2. 集美大学, 厦门市饲料检测与安全评价重点实验室, 厦门 361021;
3. 中国海洋大学, 水产动物营养与饲料农业部重点实验室, 青岛 266003
2. Xiamen Key Laboratory for Feed Quality Testing and Safety Evaluation, Jimei University, Xiamen 361021, China;
3. The Key Laboratory of Mariculture, Education Ministry of China, Ocean University of China, Qingdao 266003, China
多数情况下,硬骨鱼类体液与环境处于不等渗状态,因此需要有高效的离子渗透调节机制,以保持体内环境的稳态,从而保证机体所有生化生理过程的正常运行[1]。在淡水环境中,硬骨鱼类体液渗透压高于外界水环境,其需要抵抗体内矿物质的流失;在海水环境中,硬骨鱼类体液渗透压低于外界水环境,其需要抵御过多盐分所带来的细胞脱水状态。而广盐性硬骨鱼类能够更好地适应外界水环境盐度的变化,是由于它们有更强的调节体内渗透压的能力。 众所周知,鳃和肾脏是鱼类渗透压调节的主要器官[2, 3, 4, 5]。然而,近年来的研究发现,胃肠道作为外源性营养物质吸收的主要场所,其可通过摄取食物和水中电解质来维持体内离子的平衡[6, 7],从而参与鱼类渗透压的调节。
鱼类血浆中参与渗透压平衡调控的离子有钠离子(Na+)、钾离子(K+)、钙离子(Ca2+)、镁离子(Mg2+)、2价铁离子(Fe2+)、铜离子(Cu2+)、锰离子(Mn2+)、锌离子(Zn2+)、氯离子(Cl-)等[8]。而在这些离子中,Na+和Cl-浓度(Na+:165~285 meq/L;Cl-:129~270 meq/L)较高,因而其在鱼类体液渗透压调节中起主要作用[9, 10]。鱼类可以通过鳃吸收Na+和Cl-,亦可通过鳃将血液中过多的Na+和Cl-分泌出去。Bucking等[7]对虹鳟(Oncorhynchus mykiss)的研究发现,饲料中Na+和K+(约90%)主要是在胃部吸收;而对于无胃的模式鱼种——侧边底鳉(Fundulus heteroclitus),饲料中Na+的主要吸收位点在肠道[11]。肠道离体试验证实,淡水环境下鳉鱼肠道对Cl-的吸收能力比海水环境下高[12, 13]。另外,鱼体可以通过调节肾脏对Na+和Cl-的重吸收作用,进而调控体内矿物质的平衡,在海水环境中,Na+通过鳃向体外的运输率高于通过肾脏的运输率,而在淡水环境中,Na+通过鳃外流的速率会受到抑制,同时肾脏会从肾小球滤液中重吸收Na+来弥补自身的不足[14]。因此,鳃、胃肠道、肾脏在硬骨鱼类水盐平衡调节和渗透压平衡调节过程中都发挥着重要作用。
Na+和Cl-的平衡调节主要是通过鳃、胃肠道和肾小管上皮载体蛋白的活力调节来实现,如Na+/K+ATP酶(Na+/K+-ATPase,NKA)、Na+-K+-2Cl-协同转运蛋白(Na+-K+-2Cl- cotransporter,NKCC)、Na+/H+交换蛋白(Na+/H+ exchanger,NHE)和囊性纤维化跨膜调控子(cystic fibrosis transmembrane conductance regulator,CFTR)等,本文对参与Na+和Cl-代谢的主要载体蛋白的功能和调控机制进行综述,以期为鱼类渗透压调节的研究和实践提供理论支持。
1 NKANKA是一种P型且包含4个α亚单位和3个β亚单位的(αβ)2蛋白[15]。作为跨膜蛋白,且作为硬骨鱼类渗透调节组织中提供离子运输动力的一个主要的活跃泵[5],其主要功能是将3个Na+运出胞外,同时将2个K+运进胞内(图1和图2),该酶不仅可以维持细胞的内稳态,还可以为许多运输系统提供能量;大多数广盐性硬骨鱼类可以通过调节NKA的活力来适应外界环境的盐度变化[16, 17, 18, 19, 20, 21],且不论是在海水条件下还是在淡水条件下,位于广盐性硬骨鱼类上皮细胞基底膜外侧的NKA均可形成电化学梯度来转运Na+和Cl-[17, 22]。
