度,提高血浆高密度脂蛋白胆固醇(HDL-C)水平,改善血脂异常,缓解脂肪组织炎症[3-6]。烟酸分子结构如图 1所示。
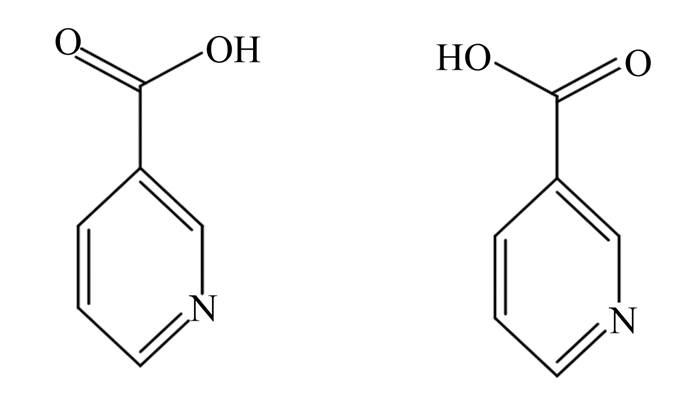
|
图 1 烟酸分子结构 Fig. 1 Molecular structure of nicotinic acid |
阿尔茨海默症(Alzheimer’s disease,AD)是一种神经退行性疾病,全世界约有3 000万人受其影响,其主要特征是β淀粉样蛋白斑块和神经纤维缠结[7],即色氨酸/烟酸缺乏导致神经退行性衰退的神经系统症状[8]。Morris等[9]用6年时间对6 158名65岁及以上的老人采用食物频数问卷确定其营养素摄入量的方法进行随访,每隔3年对所有研究参与者进行4次认知测试,对所有数据进行Logistic模型分析发现,饮食烟酸可能对AD和年龄相关的认知下降有保护作用。
帕金森综合征(Parkinson's disease,PD)是一种进行性疾病,其主要特征是α-核蛋白异常聚集、线粒体呼吸复合体1被抑制、氧化应激和神经炎症,只有5%~10%的PD是由遗传而来的,所以一些环境因素可能在PD中起了作用[10]。研究发现,烟酸是一种预防和治疗PD的因子,Wakade等[11]通过分析GPR109A(血和脑)、烟酸指数[NAD+/还原型烟酰胺腺嘌呤二核苷酸(NADH)比例]和细胞因子标记物(血液)的表达情况,测量夜间睡眠功能和感觉睡眠质量(问卷)发现,增加烟酸摄入量可以增加纹状体中的多巴胺合成,恢复线粒体复合物1活性所需的最佳烟酸指数(NAD+/NADH比例);另外研究还发现,在PD患者中,低剂量(100 mg/d)的烟酸可能通过烟酸受体GPR109A影响巨噬细胞从M1(促炎)到M2(抗炎)的转化,从而改善PD症状,促进抗炎症过程,抑制炎症反应[12]。
Cui等[13]在大脑中动脉闭塞的大鼠模型上,通过在饲粮中添加烟酸缓释制剂(40 mg/kg)发现,烟酸处理显著增加了缺血脑组织中后突触素和脑源性神经营养因子(肌钙蛋白相关激酶B)的表达,进而可治疗精神分裂症。另外,Kwon等[14]研究发现,大鼠心室颤动暂停6 min,经心肺复苏恢复后,通过口胃管给予烟酸(360 mg/kg),能改善其神经缺损量,降低海马角氨-1细胞凋亡、神经元损伤、海马轴索损伤、小胶质细胞的活化和丙二醛水平,提高脑组织谷胱甘肽、NADH和总NAD水平,抑制p38和c-Jun N端激酶(应激激活蛋白激酶)的磷酸化,进而减少大鼠心脏骤停后的脑损伤,改善大鼠神经功能。
1.2 抗炎症作用肥胖是一种以炎症细胞因子、循环免疫细胞和脂肪组织内免疫细胞增多为特征的慢性低级别炎症性疾病,慢性肥胖和脂肪组织炎症与肿瘤坏死因子-α(TNF-α)、单核细胞趋化蛋白-1(MCP-1)、C反应蛋白(CRP)、白细胞介素-6(IL-6)和促炎单核细胞循环的增加有关[15]。研究发现,烟酸[360 mg/(kg·d)]能减少小鼠脂肪组织中冠状结构的数量和降低炎症标记物整联蛋白αX(Itgax)的表达[3],可使高脂饮食喂养(HFD)的野生型小鼠血清抗炎指数脂联素浓度升高21%,减轻HFD引起的小鼠脂肪组织中MCP-1和白细胞介素-1β(IL-1β)基因表达的增加,抑制小鼠M1巨噬细胞标记物CD11c(Itgax编码)的表达,从而减轻肥胖引起的脂肪组织炎症[16]。Subramani等[17]在研究烟酸是否会延长失血性损伤大鼠的存活时间发现,烟酸能改善细胞能量学指标和减轻炎症反应。另外还有研究发现,在新西兰大白兔饲粮中添加0.6%或1.2%烟酸24 h后,显著降低其血管细胞黏附分子-1(VCAM-1)、细胞间黏附分子-1(ICAM-1)和MCP-1的表达。烟酸还能抑制内膜中性粒细胞的合成和过氧化物酶的积累,促进内皮依赖性血管舒张和环磷酸鸟苷的产生,增加血管内谷胱甘肽含量,防止次氯酸引起的内皮功能障碍和TNF-α诱导的血管炎症[18]。除此之外,烟酸还能减少促炎症介质TNF-α、IL-6和MCP-1的分泌,进而减少机体炎症反应[19]。
1.3 对脂肪代谢的调节作用脂肪代谢失衡是由多种因素引起的,严重影响动物机体的健康和产品质量,因此维持机体脂肪代谢平衡对畜禽及其肉品质十分重要。研究发现,烟酸能调节动物机体脂肪代谢(表 1),在家禽上,烟酸能降低皮下与腹部脂肪含量,增加肌内脂肪含量,降低血清TC和LDL-C等[20-22];在反刍动物上,烟酸能增加肌内脂肪含量,降低胴体脂肪含量和血清脂肪代谢相关指标等[23-27];在单胃动物上,烟酸可诱导猪Ⅱ型肌纤维转化为Ⅰ型,引起猪骨骼肌氧化代谢表型的改变,影响肉品质[28];烟酸还能显著提高家兔血浆脂联素、载脂蛋白B(ApoB)、肝瘦素受体mRNA水平,显著降低脂肪酸合成酶(FAS)mRNA水平等,抑制肝脏细胞脂肪积累[29];在水产动物上,烟酸能降低肠系膜脂肪指数和血浆TG、非酯化脂肪酸(NEFA)和LDL-C浓度等[30]。综上可知,烟酸对动物机体脂肪代谢的调节作用存在品种及部位差异。
|
|
表 1 烟酸对不同动物脂肪代谢的影响 Table 1 Effects of nicotinic acid on fat metabolism in different animals |
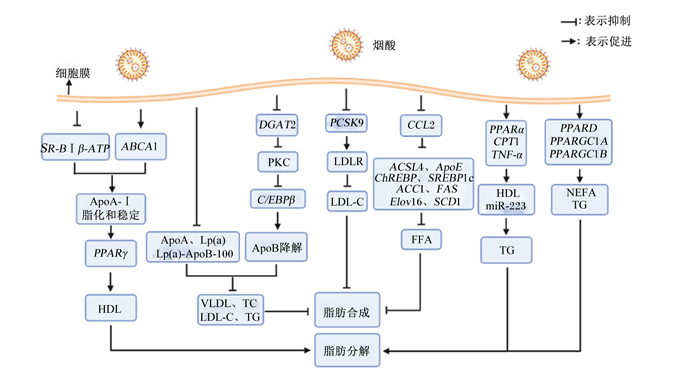
脂蛋白是一类由富含固醇脂和TG的疏水性内核以及富含蛋白质、磷脂、胆固醇等的外壳构成的球状微粒,根据密度大小可以分为乳糜微粒(CM)、极低密度脂蛋白(VLDL)、中间密度脂蛋白(IDL)、低密度脂蛋白(LDL)和高密度脂蛋白(HDL),研究发现烟酸(1~2 g/d)能够通过加速含脂蛋白(如VLDL和LDL)的ApoB在细胞内降解,降低血浆载脂蛋白A(ApoA)、脂蛋白(a)和脂蛋白(a)-ApoB-100浓度,减少VLDL的产生及其从VLDL到LDL级联的转运,抑制TC、LDL-C和TG的合成,进而抑制肝脏脂肪的合成[1, 31]。Moselhy等[32]研究发现,烟酸(8.5 mg/kg)不仅能降低HFD诱导的大鼠TG、TC、LDL-C和致动脉粥样硬化因子水平升高,抑制脂肪合成,还能通过提高HDL水平,促进脂肪分解,其主要通过降低HDL受体清道夫受体B型Ⅰ类β(SR-BⅠβ)-ATP合成酶的表达和增加ATP结合盒转运体A1(ABCA1)的表达,促进载脂蛋白A-Ⅰ(ApoA-Ⅰ)的脂化和稳定,提高过氧化物酶体增殖物激活受体γ(PPARγ)的表达,并增强巨噬细胞中PPARγ的转录活性,从而促进HDL的产生,进而促进肝脏脂肪分解[1, 33]。总之,烟酸是通过降低载脂蛋白调节脂蛋白浓度,降低VLDL和LDL浓度,提高HDL浓度,进而抑制脂肪生成,促进脂肪分解。
