骨骼肌纤维类型组成是影响畜禽肉质性状重要因素。肌纤维类型特征表现复杂且活体不易测定,分子水平寻找有效遗传选择标记和营养调控靶点成为领域研究重点。甲状腺素、胰岛素样生长因子、钙离子信号通路、Wnt信号通路、腺苷酸活化蛋白激酶(AMPK)、过氧化物酶体增殖激活受体(PPARs)及过氧化物酶体增殖激活受体γ辅助激活因子(PGC-1α)等先后被证明参与骨骼肌纤维类型转化,大部分信号通路或因子在多种组织存在且作用广泛,缺乏与骨骼肌纤维类型转化特异性关联。新近发现的可分泌型肌源细胞因子——鸢尾素(irisin),参与机体肥胖及胰岛素抗性等代谢疾病运动的治疗过程,其表达具有PGC-1α依赖和运动诱导性,并且血清鸢尾素水平与其生理功能呈正相关[1],这为骨骼肌纤维类型遗传标记和营养调控研究提供了思路。本文将对鸢尾素-Ⅲ型纤连蛋白组件包含蛋白5(fibronectin type Ⅲ domain-containing protein 5,FNDC5)途径研究最新进展及其与骨骼肌纤维类型转化分子关联作以综述介绍。
1 鸢尾素-前体基因分子结构、表达调控和生物学功能 1.1 分子结构特点鸢尾素是Boström等[1]在骨骼肌中发现的一种分泌型糖基化蛋白,由112个氨基酸构成,分子质量为12 ku。鸢尾素来源于前体蛋白FNDC5,又被称作Ⅲ型纤连蛋白重复包含蛋白2或过氧化物酶体蛋白[2, 3]。
如图1所示,FNDC5蛋白分子由信号肽(29个氨基酸)、Ⅲ型纤连蛋白组件(112个氨基酸)和C-端跨膜结构域(65个氨基酸)3部分构成,分子质量约32 ku[5],经蛋白酶水解剪切后形成分泌型FNDC5多肽片段即鸢尾素分子,进入血液循环系统。鸢尾素剪切释放过程与表皮生长因子(EGF)、转移生长因子(TGF)的剪切形成具有高度相似性[5]。Teufel等[3]研究发现,FNDC5编码基因位于第4号染色体上,长度约为5.1 kb,包含6个外显子;第1个外显子包含转录起始区,第1、2个外显子共同表达信号肽,第2、3个外显子共同表达Ⅲ型纤连蛋白组件,第4、5个外显子表达跨膜结构域。FNDC5蛋白及鸢尾素分子在不同物种间具有高度保守性[6],如在人类和啮齿类动物相 似度达到100%,高于胰岛素、胰高血糖素及瘦素 的85%、90%和83%相似度。这种高度保守性为目前鸢尾素-FNDC5基因进展用于畜禽肉质调控研究提供了参考基础。
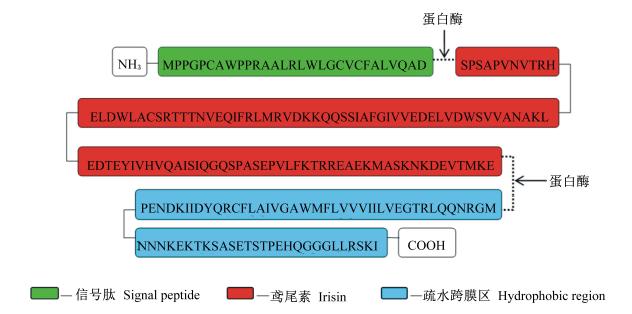 | 图1 FNDC5蛋白氨基酸序列与构成 Fig. 1 Amino acid composition and sequence of FNDC5 protein[4] |
FNDC5基因表达和鸢尾素分泌最先在人、兔及小鼠骨骼肌和血清中被检测发现[7]。Huh等[6]利用荧光定量PCR检测发现,FNDC5 mRNA主要在骨骼肌、心脏、舌和直肠等富含肌肉组织器官,以及视神经和脑组织中表达,在肾脏、肝脏及肺脏少量表达,鸢尾素分子还在皮肤真皮层及皮下组织检测发现[8],肌肉组织生长发育程度直接影响血液鸢尾素水平。有研究发现,FNDC5 mRNA和鸢尾素在啮齿类和人的脂肪组织中大量表达存在,也被称之为脂肪细胞因子[9, 10]。鸢尾素分子还在人类脑脊液、母乳及唾液中被发现[11, 12, 13],这为相关研究提供了更广阔的检测空间。
鸢尾素-FNDC5基因表达调控具有明显运动诱导和PGC-1α依赖性。Boström等[1]通过特异性剔除小鼠肌肉组织PGC-1α基因,使血清鸢尾素含量减少72%,自由跑步运动3周后增加65%。正如Hofmann等[7]报道,不同运动类型及受试个体差异对鸢尾素-FNDC5基因表达产生重要影响。在正常人中,FNDC5基因表达仅在老年人能够被耐力训练诱导增加,在年轻人或剧烈运动和高强度对抗训练者中都没有应答[14];在心脏收缩性衰竭患者中,FNDC5 mRNA表达能够被有氧运动诱导增加[15];对肥胖儿童个体进行为期1年的生活方式干预治疗,使血液鸢尾素水平升高12%[16]。有氧运动后能够使非肥胖糖尿病男性患者血清鸢尾素水平升高至2倍[17],肥胖个体血清鸢尾素水平增加更高[13],在青年小鼠也证明血清鸢尾素水平显著升高[18]。也有不同研究结果发现,Lee等[19]和Kurdiova等[20]利用人原代骨骼肌细胞模拟运动治疗,PGC-1α mRNA水平被升高2倍,FNDC5 mRNA却降低18%,培养液鸢尾素浓度降低20%;每周3次持续26周运动训练并没有改变志愿者血清鸢尾素水平。Hee等[21]研究指出,血清鸢尾素水平与受试者膳食结构无显著相关性,主要受运动类型影响。运动诱导哺乳动物鸢尾素分泌很可能是从寒颤性肌肉收缩进化而来,与棕色脂肪产热相似[22],但相关调控机理还有待证实。