众多研究发现,鱼类鳃的NKA对外界环境盐度的反应与其生态习性有关。例如:Hwang等[23]对莫桑比克罗非鱼(Oreochromis mossambicus)的研究表明,当生活于自然栖息的淡水环境下时,鳃中NKA的活力最低,当处于高渗环境下时,NKA活力则增加;Lin等[19, 24]对遮目鱼(Chanos chanos)的研究发现,在自然栖息的海水环境下,鳃中NKA的活力最低,当处于低渗环境下时,NKA活力则增加;Kang等[25]分别对自然栖息地以淡水为主的青鳉(Oryzias latipes)和以半咸水为主的黑点青鳉(Oryzias dancena)进行研究发现,当青鳉和黑点青鳉分别生活在海水和淡水条件下时,其鳃中NKA α亚基mRNA表达量最高,而这2种鱼类分别生活在淡水和半咸水条件下时,其NKA活力和α亚基蛋白丰富度是最低的。同样的现象在大马哈鱼(Oncorhynchus keta)[26]、大西洋鲑(Salmo salar)[27]、褐鳟(Salmo trutta)[28]、侧边底鳉[29]、条纹鲈(Morone saxatilis)[30]和尼罗罗非鱼(Oreochromis niloticus)[1]的研究中也有发现。由此可见,鱼类生活在自然栖息盐度环境下时,其鳃NKA活力最低,这也提示鳃NKA活力或许可以反映鱼类对环境的适应程度。
广盐性鱼类可通过调节鳃中NKA的活力来适应环境盐度的变化,而鱼类鳃中NKA活力的改变可能是其不同亚基差异表达综合表现的结果。例如:Richards等[31]对虹鳟鳃中NKA中的5种不同的α亚基(α1a、α1b、α1c、α2、α3)的研究发现,虹鳟从淡水移到含有80%海水的水体环境后,鳃中NKA α1c和α3的mRNA表达量没有显著变化,而NKA α1a的mRNA表达量降低,NKA α1b的mRNA表达量则增加;冯平等[32]对不同盐度下青鳉肠道中NKA基因表达的研究发现,肠道NKA α的表达在氯化钠(NaCl)含量为5、15和25 g/L的盐水中不变,而肠道NKA β的表达在15和25 g/L的盐水中被显著抑制。可见,盐度变化可引起鳃、肠道中NKA不同亚基的差异表达,其中肠道NKA α和β的差异表达说明NKA β只在低渗环境下起作用,而NKA α在低渗和高渗环境下都起作用;鳃NKA的α1a、α1b的差异表达表明两者可能分别调控鱼类在低渗和高渗环境下的渗透压平衡,这也解释了为何广盐性鱼类可通过NKA调节广泛地适应不同盐度环境。
外界盐度的变化不仅影响鳃中NKA的活力,也会改变鱼体肠道和肾脏中NKA的表达。Seale等[33]在对莫桑比克罗非鱼的研究中发现,海水环境下肠道NKA α的表达水平显著高于淡水环境下。Tang等[5]在研究性成熟前的日本鳗鲡(Anguilla japonica)时发现,海水环境下鳃中NKA α的表达水平高于淡水环境下,而肾脏中NKA α的表达水平低于淡水环境下。可见,在鱼类渗透压平衡调节过程中,不仅鳃中NKA起作用,其他渗透压调节组织中的NKA也发挥着重要作用,甚至有些鱼类肠道中的NKA对盐度改变的敏感性高于鳃中的NKA。例如:吴庆元等[34]对鲻鱼(Mugil cephalus)幼鱼的研究发现,在盐度为20的水体环境下,鲻鱼幼鱼肠道中NKA活力明显高于鳃中;Grosell[35]也认为,在海洋鱼类中,肠道中的NKA活力一般都比较高,有时甚至高于鳃中NKA的活力。外界盐度的改变引起多个组织中NKA活力的改变,表明NKA对稳定鱼类体液渗透压有重要作用。另外,张春晓等[36]对鲈鱼(Lateolabrax japonicus)的研究发现,低镁饲料(镁水平为0.413 g/kg)组的鲈鱼在长期适应淡水环境后,其鳃丝中NKA活力在急性盐度胁迫1 h时显著低于高镁饲料(镁水平为1.042~1.991 g/kg)组,可见 长期摄食低镁饲料会降低鲈鱼鳃丝中NKA对环境盐度刺激的敏感度,从而证实食物中离子浓度对鱼体内稳态的维持具有重要意义。因此,在对鱼类体液渗透压调节方面的研究中,除考虑水体环境因素外,食物中的矿物元素含量也不应忽视。
2 NKCC和NCC作为溶质转运体12A(SLC12A)蛋白家族的一员,NKCC是一种膜蛋白,位于上皮细胞膜的顶端或基底侧,主要作用是对离子的吸收和分泌,即负责同时将1分子的Na+、1分子的K+和2分子的Cl-通过它们的电化学梯度转移至上皮细胞内[39, 40, 41, 42](图1和图2)。在鱼类中已确定NKCC有2个亚型,分别是位于细胞基底外侧的NKCC1(作用是向体外分泌离子)和位于细胞顶膜的NKCC2(作用是向体内吸收离子),因而当鱼体内的细胞处于高渗环境下时,可激活NKCC1通过细胞向外界分泌离子来调节细胞内外渗透压的平衡(图1)[16, 43]。有学者已从欧洲鳗鲡(Anguilla anguilla)[43]和莫桑比克罗非鱼[44]体内克隆出NKCC1基因的2个亚型,即NKCC1a和NKCC1b基因。在硬骨鱼类中,NKCC1a基因在大部分的组织中都有表达,而NKCC1b基因则主要在大脑中表达[45]。另外,NKCC2基因主要在肠道和肾脏上皮细胞顶膜处表达[33, 43, 44, 46],如Tresguerres等[47]研究发现,在海洋硬骨鱼类的肠道中,NaCl从肠腔内吸收进入肠壁细胞主要是通过顶膜的NKCC2途径。