2.2 烟酸-二酰基甘油酰基转移酶(DGAT)-脂肪代谢途径DGAT1和DGAT2是脂肪酸酯化为TG的重要限速酶,催化TG合成的最后一步,特别是肝脏脂肪变性。研究发现,小鼠肝脏特异性DGAT2的过表达导致肝脏脂肪变性增加[34],烟酸可通过直接抑制棕榈酸介导的DGAT2转录,抑制蛋白激酶C(PKC)的活性,降低CCAAT/增强子结合蛋白β(C/EBPβ)mRNA的表达,导致ApoB降解,TG、VLDL和LDL合成减少[33, 35-37]。另外,Ganji等[38]以HepG2细胞为试验模型,通过在培养基中添加烟酸(0~3 mmol/L)发现,烟酸通过直接且非竞争性的抑制DGAT2的活性,而对DGAT1活性没有影响,降低TG合成和肝动脉粥样硬化脂蛋白减少,进而抑制机体组织中脂肪的生成。在人体上,Hu等[39]通过对血脂异常患者的研究发现烟酸(2 g/d)通过抑制DGAT2活性,降低了患者血浆TG浓度以及肝脏和内脏脂肪含量,进而使患者平均体重下降。
2.3 烟酸-脂肪代谢相关基因-脂肪代谢途径趋化因子(CC基序)配体2(CCL2),也被称为MCP-1[40],能够促进肝脏再生过程中的脂肪积累[41]。研究发现,烟酸(350 mmol/L)能降低CCL2的表达,抑制脂肪相关基因[长链酯酰辅酶A合成酶4(ACSL4)和载脂蛋白E(ApoE)]的表达,还能下调脂肪合成基因[碳水化合物反应元件结合蛋白(ChREBP)、胆固醇调节元件结合蛋白1c(SREBP1c)、乙酰辅酶A羧化酶l(ACC1)、FAS、超长链脂肪酸延伸酶6(ELOVL6)和硬脂酰辅酶A去饱和酶1(SCD1)表达,有效降低血浆游离脂肪酸(FFA)浓度,抑制肝脏及外周围脂肪的积累,改善胰岛素抵抗和血脂异常[42-43]。烟酸(300 μmol/L)也可通过下调前蛋白转化酶枯草杆菌蛋白酶/kexin9型(PCSK9)的表达,减少PCSK9成熟体蛋白含量,使得低密度脂蛋白受体(LDLR)降解减少,进而增加LDLR含量,促进人肝癌细胞系(HepG2)细胞摄取LDL-C,进而抑制脂肪的生成[44]。烟酸还能促进脂肪分解,研究发现,在胰岛素抵抗情况下,烟酸(1%)可以显著提高空肠过氧化物酶体增殖物激活受体α(PPARα)、肉毒碱棕榈酰转移酶1A(CPT1A)和TNF-α的表达,增加HDL和miR-223的分泌,减少与HDL相关的TG浓度,促进脂肪的分解[45]。Ringseis等[46]研究也发现,添加烟酸(750 mg/kg)能上调肌肉纤维转化关键因子[过氧化物酶体增殖物激活受体δ(PPARD)、过氧化物酶体增殖物激活受体γ辅激活因子1A(PPARGC1A)和过氧化物酶体增殖物激活受体γ辅激活因子1B(PPARGC1B)的表达,增强肌肉氧化利用脂肪酸的能力,降低肝脏NEFA和TG含量,进而促进脂肪的降解。
综上所述,烟酸可通过调节脂蛋白水平,影响脂肪代谢,也可以通过影响DGAT活性,进而影响脂肪代谢相关基因的表达,达到调节脂肪生成的目的;此外,烟酸还可直接影响脂肪代谢相关基因,调节脂肪的生成和分解(图 2)。
|
SR-BⅠβ-ATP:高密度脂蛋白受体清道夫受体B型Ⅰ类β-ATP合成酶high density lipoprotein receptor scavenger receptor class B type Ⅰ β-ATP synthase;ABCA1:ATP结合盒转运体A1 ATP binding box transporter A1;ApoA-Ⅰ:载脂蛋白A-Ⅰ apolipoprotein A-Ⅰ;PPARγ:过氧化物酶体增殖物激活受体γ peroxisome proliferator-activated receptor γ;HDL:高密度脂蛋白high density lipoprotein;ApoA:载脂蛋白A apolipoprotein A;Lp(a):脂蛋白(a) lipoprotein (a);Lp(a)-ApoB-100:脂蛋白(a)-载脂蛋白B-100 lipoprotein (a)-apolipoprotein B-100;VLDL:极低密度脂蛋白very low density lipoprotein;TC:总胆固醇total cholesterol;LDL-C:低密度脂蛋白胆固醇low density lipoprotein cholesterol;TG:甘油三酯triglyceride;DGAT2:二酰基甘油酰基转移酶2 diacylglycerol acyltransferase 2;PKC:蛋白激酶C protein kinase C;C/EBPβ:CCAAT/增强子结合蛋白β CCAAT/enhancer binding protein β;ApoB;载脂蛋白B apolipoprotein B;PCSK9;前蛋白转化酶枯草杆菌蛋白酶/kexin9型proprotein convertase subtilisin/kexin type 9;LDLR:低密度脂蛋白受体low density lipoprotein receptor;CCL2:趋化因子(CC基序)配体2 chemokine (CC motif) ligand 2;ACSL4:长链酯酰辅酶A合成酶4 long-chain acyl-CoA synthetase 4;ApoE:载脂蛋白E apolipoprotein E;SREBP1c:胆固醇调节元件结合蛋白1c sterol regulatory element binding protein 1c;ACC1:乙酰辅酶A羧化酶l acetyl-CoA carboxylase 1;FAS:脂肪酸合成酶fatty acid synthase;ELOVL6:超长链脂肪酸延伸酶6 elongase of very long chain fatty acid 6;SCD1:硬脂酰辅酶A去饱和酶1 stearyl CoA desaturase 1;FFA:游离脂肪酸free fatty acid;PPARα:过氧化物酶体增殖物激活受体α peroxisome proliferator-activated receptor α;CPT1:肉毒碱棕榈酰转移酶1 carnitine palmityl transferase 1;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;PPARD:过氧化物酶体增殖物激活受体δ peroxisome proliferator-activated receptor δ;PPARGC1A:过氧化物酶体增殖物激活受体γ辅激活因子1A peroxisome proliferators-activated receptor-γ coactivator 1A;PPARGC1B:过氧化物酶体增殖物激活受体γ辅激活因子1B peroxisome proliferators-activated receptor-γ coactivator 1B;NEFA:非酯化脂肪酸non-esterified fatty acid。 图 2 烟酸调节脂肪代谢的作用机制 Fig. 2 Mechanism of niacin regulating fat metabolism |