1.3 生物学功能鸢尾素-FNDC5参与机体产热、脂肪转化及肥胖发生等过程。Boström等[1]给正常饮食和高脂诱导肥胖小鼠静脉注射FNDC5全长腺病毒发现:正常饮食小鼠皮下脂肪组织的FNDC5 mRNA水平增加15倍,鸢尾素全血水平增加3~4倍,注射10 d后可检测到解偶联蛋白1(uncoupling protein 1,UCP1) mRNA水平增加13倍,细胞凋亡诱导因子(cell death-inducing Dff45 like effector,Cidea)表达显著增加;肥胖小鼠皮下脂肪组织表现相似的棕色化模型,Cidea表达升高3倍,伴随着耗氧量增加、体重减轻、糖耐量改善、空腹胰岛素水平降低。Cidea主要在成年小鼠棕色脂肪组织表达,能够调节机体脂类代谢与肥胖发生[22]。Boström等[1]还发现,20 nmol/L的FNDC5蛋白可使UCP1 mRNA表达水平升高7~1 500倍,导致ATP合成受阻,产热增加,消耗更多机体能量贮备即脂肪。
血清鸢尾素水平与肥胖、糖尿病等代谢疾病发生存在相关性。肥胖患者血清鸢尾素水平与体重指数、血糖水平等呈正相关,与年龄、胰岛素、胆固醇等指标呈负相关[6];Ⅱ型糖尿病患者血清鸢尾素水平较低,与新发Ⅱ型糖尿病发病率呈负相关[23, 24];非酒精性脂肪肝肥胖患者血清鸢尾素水平相对较低,伴随肝内三酰甘油水平升高而逐渐减少,与血清谷丙转氨酶及谷草转氨酶的活性呈负相关[25]。与普通猪比较,高胆固醇血症家族猪的肌肉和脂肪组织FNDC5基因mRNA表达水平没有差异,但鸢尾素水平显著升高[26]。与正常胎儿相比,宫内迟缓发育胎儿脐带血鸢尾素表达降低,大型胎儿鸢尾素表达水平没有显著变化;血清鸢尾素水平与胎儿初生体重呈正相关,在正常和大型胎儿组与胰岛素水平呈正相关[27, 28]。这可能与个体生长后期代谢疾病发生存在某种关联。一方面,鸢尾素水平降低将导致新生儿寒颤性产热较少,脂肪组织代偿性沉积加强,从而增加代谢疾病发生风险;相反,鸢尾素水平提高通过增加寒颤性产热而降低脂肪沉积,从而减少代谢疾病发生可能。
此外,鸢尾素还与机体氧化应激、甲状腺功能紊乱、肌肉损伤、心血管疾病及癌症发生等有关[29]。通过外源补充鸢尾素可以降低相关疾病损伤,如:重组鸢尾素可以抑制非酒精性脂肪肝细胞的精氨酸甲基转移酶-3活性,进而减少细胞氧化应激、降低脂类合成与积累[30];腹腔注射鸢尾素可以抑制高脂膳食Ⅱ型糖尿病小鼠的蛋白激酶C-β(PKC-β)/还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶活性、核转录因子-κB(NF-κB)/诱导型一氧化氮合酶(iNOS)信号通路及过氧化亚硝基阴离子形成,从而改善血管内皮细胞功能[31]。另有研究发现,通过给不运动小鼠注射FNDC5蛋白,可以激活脑源性神经因子表达,促进学习和记忆神经元生长,展现出在治疗老年认知减退、阿尔茨海默病以及帕金森综合症等神经退行性疾病方面的潜在作用[32]。目前报道显示,鸢尾素可能通过p38分裂原激活的蛋白激酶(MAPK)、细胞外信号调节激酶(ERK)-MAPK及PPARα等信号通路发挥作用[33, 34]。随着鸢尾素-FNDC5基因表达、分泌在更多组织被发现,将有更多鸢尾素-FNDC5生物学功能及作用机制被研究证实。
2 骨骼肌纤维类型转化机制根据酶组化学反应特性(三磷酸腺苷酶结合琥珀酸脱氢酶)或肌球蛋白重链(MyHC)亚基种类(Ⅰ、Ⅱa、Ⅱb和Ⅱx型)组成差异,动物骨骼肌纤维被划分成不同类型,在成年哺乳动物包括快速氧化、慢速氧化、中间型和快速酵解4种不同类型[35]。不同类型肌纤维可以通过细胞内外多种信号通路相互转化,如经典的钙离子(Ca2+)信号通路。在神经冲动和激素诱导等细胞外信号刺激下,细胞内Ca2+或钙调素(CAMK)浓度升高;钙调神经磷酸酶(CaN)被激活,使活化T细胞核因子(NFATs)发生去磷酸化作用;去磷酸化NFATs进入细胞核,与肌细胞增强子结合因子2(MEF2)、生肌决定因子(MyoD)及PGC-1α等核转录因子相互作用[36],参与骨骼肌纤维类型转化过程。
NFATs包含NFATc1等5个蛋白家族成员[37],一方面可以选择性激活慢肌纤维相关基因表达[38];另一方面通过破坏MyoD与辅助转录激活因子p300的结合,抑制MyoD依赖性快肌纤维相关基因启动子活性,导致快肌纤维类型比例降低[39]。去磷酸化NFATs与核转录因子激活蛋白1(AP1)、MEF2及转录因子GATA2/4结合共同激活基因转录,经糖原合成酶激酶-3β(GSK3-β)、蛋白激酶A(PKA)、促分裂原活化蛋白激酶(p38-MAP)和酪蛋白激酶等磷酸化作用后返回细胞质,停止转录激活[40]。IGF-1通过增强NFATc1和GATA2活性刺激骨骼肌肥大增生,强直运动神经通过增加NFATs与MEF2结合活性促进慢速氧化型肌纤维形成[35]。
MEF2是一类转录因子,可以激活肌肉组织中富含A/T顺式调节元件的特异性基因表达[41],并在p38MAP和Ca2+/CaMK/CaN介导的肌肉细胞终末分化中发挥作用。同NFATc1一样,MEF2可以与MyoD家族成员结合形成复合体,抑制MyoD依赖性快肌纤维基因表达[42]。叉形头转录因子(FoxO)是胰岛素/蛋白激酶B(Akt)信号通路的下游调控基因,被Akt磷酸化后失去结合目的基因活性[43]。人源FoxO1超表达转基因小鼠骨骼肌组织块有所减少,肌肉颜色较白,Ⅰ型肌纤维表达显著降低,但Ⅱ型纤维表达未发生变化[44];FoxO1在比目鱼肌(Ⅰ型肌纤维为主)中表达丰度 较低,在趾长伸肌(Ⅱ型肌纤维为主)中表达丰度较高[45];运用RNAi技术抑制成肌细胞内FoxO1表达,可使MEF2、CaMKⅡ、NFATs和MyoD等肌纤维类型相关基因上调表达[46]。因此FoxO1可能通过抑制MEF2、CaMK和NFATs等相关途径降低Ⅰ型肌纤维含量。