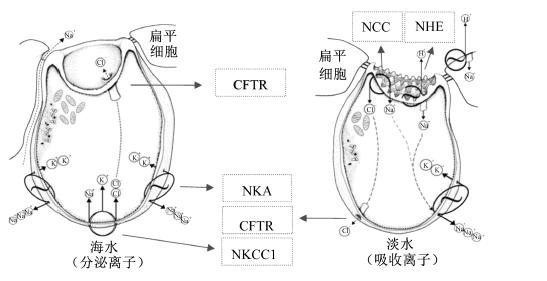 | NCC:Na+/Cl-协同转运蛋白 Na+/Cl- cotransporter;NHE:Na+/H+交换蛋白 Na+/H+ exchanger;CFTR:囊性纤维化跨膜调控子 cystic fibrosis transmembrane conductance regulator;NKA:Na+/K+ATP酶 Na+/K+ATPase;NKCC1:Na+K+2Cl-协同转运蛋白1 Na+K+2Cl- cotransporter 1。图2同 The same as Fig.2。 图1 海水和淡水环境下鱼鳃中泌氯细胞的形态及转运机制(基于McCormick[37],略有修改) Fig. 1 Morphology and transport mechanisms of gill chloride cells in seawater and fresh water (to slightly change something based on the McCormick[37]) |
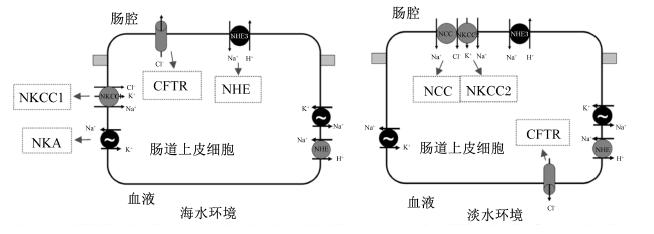 | NKCC2:Na+K+2Cl-协同转运蛋白2 Na+K+2Cl- cotransporter 2;NHE3:Na+/H+交换蛋白 Na+/H+ exchanger 3。 图2 鱼肠道中离子交换转运机制概念模型(基于Grosell等[38],略有修改) Fig. 2 Conceptual model of transport processes involved in intestinal ions exchange in |
多数研究发现,改变水体盐度可以影响鱼体组织中NKCC基因的表达。例如,侧边底鳉在从淡水转移至海水后,鳃上皮细胞中NKCC1的mRNA表达量增加[29, 48]。当萨罗罗非鱼(Sarotherodon melanothern)生活于136盐度环境下时,其鳃中NKCC1a的mRNA表达量极显著高于0盐度环境下[49]。另外,Hiroi等[50]在对3种鲑科鱼类[湖红点鲑(Salvelinus namaycush)、美洲红点鲑(Salvelinus fontinalis)和大西洋鲑]的研究中发现,随着外界环境盐度的升高,3种鱼鳃中NKCC基因的表达呈现上调的趋势,这与NKA活力的变化趋势相同。Lorin-Nebel等[51]采用分子生物学方法对欧洲鲈鱼(Dicentrarchus labrax)不同组织中NKCC1基因分析后发现,淡水环境下,鲈鱼鳃中NKCC1的mRNA表达量显著低于海水环境下,而后肾和后肠中NKCC(NKCC1和NKCC2)的mRNA表达量受水体盐度的影响不大。同时,范武江等[49]研究也发现,136盐度环境下萨罗罗非鱼鳃中NKCC1a的mRNA表达量极显著高于肠道和肾脏中,说明鳃是鱼类在海水环境中向外分泌离子的主要部位。Cutler等[43]对欧洲鳗的研究发现,当非迁徙的黄鳗(性成熟前的欧洲鳗鲡腹部为黄色)移至海水环境下2 d时,其鳃中NKCC1a的mRNA表达量上调了4.3倍,而且3周后达到近6倍高,而肾脏和中肠中NKCC1a的mRNA表达量则均有所降低;对于性成熟的银鳗(性成熟后的欧洲鳗鲡腹部为银色),移至海水环境下其鳃中NKCC1a的mRNA表达量并没有显著差异,而肾脏中NKCC1a的mRNA表达量在下调,且显著低于海水环境下黄鳗肾脏中NKCC1a的mRNA表达量;此外,中肠中NKCC1a的mRNA表达量和移至海水环境后的黄鳗相比无显著差异。可见,当欧洲鳗处于高渗环境下时,鳃中NKCC1a向外分泌过多的盐,进而调节体液渗透压平衡,而肠道和肾小管通过NKCC1a分泌盐的能力相对较低,而且不同的生态习性也会影响欧洲鳗组织中NKCC1a的mRNA表达。除了水体盐度外,一些激素也会影响组织中NKCC基因的表达。Tipsmark等[28]采用离体试验对棕鳟(Salmo trutta)和大西洋鲑研究发现,皮质醇可以直接刺激鳃中NKCC1的mRNA表达。Seale等[33]对莫桑比克罗非鱼的研究发现,由脑垂体分泌的催乳素可以调控肠道中NKCC2的mRNA表达,从而适应外界环境盐度的变化。