GPR109A(编码基因:小鼠,PUMA-G;人类,HM74A),又名HCAR2,是Gi家族的七跨膜G蛋白偶联受体,作为一种已知的烟酸受体,主要在白色和棕色脂肪组织中表达,在各种生命活动中发挥重要作用;GPR109A在脂肪代谢中起着重要作用,是烟酸介导的脂肪生成减少的重要受体[47-48]。烟酸进入体内可立即结合GPRl09A,并激活抗脂解的Gαi偶联受体,抑制脂肪细胞脂解,随后抑制环磷酸腺苷(cAMP)形成,最终减少脂肪酸水解为TG[49]。烟酸不仅可以通过GPR109A减弱脂肪组织的脂解作用,还可以增加脂联素的分泌[50]。脂联素主要由白色脂肪组织分泌并且是烟酸的主要靶标,烟酸激活GRP109A后,通过增加大鼠血清总脂联素浓度,降低NEFA浓度,进而减弱大鼠脂肪组织的脂解作用[51]。GPR109A的过表达可降低大鼠血浆TG浓度[52],GPR109A基因敲除可导致小鼠肝脏中SREBP1c、FAS和乙酰辅酶A羧化酶(ACC)的表达显著上调,PPARα、酰基辅酶A氧化酶1(ACOX1)和肉毒碱棕榈酰转移酶1(CPT1)显著下调,从而促进肝脂肪变性[53]。总而言之,烟酸及其受体GPR109A在脂肪代谢中起着重要作用。
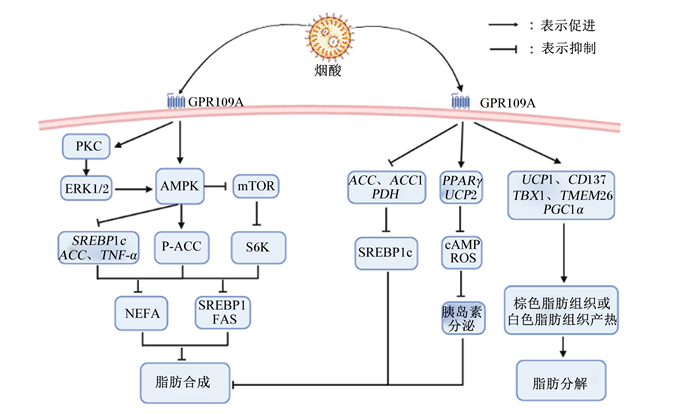
3.1 烟酸-GPR109A-单磷酸腺苷活化蛋白激酶(AMPK)-脂肪代谢途径AMPK是能量代谢中的关键因子,通过调节脂肪细胞中脂肪代谢相关因子发挥细胞传感器的作用[54]。哺乳动物雷帕霉素靶蛋白(mTOR)-核糖体蛋白S6激酶(S6K)信号通路与肥胖的发病机制有关,mTOR在蛋白质合成、脂肪合成和线粒体代谢中起着重要作用[55],其可以促进肝脏中胆固醇调节元件结合蛋白1(SREBP1)的表达,从而促进脂肪生成[56]。S6K是mTOR信号通路的效应蛋白,在细胞生长、繁殖和能量代谢中起着重要作用[57]。Wang等[58]研究发现,烟酸(0.5 mmol/L)通过GPR109A激活AMPK-ACC信号通路并抑制AMPK-mTOR-S6K信号通路,降低SREBP1和FAS的表达,从而减少牛乳腺上皮细胞中乳脂的合成。烟酸通过GPR109A介导PKC-细胞外信号调节激酶1/2(ERK1/2)-AMPK信号通路,促进肝脏中ERK1/2、AMPK和蛋白激酶B(Akt)磷酸化,促进AMPK下游靶标ACC磷酸化,下调SREBP1c和ACC的表达;还能显著降低TNF-α,减轻TNF-α对脂肪细胞表面的Gi蛋白偶联腺苷受体表达的抑制效果,从而减少脂肪细胞释放出FFA,进而抑制肝脏脂肪生成[47, 59]。
3.2 烟酸-GPR109A-脂肪代谢相关基因-脂肪代谢途径解偶联蛋白(UCP)是一种线粒体内膜蛋白,可以增加机体耗氧量,促进产热。Ye等[48]研究发现,烟酸(50 mmol/L)通过激活GPR109A,促进UCP1 mRNA和白色脂肪组织中CD137、T盒转录因子1(TBX1)、跨膜蛋白26(TMEM26)和过氧化物酶体增殖物激活受体γ辅助活化因子1α(PGC1α)表达,触发棕色脂肪组织或白色脂肪组织产热,进而促进脂肪氧化分解,同时烟酸激活GPR109A还能显著降低ACC、ACC1和丙烷脱氢酶(PDH)活性,下调SREBP1c mRNA转录,抑制肝脏脂肪的生成。Chen等[60]研究表明,添加烟酸(30 mg/kg)能激活GPR109A,促进细胞中PPARγ和UCP2的表达,增加cAMP积累,短暂增加活性氧(ROS)的产生,降低线粒体膜电位,显著降低葡萄糖诱导的胰岛素分泌,进而减少脂肪合成,其通路可能是烟酸激活胰岛素B细胞GPR109A诱导的ROS-PPARγ-UCP2途径调节的。
综上所述,GPR109A在动物机体发挥调节脂肪代谢的作用可通过AMPK通路,影响该途径上的脂肪代谢相关基因,调节脂肪生成;也可以直接影响脂肪代谢相关基因的表达,调节机体脂肪代谢(图 3)。
|
GPR109A:G蛋白偶联受体109A G protein-coupled receptor 109A;PKC:蛋白激酶C protein kinase C;EPK1/2:细胞外信号调节激酶1/2 extracellular signal-regulated kinase 1/2;AMPK:单磷酸腺苷活化蛋白激酶adenosine monophosphate activated protein kinase;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;SREBP1c:胆固醇调节元件结合蛋白1c sterol regulatory element binding protein 1c;ACC:乙酰辅酶A羧化酶acetyl-CoA carboxylase;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;P-ACC:磷酸化乙酰辅酶A羟化酶phosphorylated acetyl-CoA carboxylase;S6K:核糖体蛋白S6激酶ribosomal protein S6 kinase;NEFA:非酯化脂肪酸non-esterified fatty acid;SREBP1:胆固醇调节元件结合蛋白1 sterol regulatory element binding protein 1;FAS:脂肪酸合成酶fatty acid synthase;ACC1:乙酰辅酶A羧化酶1 acetyl-CoA carboxylase 1;PDH:丙烷脱氢酶propane dehydrogenase;PPARγ:过氧化物酶体增殖物激活受体γ peroxisome proliferator-activated receptor γ;UCP2:解偶联蛋白2 uncoupling protein 2;cAMP:环磷酸腺苷cyclic adenosine monophosphate;ROS:活性氧reactive oxygen species;UCP1:解偶联蛋白1 uncoupling protein 1;TBX1:T盒转录因子1 T-box transcription factor 1;TMEM26:跨膜蛋白26 transmembrane protein 26;PGC1α:过氧化物酶体增殖物激活受体γ共激活因子1α peroxisome proliferator-activated receptor γ coactivator 1α。 图 3 烟酸受体在脂肪代谢中的作用 Fig. 3 Roles of nicotinic acid receptors in fat metabolism |