如图2所示,PGC-1α基因启动子存在MEF2、FoxO1和环磷酸腺苷反应序列(CRE)结合区,其表达受胰岛素-Akt-FoxO1、细胞因子\运动-p38MAPK-MEF2\ATF2、运动-CaN\CaMKⅣ-MEF2\环磷腺苷效应元件结合蛋白(CREB)、寒冷刺激-β3肾上腺素受体(β3-AR)-PKA-CREB、胰高血糖素-GLGNR-PKA-CREB等信号通路调控[47]。通过PGC-1α转基因小鼠研究证明,超表达PGC-1α可以促进骨骼肌Ⅰ型和Ⅱa型纤维比例增加[48],MyHC Ⅰ和Ⅱa mRNA表达增加、MyHCⅡb和Ⅱx mRNA表达降低[49];敲除PGC-1α基因可以降低Ⅰ型肌纤维氧化呼吸作用、线粒体数量、耐力训练和抗疲劳能力[50]。显然,PGC-1α成为调控骨骼肌纤维类型转化(特别是氧化型纤维形成)的关键因子。
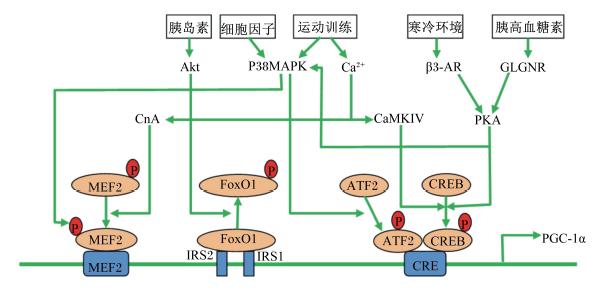 | 图2 PGC-1α与骨骼肌纤维类型转化信号通路基因的分子关联 Fig. 2 Intracellular signaling underlying muscle fiber types determination[46] |
PGC-1α是鸢尾素途径与骨骼肌纤维类型转化的主要关联分子。PGC-1α在骨骼肌代谢调节中具有非常广泛的生物学作用,如:糖原合成、脂肪酸转运与氧化、线粒体合成与修复、葡萄糖摄取、脂类合成、细胞自噬、增生性细胞因子分泌、肌纤维类型转化、神经肌肉接头基因表达诱导、肌源因子分泌、促炎细胞因子分泌等,主要生理功能表现为促进血管生成、提高氧化代谢能力、改善运动功能、缓解肌肉萎缩和营养不良及调节胰岛素敏感性,可以改善衰老性肌肉减少症、线粒体功能缺乏及系统性炎症等[51],已成为相关代谢疾病治疗的重要靶点,也是理解PGC-1α关联骨骼肌纤维类型与鸢尾素-FNDC5途径的重要基础。
如图3所示,有氧运动首先通过AMPK途径刺激PGC-1α表达,后者一方面促进FNDC5基因表达和鸢尾素分泌[52],另一方面参与骨骼肌纤维类型转化等代谢调节作用。同时,运动训练也是改变肌肉纤维类型组成的外界因素。如:耐力训练、拉伸和机械负荷等能够增加肌肉非酵解型纤维比例,减少酵解型纤维比例[53, 54];户外散养、寒冷环境能够增加猪肌肉氧化型纤维比例,降低酵解型纤维比例[55],与骨骼肌寒颤性收缩运动有关。此外,线粒体增多是氧化型肌纤维形成的重要标志,也是鸢尾素作用的显著结果。这些研究提示,鸢尾素-FNDC5途径可能与氧化型肌纤维形成存在关联,一方面受共同上游基因PGC-1α调控,另一方面鸢尾素也可能直接参与了骨骼肌纤维类型特征形成,有待深入研究。
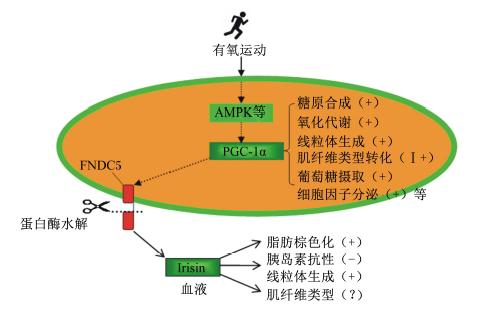 | 图3 PGC-1α成为鸢尾素-FNDC5途径与骨骼肌纤维类型转化的关联分子 Fig. 3 PGC-1α as an associated molecule between irisin-FNDC5 and muscle fiber types[7, 39] |
不同纤维类型组成肌肉,通过宰后糖原酵解与磷酸肌酸转化(ATP-CP)能力差异影响pH下降和白肌肉(PSE肉)形成,通过脂类氧化代谢差异影响肌内脂肪沉积,通过细胞骨架及间质形成、抗氧化状态、线粒体ATP生成等进一步影响肉质性状[56],显示出在畜禽肉质调控中重要性。
由于鸢尾素及其前体基因具有糖脂代谢调节功能,且与骨骼肌纤维类型转化相互关联,也必将在肉质形成与调控中发挥作用。一方面,可能成为骨骼肌纤维类型组成或肉质性状的重要分子标记,如:FNDC5基因多态性、血清鸢尾素水平等。在人医研究表明,FNDC5基因多态性与女性糖尿病患者血压、脂类分布及血糖含量存在相关性[57, 58],血清鸢尾素水平存在人种差异[21]。另一方面,为肉质营养调控研究提供新依据。虽然鸢尾素-FNDC5基因特异性调控营养素未见报道,由于FNDC5参与PGC-1α调控心肌分化和线粒体形成等过程[59],PGC-1α天然配体或线粒体营养素可直接提供相关参考。这些应用研究还有待于鸢尾素-FNDC5基因在骨骼肌纤维类型及肉质形成中作用机制证实。
5 小结与展望综上所述,鸢尾素作为一种新发现肌源细胞因子,在抵抗肥胖和糖尿病治疗等方面表现较高学术研究价值。鸢尾素-FNDC5表达调控及作用机制尚不清楚,如:调控鸢尾素-FNDC5表达分泌的细胞信号通路、FNDC5蛋白及鸢尾素作用靶点和功能效应、影响鸢尾素水解分泌因素等问题,限制了在相关领域的进一步应用。目前鸢尾素的研究仅限于试验动物和人医方面,在畜禽肉质形成与改良中鲜有报道,深入研究鸢尾素-FNDC5途径在骨骼肌纤维类型分化和代谢特征形成中的调控作用,必将为今后畜禽肉质改良提供新的科学依据。