一些淡水鱼类的渗透压组织和卵黄囊膜顶膜中存在另一种SLC12A蛋白家族成员,即Na+/Cl-协同转运蛋白(NCC)(图1和图2),其可以同时促进Na+和Cl-的吸收,是一种电中性的离子转运蛋白,在海水适应过程中,NCC活力较低[44, 52, 53, 54]。邵占涛[52]在鲈鱼的研究中发现,鱼体进入淡水环境后,鳃、肠道、肾脏组织中NCC的mRNA表达量显著高于海水环境下,且在第3天达到最大值。Hiroi等[44]对莫桑比克罗非鱼的研究发现,鱼体进入淡水环境后,鳃中NCC的mRNA表达量显著高于海水环境下。此外,Inokuchi等[55]对莫桑比克罗非鱼的研究发现,当莫桑比克罗非鱼生活于“正常Na+/低Cl-”环境中时,其鳃中NCC的mRNA表达量相对于对照组(生活于“正常Na+/正常Cl-”环境中)显著增加,可见NCC的主要功能是促进Cl-的吸收。有研究指出,位于基底膜外侧的NKCC1a主要在海水环境下表达,起向体外分泌离子的作用且存在于Ⅳ型富含线粒体的细胞中;而位于顶膜的NCC主要在淡水环境下表达,起吸收离子作用且存在于Ⅱ型富含线粒体的细胞中[44]。可见,当鱼类生活于海水环境中时,其较多的是调用细胞基底膜外侧的NKCC1向体外分泌离子,而当鱼类生活在淡水环境中时,较多的是调用细胞顶膜处的NKCC2和NCC向体内吸收离子。在鱼类中,关于NKCC和NCC在渗透压调节方面已有大量文献报道,但研究较多的是外界盐度的变化对其活力及mRNA表达的影响,而食物中矿物元素对其活力的影响还需进一步研究。
3 NHENHE是一种双向离子交换载体蛋白,位于细胞的顶膜或基底膜外侧,其可以将Na+转运至细胞中并交换转运出H+(或NH+4),并且调控Na+和H+以1 ∶ 1的比例进行电中性交换[56, 57, 58](图1和图2)。从早期Krogh[59]提出Na+的吸收和酸的分泌存在偶联开始,Na+和H+的交换模式就已被公认为Na+吸收的一种潜在机制。Yan等[60]对斑马鱼红细胞、鳃、肾脏以及大脑中的8种NHE亚型(NHE1、2、3a、3b、5、6、7、8)进行克隆分析发现,在鳃组织中表达量较高的是NHE2和NHE3b,表达量较低的是NHE5和NHE6;而肾脏中主要存在的亚型是NHE3a和NHE3b;另外,NHE8在任何组织中都存在表达。Esaki等[61]通过选用不同抑制剂来抑制斑马鱼幼鱼阶段NKA、NKCC、NHE和H+-ATP酶(H+-ATPase)的活力,结果发现NHE和H+-ATPase在线粒体丰富细胞中对Na+吸收起着重要的作用;此外,在富含H+-ATPase的线粒体丰富细胞中存在一种高表达的转录因子foxi3a,该转录因子的缺失会抑制线粒体丰富细胞的分化,且降低Na+的吸收。
有研究表明,鱼类组织中的NHE mRNA的表达也容易受到外界盐度的影响。例如,Choe等[62]对板鳃亚纲的黄貂鱼(Dasyatis sabina)的研究发现,当该鱼生活于低盐度的水体中时,鳃线粒体丰富细胞顶膜处NHE3的表达会增加,从而促进对水体Na+的吸收。在莫桑比克罗非鱼的研究中也发现,淡水环境下罗非鱼鳃中NHE3的mRNA表达量高于海水环境下[55, 63]。而Grosell等[38]对虹鳟的研究发现,当虹鳟从淡水环境移至含有65%海水的水体环境后,其肠道中NHE3的mRNA表达量在上调,进而促进肠道分泌H+以中和肠腔上皮细胞分泌的HCO-3来维持肠腔内酸碱平衡,说明NHE3参与机体渗透压与酸碱平衡的调节。又如,当水体中二氧化碳(CO2)浓度过高时,鱼体血浆中碳酸盐的浓度会增加[64],而较高的血浆碳酸盐浓度可刺激血浆中皮质醇浓度的升高,皮质醇浓度升高会显著提高肾脏中NHE的mRNA表达和蛋白丰富度[65]。另外,Hirata等[66]对极度耐酸的雅罗鱼(Tribolodon hakonensis)的研究发现,在雅罗鱼移至酸性环境时,其鳃中NHE3 mRNA的表达量显著增加,极大的促进了H+的排出和Na+的吸收。上述研究结果进一步表明,NHE不仅在鱼体渗透压的平衡调节过程中起作用,也参与了体液酸碱平衡的调节,而激素、水体盐度和pH皆可影响鱼类NHE活力,表明NHE的调控可能存在多种途径。此外,也有研究者发现相同处理条件下,NHE不同亚型mRNA的表达存在差异。如Scott等[67]将侧边底鳉鱼从含有10%海水的水体环境移至淡水环境12 h后发现,鳃中NHE2基因的表达量显著高于NHE3基因的表达量;Ivanis等[65]在对虹鳟的研究中发现,虹鳟肾脏中NHE3的mRNA表达量比NHE2高,说明NHE不同亚型的功能可能存在差异。
由此可见,NHE参与Na+的吸收和H+的分泌,进而维持体液渗透压和酸碱平衡。但有研究指出鱼类在海水环境下NHE的主要功能是维持体液的酸碱平衡[63, 68, 69];而在淡水环境中,NHE的主要功能是维持体液的渗透压平衡[58]。因而,在通过NHE途径研究食物中矿物元素调控鱼体液渗透压平衡时,应考虑鱼体所处环境的盐度。此外,与NKA相同,NHE也存在多种亚型,而这些亚型受外界条件的影响所产生的表达各不相同,因此在渗透压平衡调节的研究中,还应注意NHE不同亚基表达差异的影响。