烟酸具有保护动物机体神经系统、缓解炎症反应和维持脂肪代谢平衡等作用。目前,国内外研究发现烟酸主要是通过烟酸-脂蛋白-脂肪代谢、烟酸-DGAT-脂肪代谢及烟酸-GPR109A-AMPK-脂肪代谢等途径或烟酸直接影响转录因子及脂肪代谢相关基因的表达,进而调控脂肪代谢。目前,关于烟酸的功能及其对脂肪代谢的影响进行了广泛的研究,但仍存在许多问题值得进一步深入研究,包括探究烟酸对不同部位脂肪代谢差异性影响的分子机制,探明烟酸对胰岛素抵抗双重影响的作用过程等。脂肪代谢途径十分复杂,深入探明烟酸对脂肪代谢影响的分子机制仍是今后研究的重点。本文从神经系统、抗炎症及调节脂肪代谢等3个方面总结了烟酸的功能,并从烟酸及其受体等多条信号通路总结其影响脂肪代谢的分子机制,为烟酸在维持畜禽脂肪代谢平衡及提高畜产品质量的应用提供了理论依据。
| [1] |
GILLE A, BODOR E T, AHMED K, et al. Nicotinic acid: pharmacological effects and mechanisms of action[J]. Annual Review of Pharmacology and Toxicology, 2008, 48: 79-106. DOI:10.1146/annurev.pharmtox.48.113006.094746 |
| [2] |
李慧瑾, 金曦, 李鹏权, 等. 烟酸和烟酸受体激动剂在动脉粥样硬化中的研究进展[J]. 中国药理学通报, 2020, 36(10): 1341-1345. LI H J, JIN X, LI P Q, et al. Research progress on niacin and niacin receptor agonists in atherosclerosis[J]. Chinese Pharmacological Bulletin, 2020, 36(10): 1341-1345 (in Chinese). |
| [3] |
GRAFF E C, FANG H, WANDERS D, et al. The absence of adiponectin alters niacin's effects on adipose tissue inflammation in mice[J]. Nutrients, 2020, 12(8): 2427. DOI:10.3390/nu12082427 |
| [4] |
KASHYAP M L, GANJI S, NAKRA N K, et al. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): novel use for an old drug?[J]. Journal of Clinical Lipidology, 2019, 13(6): 873-879. DOI:10.1016/j.jacl.2019.10.006 |
| [5] |
ROBERTS W C. From the editor pellagra, osler, roberts, goldberger, the atherosclerotic diet, niacin, the beginning of the atherosclerotic epidemic, and the first lipid-altering drug[J]. The American Journal of Cardiology, 2019, 123(4): 697-700. DOI:10.1016/j.amjcard.2018.11.003 |
| [6] |
SCHANDELMAIER S, BRIEL M, SACCILOTTO R, et al. Niacin for primary and secondary prevention of cardiovascular events[J]. Cochrane Database of Systematic Reviews, 2017, 6(6): CD009744.. |
| [7] |
KUMAR A, SINGH A, Ekavali. A review on Alzheimer's disease pathophysiology and its management: an update[J]. Pharmacological Reports, 2015, 67(2): 195-203. DOI:10.1016/j.pharep.2014.09.004 |
| [8] |
WILLIAMS A C, HILL L J, RAMSDEN D B. Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: hypotheses and lessons from pellagra[J]. Current Gerontology and Geriatrics Research, 2012, 2012: 302875. |
| [9] |
MORRIS M C, EVANS D A, BIENIAS J L, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2004, 75(8): 1093-1099. DOI:10.1136/jnnp.2003.025858 |
| [10] |
AASETH J, DUSEK P, ROOS P M. Prevention of progression in Parkinson's disease[J]. BioMetals, 2018, 31(5): 737-747. DOI:10.1007/s10534-018-0131-5 |
| [11] |
WAKADE C, CHONG R, BRADLEY E, et al. Upregulation of GPR109A in Parkinson's disease[J]. PLoS One, 2014, 9(10): e109818. DOI:10.1371/journal.pone.0109818 |
| [12] |