| [1] | BOSTRÖM P,WU J,JEDRYCHOWSKI M P,et al.A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis[J]. Nature,2012,481(7382):463-468. ( 5) 5)
|
| [2] | FERRER-MARTÍNEZ A,RUIZ-LOZANO P,CHIEN K R.Mouse PeP:a novel peroxisomal protein linked to myoblast differentiation and development[J]. Developmental Dynamics,2002,224(2):154-167. ( 1) 1)
|
| [3] | TEUFEL A,MALIK N,MUKHOPADHYAY M,et al.Frcp1 and Frcp2,two novel fibronectin type Ⅲ repeat containing genes[J]. Gene,2002,297(1/2):79-83. ( 2) 2)
|
| [4] | 周伟,陈俊.运动与PGC-1α依赖性肌肉因子鸢尾素研究进展[J]. 中国运动医学杂志,2014,33(7):746-752. ( 1) 1)
|
| [5] | ERICKSON H P.Irisin and FNDC5 in retrospect:an exercise hormone or a transmembrane receptor?[J]. Adipocyte,2013,2(4):289-293. ( 2) 2)
|
| [6] | HUH J Y,PANAGIOTOU G,MOUGIOS V,et al.FNDC5 and irisin in humans: Ⅰ.Predictors of circulating concentrations in serum and plasma and Ⅱ.mRNA expression and circulating concentrations in response to weight loss and exercise[J]. Metabolism,2012,61(12):1725-1738. ( 3) 3)
|
| [7] | HOFMANN T,ELBELT U,STENGEL A.Irisin as a muscle-derived hormone stimulating thermogenesis-A critical update[J]. Peptides,2014,54:89-100. ( 3) 3)
|
| [8] | KULOGLU T,AYDIN S,EREN M N,et al.Irisin:a potentially candidate marker for myocardial infarction[J]. Peptides,2014,55:85-91. ( 1) 1)
|
| [9] | ROCA-RIVADA A,CASTELAO C,SENIN L L,et al.FNDC5/irisin is not only a myokine but also an adipokine[J]. PLoS One,2013,8(4):e60563. ( 1) 1)
|
| [10] | MORENO-NAVARRETE J M,ORTEGA F,SERRANO M,et al.Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance[J]. Journal of Clinical Endocrinology and Metabolism,2013,98(4):E769-E778. ( 1) 1)
|
| [11] | PIYA M K,HARTE A L,SIVAKUMAR K,et al.The identification of irisin in human cerebrospinal fluid:influence of adiposity,metabolic markers,and gestational diabetes[J]. American Journal of Physiology Endocrinology and Metabolism,2014,306(5):E512-E518. ( 1) 1)
|
| [12] | AYDIN S,KULOGLU T,AYDIN S.Copeptin,adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus[J]. Peptides,2013,47:66-70. ( 1) 1)
|