4 CFTRCFTR是一种调节Cl-转运的阴离子通道蛋白(图1和图2),主要位于硬骨鱼类鳃组织中富含线粒体的泌氯细胞以及鳃盖上皮细胞中,CFTR是通过环腺苷酸(cAMP)和蛋白激酶A(PKA)来激活的[70]。Singer等[71]对不同盐度下的侧边底鳉体组织中CFTR基因克隆分析发现,当淡水环境中的鳉鱼突然移至海水环境后,其鳃、鳃盖上皮细胞以及肠道中CFTR基因的表达量显著升高。Scott等[29]研究发现,当鳉鱼从海水环境中移至淡水环境中时,24 h内CFTR基因的表达量显著降低甚至消失,同样的现象在罗非鱼[72]中也有发现。鱼类鳃组织的CFTR基因在海水中高表达,在淡水中低表达,说明CFTR是广盐性硬骨鱼类鳃线粒体丰富细胞分泌Cl-,维持体内Cl-平衡的重要调控途径。另外,Singer等[73]对大西洋鲑的研究发现,CFTR也存在2种亚型,当该鱼从淡水环境中移至海水环境中2周内,其鳃中CFTRⅠ的表达量显著上调,而CFTRⅡ的表达量只是在最开始的24 h内出现短暂的上调,说明CFTRⅡ的主要功能是应对环境盐度的突变。因此,在研究CFTR调控Cl-代谢而参与体液渗透压平衡时,不仅要考虑CFTR活力,还应考虑采样时间和CFTR不同亚型的差异表达所带来的影响。
5 小 结根据以上研究报道,与Na+和Cl-转运相关的载体蛋白NKA、NKCC、NCC、NHE和CFTR均参与广盐性硬骨鱼类的机体渗透压调节。其中位于膜基底侧的NKA主要功能是水解ATP提供能量,并将细胞中的Na+运输到细胞外,形成Na+浓度梯度差,进而调节膜上NHE、NKCC、NCC调节转运细胞内外环境中的Na+和Cl-。此外,位于鳃的泌氯细胞和鳃盖上皮细胞中的CFTR主要调控Cl-的代谢,当生活在高渗环境下,CFTR可以调节Cl-向机体外运输,来维持机体内的渗透压稳态。目前关于鱼类体液渗透压平衡调节的研究,较多的关注水体盐度的改变对Na和Cl转运载体的影响,而对于食物中矿物质因素对渗透压影响的研究较少。因此,通过对Na和Cl转运载体的功能及调控机制研究的总结,将为探讨饲料矿物质调节鱼类渗透压平衡机制提供理论依据。
| [1] | 庄青青.盐度胁迫下尼罗罗非鱼鳃离子细胞和Na+-K+-ATPase a1的渗透调节[D]. 硕士学位论文.上海:上海海洋大学,2013. ( 2) 2)
|
| [2] | KARNAKY K J,ERNST S A,PHILPOTT C W.Teleost chloride cell.Ⅰ.Response of pupfish Cyprinodon variegatus gill Na,K-ATPase and chloride cell fine structure to various high salinity environments[J]. The Journal of Cell Biology,1976,70(1):144-156. ( 1) 1)
|
| [3] | MACHADO M R.Uso de brânquias de peixes como indicadores de qualidade das águas[J]. Unopar Científica Ciências Biológicas e da Saúde,1999,1(1):63-76. ( 1) 1)
|
| [4] | 李加儿,刘匆,段彪.提高鱼类渗透压调节能力研究进展[J]. 水产养殖,2002(3):30-32. ( 1) 1)
|
| [5] | TANG C H,LAI D Y,LEE T H.Effects of salinity acclimation on Na+/K+-ATPase responses and FXYD11 expression in the gills and kidneys of the Japanese eel (Anguilla japonica)[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2012,163(3/4):302-310. ( 3) 3)
|
| [6] | BUCKING C,WOOD C M.Water dynamics in the digestive tract of the freshwater rainbow trout during the processing of a single meal[J]. Journal of Experimental Biology,2006,209(10):1883-1893. ( 1) 1)
|
| [7] | BUCKING C,WOOD C M.Gastrointestinal processing of Na+,Cl-,and K+ during digestion:implications for homeostatic balance in freshwater rainbow trout[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2006,291(6):1764-1772. ( 2) 2)