WAKADE C, GIRI B, MALIK A, et al. Niacin modulates macrophage polarization in Parkinson's disease[J]. Journal of Neuroimmunology, 2018, 320: 76-79. DOI:10.1016/j.jneuroim.2018.05.002 |
| [13] |
CUI X, CHOPP M, ZACHAREK A, et al. Niacin treatment of stroke increases synaptic plasticity and axon growth in rats[J]. Stroke, 2010, 41(9): 2044-2049. DOI:10.1161/STROKEAHA.110.589333 |
| [14] |
KWON W Y, SUH G J, KIM K S, et al. Niacin suppresses the mitogen-activated protein kinase pathway and attenuates brain injury after cardiac arrest in rats[J]. Critical Care Medicine, 2013, 41(9): e223-e232. DOI:10.1097/CCM.0b013e31828a2394 |
| [15] |
GHANIM H, ALJADA A, HOFMEYER D, et al. Circulating mononuclear cells in the obese are in a proinflammatory state[J]. Circulation, 2004, 110(12): 1564-1571. DOI:10.1161/01.CIR.0000142055.53122.FA |
| [16] |
WANDERS D, GRAFF E C, WHITE B D, et al. Niacin increases adiponectin and decreases adipose tissue inflammation in high fat diet-fed mice[J]. PLoS One, 2013, 8(8): e71285. DOI:10.1371/journal.pone.0071285 |
| [17] |
SUBRAMANI K, CHU X G, WARREN M, et al. Deficiency of metabolite sensing receptor HCA2 impairs the salutary effect of niacin in hemorrhagic shock[J]. Biochimica et Biophysica Acta: Molecular Basis of Disease, 2019, 1865(3): 688-695. DOI:10.1016/j.bbadis.2019.01.009 |
| [18] |
WU B J, YAN L, CHARLTON F, et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction Independent of changes in plasma lipids[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2010, 30(5): 968-975. DOI:10.1161/ATVBAHA.109.201129 |
| [19] |
DIGBY J E, MARTINEZ F, JEFFERSON A, et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2012, 32(3): 669-676. DOI:10.1161/ATVBAHA.111.241836 |
| [20] |
ZHANG B B, HAO J Z, YIN H J, et al. Effects of dietary nicotinic acid supplementation on meat quality, carcass characteristics, lipid metabolism, and tibia parameters of Wulong geese[J]. Poultry Science, 2021, 100(11): 101430. DOI:10.1016/j.psj.2021.101430 |
| [21] |
ADEBOWALE T O, LIU H N, OSO A O, et al. Effect of dietary niacin supplementation on performance, total tract nutrient retention, carcass yield and meat lipid profile of growing turkeys[J]. Animal Production Science, 2018, 59(6): 1098-1107. |
| [22] |
ADEBOWALE T, OSO A, LIU H N, et al. Effect of dietary niacin supplementation on growth performance, nutrient digestibility, hematology, and lipoprotein concentrations of young turkeys, Meleagris gallopavo[J]. The Journal of Poultry Science, 2019, 56(2): 112-119. DOI:10.2141/jpsa.0170212 |
| [23] |
YANG Z Q, BAO L B, ZHAO X H, et al. Nicotinic acid supplementation in diet favored intramuscular fat deposition and lipid metabolism in finishing steers[J]. Experimental Biology and Medicine, 2016, 241(11): 1195-1201. DOI:10.1177/1535370216639395 |
| [24] |