| [13] | AYDIN S,AYDIN S,KULOGLU T,et al.Alterations of irisin concentrations in saliva and serum of obese and normal-weight subjects,before and after 45 min of a Turkish bath or running[J]. Peptides,2013,50:13-18. ( 2) 2)
|
| [14] | TIMMONS J A,BAAR K,DAVIDSEN P K.Is irisin a human exercise gene?[J]. Nature,2012,488(7413):E9-E10. ( 1) 1)
|
| [15] | LECKER S H,ZAVIN A,CAO P R,et al.Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure[J]. Circulation:Heart Failure,2012,5:812-818. ( 1) 1)
|
| [16] | LAWSON E A,ACKERMAN K E,SLATTERY M,et al.Oxytocin secretion is related to measures of energy homeosta-sis in young amenorrheic athletes[J]. Journal of Clinical Endocrinology and Metabolism,2014,99(5):E881-E885. ( 1) 1)
|
| [17] | BESSE-PATIN A,MONTASTIER E,VINEL C,et al.Effect of endurance training on skeletal muscle myokine expression in obese men:identification of apelin as a novel myokine[J]. International Journal of Obesity,2014,38(5):707-713. ( 1) 1)
|
| [18] | AYDIN S,KULOGLU T,AYDIN S,et al.Cardiac,skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats:cardiac muscle produces more irisin than skeletal muscle[J]. Peptides,2013,52:68-73. ( 1) 1)
|
| [19] | LEE P,LINDERMAN J D,SMITH S,et al.Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans[J]. Cell Metabolism,2014,19(2):302-309. ( 1) 1)
|
| [20] | KURDIOVA T,BALAZ M,MAYER A,et al.Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes[J]. Peptides,2014,56:1-7. ( 1) 1)
|
| [21] | HEE P H,ZAICHENKO L,PETER P,et al.Diet quality is associated with circulating C-reactive protein but not irisin levels in humans[J]. Metabolism Clinical and Experimental,2014,63(2):233-241. ( 2) 2)
|
| [22] | ITO M,NAGASAWA M,OMAE N,et al.Differential regulation of CIDEA and CIDEC expression by insulin via Akt1/2- and JNK2-dependent pathways in human adipocytes[J]. Journal of Lipid Research,2011,52:1450-1460. ( 2) 2)
|
| [23] | CHOI Y K,KIM M K,BAE K H,et al.Serum irisin levels in new-onset type 2 diabetes[J]. Diabetes Research and Clinic Practice,2013,100(1):96-101. ( 1) 1)
|
| [24] | LIU J J,WONG M D S,TOY W C,et al.Lower circulating irisin is associated with type 2 diabetes mellitus[J]. Journal of Diabetes and Its Complications,2013,27(4):365-369. ( 1) 1)