|
| [8] | STURROCK A M,HUNTER E,MILTON J A,et al.Analysis methods and reference concentrations of 12 minor and trace elements in fish blood plasma[J]. Journal of Trace Elements in Medicine and Biology,2013,27(4):273-285. ( 1) 1)
|
| [9] | NORDLIE F G.Plasma osmotic,Na+ and Cl- regulation under euryhaline conditions in Cyprinodon variegatus lacépède[J]. Comparative Biochemistry and Physiology Part A:Physiology,1987,86(1):57-61. ( 1) 1)
|
| [10] | EVANS D H.Teleost fish osmoregulation:what have we learned since August Krogh,Homer Smith,and Ancel Keys[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2008,295(2):R704-R713. ( 1) 1)
|
| [11] | BUCKING C,WOOD C M,GROSELL M.Uptake,handling and excretion of Na+ and Cl- from the diet in vivo in freshwater- and seawater-acclimated killifish,Fundulus heteroclitus,an agastric teleost[J]. Indian Journal of Experimental Biology,2013,216(20):3925-3936. ( 1) 1)
|
| [12] | SCOTT G R,SCHULTE P M,WOOD C M.Plasticity of osmoregulatory function in the killifish intestine:drinking rates,salt and water transport,and gene expression after freshwater transfer[J]. Journal of Experimental Biology,2006,209(20):4040-4050. ( 1) 1)
|
| [13] | WOOD C M,BUCKING C,GROSELL M.Acid-base responses to feeding and intestinal Cl- uptake in freshwater-and seawater-acclimated killifish,Fundulus heteroclitus,an agastric euryhaline teleost[J]. Blades on Ice,2010,213(Pt 15):2681-2692. ( 1) 1)
|
| [14] | EPSTEIN F H,MANITIUS A,WEINSTEIN E,et al.Sodium- and potassium-activated adenosine triphosphatase in kidneys of Fundulus heteroclitus adapted to fresh and salt water[J]. Yale Journal of Biology & Medicine,1969,41(5):388-393. ( 1) 1)
|
| [15] | HWANG P P,LEE T H.New insights into fish ion regulation and mitochondrion-rich cells[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2007,148(3):479-497. ( 1) 1)
|