YANG Z Q, ZHAO X H, XIONG X W, et al. Uncovering the mechanism whereby dietary nicotinic acid increases the intramuscular fat content in finishing steers by RNA sequencing analysis[J]. Animal Production Science, 2019, 59(9): 1620-1630. DOI:10.1071/AN18205 |
| [25] |
王雪莹. 烟酸对牦牛热应激期生长性能、营养物质表观消化率和脂质代谢的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2019. WANG X Y. Effects of niacin on grouth performance, apparent digestibility of nutrients and lipid metabolism in yak under heat-stress[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2019. (in Chinese) |
| [26] |
HRISTOVSKA T, CINCOVIĆ M R, BELIĆ B, et al. Effects of niacin supplementation on the insulin resistance in Holstein cows during early lactation[J]. Acta Veterinaria Brno, 2017, 86(3): 231-238. DOI:10.2754/avb201786030231 |
| [27] |
PIRES J A A, STUMPF L F, SOUTULLO I D, et al. Effects of abomasal infusion of nicotinic acid on responses to glucose and β-agonist challenges in underfed lactating cows[J]. Journal of Dairy Science, 2016, 99(3): 2297-2307. DOI:10.3168/jds.2015-10308 |
| [28] |
KHAN M, RINGSEIS R, MOOREN F C, et al. Niacin supplementation increases the number of oxidative type Ⅰ fibers in skeletal muscle of growing pigs[J]. BMC Veterinary Research, 2013, 9: 177. DOI:10.1186/1746-6148-9-177 |
| [29] |
LIU L, LI C Y, FU C Y, et al. Dietary niacin supplementation suppressed hepatic lipid accumulation in rabbits[J]. Asian-Australasian Journal of Animal Sciences, 2016, 29(12): 1748-1755. DOI:10.5713/ajas.15.0824 |
| [30] |
LI X F, WANG T J, QIAN Y, et al. Dietary niacin requirement of juvenile blunt snout bream Megalobrama amblycephala based on a dose-response study[J]. Aquaculture Nutrition, 2017, 23(6): 1410-1417. DOI:10.1111/anu.12516 |
| [31] |
OOI E M, WATTS G F, CHAN D C, et al. Effects of extended-release niacin on the postprandial metabolism of Lp (a) and ApoB-100-containing lipoproteins in statin-treated men with type 2 diabetes mellitus[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2015, 35(12): 2686-2693. DOI:10.1161/ATVBAHA.115.306136 |
| [32] |
MOSELHY S S, KAMAL I H, KUMOSANI T A, et al. Possible inhibition of hydroxy methyl glutaryl CoA reductase activity by nicotinic acid and ergosterol: as targeting for hypocholesterolemic action[J]. African Health Sciences, 2016, 16(1): 319-324. DOI:10.4314/ahs.v16i1.42 |
| [33] |
ROMANI M, HOFER D C, KATSYUBA E, et al. Niacin: an old lipid drug in a new NAD+ dress[J]. Journal of Lipid Research, 2019, 60(4): 741-746. DOI:10.1194/jlr.S092007 |
| [34] |
MONETTI M, LEVIN M C, WATT M J, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver[J]. Cell Metabolism, 2007, 6(1): 69-78. DOI:10.1016/j.cmet.2007.05.005 |
| [35] |
UMAYAHARA Y, KAJIMOTO Y, FUJITANI Y, et al. Protein kinase C-dependent, CCAAT/enhancer-binding protein beta-mediated expression of insulin-like growth factor Ⅰ gene[J]. Journal of Biological Chemistry, 2002, 277(18): 15261-15270. DOI:10.1074/jbc.M110827200 |