|
| [25] | ZHANG H J,ZHANG X F,MA Z M,et al.Irisin is inversely associated with intrahepatic triglyceride contents in obese adults[J]. Journal of Hepatology,2013,59(3):557-562. ( 1) 1)
|
| [26] | FAIN J N,COMPANY J M,BOOTH F W,et al.Exercise training does not increase muscle FNDC5 protein or mRNA expression in pigs[J]. Metabolism Clinical and Experimental,2013,62(10):1503-1511. ( 1) 1)
|
| [27] | BAKA S,MALAMITSI-PUCHNER A,BOUTSIKOU T,et al.Cord blood irisin at the extremes of fetal growth[J]. Metabolism Clinical and Experimental,2015,64(11):1515-1520. ( 1) 1)
|
| [28] | JOUNG K E,PARK K H,FILIPPAIOS A,et al.Cord blood irisin levels are positively correlated with birth weight in newborn infants[J]. Metabolism Clinical and Experimental,2015,64(11):1507-1515. ( 1) 1)
|
| [29] | HUH J Y,MANTZOROS C S.Irisin physiology,oxidative stress,and thyroid dysfunction:what next?[J]. Metabolism Clinical and Experimental,2015,64(7):765-767. ( 1) 1)
|
| [30] | PARK M J,KIMA D I,CHOI J H,et al.New role of irisin in hepatocytes:the protective effect of hepatic steatosis in vitro[J]. Cellular Signalling,2015,27(9):1831-1839. ( 1) 1)
|
| [31] | ZHU D,WANG H C,ZHANG J L,et al.Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses[J]. Journal of Molecular and Cellular Cardiology,2015,87:138-147. ( 1) 1)
|
| [32] | WRANN C D,WHITE J P,SALOGIANNNIS J,et al.Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway[J]. Cell Metabolism,2013,18(5):649-659. ( 1) 1)
|
| [33] | HIUKKA A,MARANGHI M,MATIKAINEN N,et al.PPARα:an emerging therapeutic target in diabetic microvascular damage[J]. Nature Reviews Endocrinology,2010,6(8):454-463. ( 1) 1)
|
| [34] | ZHANG Y,LI R,MENG Y,et al.Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling[J]. Diabetes,2014,63(2):514-525. ( 1) 1)
|
| [35] | LEFAUCHEUR L,HOFFMAN R K,GERRARD D E,et al.Evidence for three adult fast myosin heavy chain isoforms in type Ⅱ skeletal muscle fibers in pigs[J]. Journal of Animal Science,1998,76(6):1584-1593. ( 2) 2)
|
| [36] | SCHULZ R A,YUTZEY K E.Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development[J]. Developmental Biology,2004,266(1):1-16. ( 1) 1)