| [16] | CUTLER C P,CRAMB G.Molecular physiology of osmoregulation in eels and other teleosts:the role of transporter isoforms and gene duplication[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2001,130(3):551-564. ( 2) 2)
|
| [17] | MARSHALL W S.Na+,Cl-,Ca2+ and Zn2+ transport by fish gills:retrospective review and prospective synthesis[J]. Journal of Experimental Zoology,2002,293(3):264-283. ( 2) 2)
|
| [18] | EVANS D H,PIERMARINI P M,CHOE K P.The multifunctional fish gill:dominant site of gas exchange,osmoregulation,acid-base regulation,and excretion of nitrogenous waste[J]. Physiological Reviews,2005,85(1):97-177. ( 1) 1)
|
| [19] | LIN Y M,CHEN C N,LEE T H.The expression of gill Na,K-ATPase in milkfish,Chanos chanos,acclimated to seawater,brackish water and fresh water[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2003,135(3):489-497. ( 2) 2)
|
| [20] | TANG C H,LEE T H.The effect of environmental salinity on the protein expression of Na+/K+-ATPase,Na+/K+/2Cl- cotransporter,cystic fibrosis transmembrane conductance regulator,anion exchanger 1,and chloride channel 3 in gills of a euryhaline teleost,Tetraodon nigroviridis[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2007,147(2):521-528. ( 1) 1)
|
| [21] | LIN C H,LEE T H.Sodium or potassium ions activate different kinetics of gill Na,K-ATPase in three seawater- and freshwater-acclimated euryhaline teleosts[J]. Journal of Experimental Zoology Part A:Comparative Experimental Biology,2005,203(1):57-65. ( 1) 1)
|
| [22] | HIROSE S,KANEKO T,NAITO N,et al.Molecular biology of major components of chloride cells[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2003,136(4):593-620. ( 1) 1)
|
| [23] | HWANG P P,SUN C M,WU S M.Changes of plasma osmolality,chloride concentration and gill Na+-K+-ATPase activity in tilapia Oreochromis mossambicus during seawater acclimation[J]. Marine Biology,1989,100(3):295-299. ( 1) 1)
|
| [24] | LIN Y M,CHEN C N,YOSHINAGA T,et al.Short-term effects of hyposmotic shock on Na+/K+-ATPase expression in gills of the euryhaline milkfish,Chanos chanos[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2006,143(3):406-415. ( 1) 1)