| [36] |
TAN S H, SHUI G H, ZHOU J, et al. Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin)[J]. Journal of Biological Chemistry, 2012, 287(18): 14364-14376. DOI:10.1074/jbc.M111.294157 |
| [37] |
GANJI S H, KASHYAP M L, KAMANNA V S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: impact on non-alcoholic fatty liver disease[J]. Metabolism, 2015, 64(9): 982-990. DOI:10.1016/j.metabol.2015.05.002 |
| [38] |
GANJI S H, TAVINTHARAN S, ZHU D M, et al. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells[J]. Journal of Lipid Research, 2004, 45(10): 1835-1845. DOI:10.1194/jlr.M300403-JLR200 |
| [39] |
HU M, CHU W C W, YAMASHITA S, et al. Liver fat reduction with niacin is influenced by DGAT-2 polymorphisms in hypertriglyceridemic patients[J]. Journal of Lipid Research, 2012, 53(4): 802-809. DOI:10.1194/jlr.P023614 |
| [40] |
BONYADI M, MOHAMMADIAN T, RAFEEY M, et al. Chemokine MCP1/CCL2 gene polymorphism influences Henoch-Schönlein purpura susceptibility in Iranian Azeri-Turkish patients[J]. International Journal of Dermatology, 2015, 54(11): 1269-1274. DOI:10.1111/ijd.12940 |
| [41] |
邢雪琨, 武红艳, 林俊堂, 等. 趋化因子2促进肝再生中脂肪的形成[J]. 解剖学报, 2016, 47(5): 697-702. XING X K, WU H Y, LIN J T, et al. Effect of chemokine 2 on the formation of lipid on liver regeneration[J]. Acta Anatomica Sinica, 2016, 47(5): 697-702 (in Chinese). |
| [42] |
XING X K, WANG H, ZHAO L, et al. Niacin downregulates chemokine (c-c motif) ligand 2(CCL2) expression and inhibits fat synthesis in rat liver cells[J]. Tropical Journal of Pharmaceutical Research, 2020, 19(5): 977-982. DOI:10.4314/tjpr.v19i5.10 |
| [43] |
KROON T, BACCEGA T, OLSÉN A, et al. Nicotinic acid timed to feeding reverses tissue lipid accumulation and improves glucose control in obese Zucker rats[J]. Journal of Lipid Research, 2017, 58(1): 31-41. DOI:10.1194/jlr.M068395 |
| [44] |
欧露, 麻燕妮, 张彩平, 等. 烟酸通过下调PCSK9的表达促进HepG2细胞摄取LDL-C[J]. 中国药理学通报, 2017, 33(2): 243-248. OU L, MA Y N, ZHANG C P, et al. Niacin accelerates LDL-C uptake in HepG2 cells via downregulation of PCSK9[J]. Chinese Pharmacological Bulletin, 2017, 33(2): 243-248 (in Chinese). |
| [45] |
MANGAT R, BORTHWICK F, HAASE T, et al. Intestinal lymphatic HDL miR-223 and ApoA-Ⅰ are reduced during insulin resistance and restored with niacin[J]. FASEB Journal, 2018, 32(3): 1602-1612. DOI:10.1096/fj.201600298RR |
| [46] |
RINGSEIS R, ROSENBAUM S, GESSNER D K, et al. Supplementing obese Zucker rats with niacin induces the transition of glycolytic to oxidative skeletal muscle fibers[J]. Journal of Nutrition, 2013, 143(2): 125-131. DOI:10.3945/jn.112.164038 |
| [47] |
YE L Y, CAO Z, LAI X R, et al. Niacin ameliorates hepatic steatosis by inhibiting de novo lipogenesis via a GPR109A-mediated PKC-ERK1/2-AMPK signaling pathway in C57BL/6 mice fed a high-fat diet[J]. The Journal of Nutrition, 2020, 150(4): 672-684. DOI:10.1093/jn/nxz303 |