|
| [37] | HOGAN P G,CHEN L,NARDONE J,et al.Transcriptional regulation by calcium,calcineurin,and NFAT[J]. Genes & Development,2003,17(18):2205-2232. ( 1) 1)
|
| [38] | FRAYSSE B,DESAPHY J F,PIERNO S,et al.Decrease in resting calcium and calcium entry associated with slow-to-fast transition in unloaded rat soleus muscle[J]. The FASEB Journal,2003,17(13):1916-1918. ( 1) 1)
|
| [39] | EHLERS M L,CELONA B,BLACK B L.NFATc1 controls skeletal muscle fiber type and is a negative regulator of MyoD activity[J]. Cell Reports,2014,8(6):1639-1648. ( 2) 2)
|
| [40] | HORSLEY V,JANSEN K M,MILLS S T,et al.IL-4 acts as a myoblast recruitment factor during mammalian muscle growth[J]. Cell,2003,113(4):483-494. ( 1) 1)
|
| [41] | NAYA F J,OLSON E.MEF2:a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation[J]. Current Opinion in Cell Biology,1999,11(6):683-688. ( 1) 1)
|
| [42] | WU H,ROTHERMEL B,KANATOUS S,et al.Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway[J]. The EMBO Journal,2001,20(22):6414-6423. ( 1) 1)
|
| [43] | NISSIM H.Biochimica et biophysica acta (BBA)-molecular[J]. Cell Research,2011(1813):1965-1970. ( 1) 1)
|
| [44] | KAMEI Y,MIURA S,SUZUKI M,et al.Skeletal muscle FoxO1(FKHR)-transgenic mice have less skeletal muscle mass,down-regulated type I (slow twitch/red muscle) fiber genes,and impaired glycemic control[J]. Journal of Biological Chemistry,2004,279(39):41114-41123. ( 1) 1)
|
| [45] | 杨燕军,庞卫军,白亮,等.八眉猪、长白猪及长×八杂交猪肌肉组织中FoxO1基因的表达[J]. 遗传,2008,30(2):185-189. ( 1) 1)
|
| [46] | 庞卫军.猪FoxO1基因cDNA的克隆及对前体脂肪细胞和成肌细胞分化的调控作用[D]. 博士学位论文.杨凌:西北农林科技大学,2007. ( 2) 2)
|
| [47] | FERNANDEZ-MARCOS P J,AUWERX J.Regulation of PGC-1α,a nodal regulator of mitochondrial biogenesis[J]. American Society for Nutrition,2011,93(4):884S-890S. ( 1) 1)
|
| [48] | HANDSCHIN C,CHIN S,LI P,et al.BM.Skeletal muscle fiber-type switching,exercise in tolerance,and myopathy in PGC-1α muscle-specific knock-out animals[J]. Journal of Biological Chemistry,2007,282(41):30014-30021. ( 1) 1)
|
| [49] | MORTENSEN O H,FRANDSEN L,SCHJERLING P,et al.PGC-1α and PGC-1β have both similar and distinct effects on myofiber switching toward an oxidative phenotype[J]. American Journal of Physiology Endocrinology and Metabolism,2006,291(4):E807-E816. ( 1) 1)