| [48] |
YE L Y, CAO Z, LAI X R, et al. Niacin fine-tunes energy homeostasis through canonical GPR109A signaling[J]. FASEB Journal, 2019, 33(4): 4765-4779. DOI:10.1096/fj.201801951R |
| [49] |
ADEPU K K, KACHHAP S, ANISHKIN A, et al. Structural and energetic insights into the interaction of niacin with the GPR109A receptor[J]. Bioinformatics and Biology Insights, 2021, 15: 11779322211056122. |
| [50] |
KOPP C, HOSSEINI A, SINGH S P, et al. Nicotinic acid increases adiponectin secretion from differentiated bovine preadipocytes through G-protein coupled receptor signaling[J]. International Journal of Molecular Sciences, 2014, 15(11): 21401-21418. DOI:10.3390/ijms151121401 |
| [51] |
PLAISANCE E P, LUKASOVA M, OFFERMANNS S, et al. Niacin stimulates adiponectin secretion through the GPR109A receptor[J]. American Journal of Physiology: Endocrinology and Metabolism, 2009, 296(3): E549-E558. DOI:10.1152/ajpendo.91004.2008 |
| [52] |
MASUDA Y, KURIKAWA N, NISHIZAWA T. Overexpressing human GPR109A leads to pronounced reduction in plasma triglyceride levels in BAC transgenic rats[J]. Atherosclerosis, 2018, 272: 182-192. DOI:10.1016/j.atherosclerosis.2018.03.041 |
| [53] |
JADEJA R N, JONES M A, FROMAL O, et al. Loss of GPR109A/HCAR2 induces aging-associated hepatic steatosis[J]. Aging, 2019, 11(2): 386-400. DOI:10.18632/aging.101743 |
| [54] |
LAGE R, DIÉGUEZ C, VIDAL-PUIG A, et al. AMPK: a metabolic gauge regulating whole-body energy homeostasis[J]. Trends in Molecular Medicine, 2008, 14(12): 539-549. DOI:10.1016/j.molmed.2008.09.007 |
| [55] |
SWITON K, KOTULSKA K, JANUSZ-KAMINSKA A, et al. Molecular neurobiology of mTOR[J]. Neuroscience, 2017, 341: 112-153. DOI:10.1016/j.neuroscience.2016.11.017 |
| [56] |
YIN F, SHAREN G, YUAN F, et al. TIP30 regulates lipid metabolism in hepatocellular carcinoma by regulating SREBP1 through the Akt/mTOR signaling pathway[J]. Oncogenesis, 2017, 6(6): e347. DOI:10.1038/oncsis.2017.49 |
| [57] |
MAGNUSON B, EKIM B, FINGAR D C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks[J]. Biochemical Journal, 2012, 441(1): 1-21. DOI:10.1042/BJ20110892 |
| [58] |
WANG J X, CAO Y, FU S P, et al. Niacin inhibits the synthesis of milk fat in BMECs through the GPR109A-mediated downstream signalling pathway[J]. Life Sciences, 2020, 260: 118415. DOI:10.1016/j.lfs.2020.118415 |
| [59] |
GASIC S, TIAN B, GREEN A. Tumor necrosis factor α stimulates lipolysis in adipocytes by decreasing Gi protein concentrations[J]. Journal of Biological Chemistry, 1999, 274(10): 6770-6775. DOI:10.1074/jbc.274.10.6770 |
| [60] |
CHEN L H, SO W Y, LI S Y T, et al. Niacin-induced hyperglycemia is partially mediated via niacin receptor GPR109a in pancreatic islets[J]. Molecular and Cellular Endocrinology, 2015, 404: 56-66. DOI:10.1016/j.mce.2015.01.029 |