|
| [50] | LEONE T C,LEHMAN J J,FINCK B N,et al.PGC-1α deficiency causes multi-system energy metabolic derangements:muscle dysfunction,abnormal weight control and hepatic steatosis[J]. PLoS Biology,2005,3(4):672-687. ( 1) 1)
|
| [51] | SVENSSON K,HANDSCHIN C.Modulation of PGC-1α activity as a treatment for metabolic and muscle-related diseases[J]. Drug Discovery Today,2014,19(7):1024-1029. ( 1) 1)
|
| [52] | HUH J Y,DINCER F,MESFUM E,et al.Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans[J]. International Journal of Obese,2014,38(12):1538-1544. ( 1) 1)
|
| [53] | STARON R S,KARAPONDO D L,KRAEMER W J,et al.Skeletal muscle adaptations during early phase of heavy-resistance training in men and women[J]. Journal of Applied Physiology,1994,76(3):1247-1255. ( 1) 1)
|
| [54] | CAIOZZO V J,HADDAD F,BAKER M J,et al.Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle[J]. Journal of Applied Physiology,1996,81(1):123-132. ( 1) 1)
|
| [55] | BEE G.Effect of early-gestation feeding,birth weight,and gender of progeny on muscle fiber characteristics of pigs at slaughter[J]. Journal of Animal Science,2004,82(3):826-836. ( 1) 1)
|
| [56] | 徐子伟,门小明,齐珂珂.猪肌肉纤维类型及其代谢特征与肉质形成的关系及机理探讨[C]//动物营养研究进展(2012年版).北京:中国农业科学技术出版社,2012:85-96. ( 1) 1)
|
| [57] | BRONDANI L A,BOELTER G,ASSMANN T S,et al.Irisin-encoding gene (FNDC5) variant is associated with changes in blood pressure and lipid profile in type 2 diabetic women but not in men[J]. Metabolism Clinical and Experimental,2015,64(9):952-957. ( 1) 1)
|
| [58] | TANISAWA K,TANIGUCHI H,SUN X M,et al.Common single nucleotide polymorphisms in the FNDC5 gene are associated with glucose metabolism but do not affect serum irisin levels in Japanese men with low fitness levels[J]. Metabolism Clinical and Experimental,2014,63(4):574-583. ( 1) 1)
|
| [59] | ZADEGAN F G,GHAEDI K,KALANTAR S M,et al.Cardiac differentiation of mouse embryonic stem cells is influenced by a PPARγ/PGC-1α-FNDC5 pathway during the stage of cardiac precursor cell formation[J]. European Journal of Cell Biology,2015,94(6):257-266. ( 1) 1)
|