|
| [25] | KANG C K,TSAI S C,LEE T H,et al.Differential expression of branchial Na+/K+-ATPase of two medaka species,Oryzias latipes and Oryzias dancena,with different salinity tolerances acclimated to fresh water,brackish water and seawater[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2008,151(4):566-575. ( 1) 1)
|
| [26] | UCHIDA K,KANEKO T,TAGAWA M,et al.Localization of cortisol receptor in branchial chloride cells in chum salmon fry[J]. General and Comparative Endocrinology,1998,109(2):175-185. ( 1) 1)
|
| [27] | D'COTTA H,VALOTAIRE C,GAC F L,et al.Synthesis of gill Na+-K+-ATPase in Atlantic salmon smolts:differences in α-mRNA and α-protein levels[J]. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology,2000,278(1):R101-R110. ( 1) 1)
|
| [28] | TIPSMARK C K,MADSEN S S,SEIDELIN M,et al.Dynamics of Na+,K+,2Cl- cotransporter and Na+,K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and atlantic salmon (Salmo salar)[J]. Journal of Experimental Zoology,2002,293(2):106-118. ( 2) 2)
|
| [29] | SCOTT G R,RICHARDS J G,FORBUSH B,et al.Changes in gene expression in gills of the euryhaline killifish (Fundulus heteroclitus) after abrupt salinity transfer[J]. American Journal of Physiology:Cell Physiology,2004,287(2):C300-C309. ( 3) 3)
|
| [30] | TIPSMARK C K,MADSEN S S,BORSKI R J.Effect of salinity on expression of branchial ion transporters in striped bass (Morone saxatilis)[J]. Journal of Experimental Zoology Part A:Comparative Experimental Biology,2004,301(12):979-991. ( 1) 1)
|
| [31] | RICHARDS J G,SEMPLE J W,BYSTRIANSKY J S,et al.Na+/K+-ATPase ɑ-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer[J]. Journal of Experimental Biology,2003,206(24):4475-4486. ( 1) 1)
|
| [32] | 冯平,王峰,范光丽.盐度对青鳉鱼肠内Na+-K+-ATPase基因表达的影响[J]. 西北农业学报,2006,15(6):24-27. ( 1) 1)
|
| [33] | SEALE A P,STAGG J J,YAMAGUCHI Y,et al.Effects of salinity and prolactin on gene transcript levels of ion transporters,ion pumps and prolactin receptors in Mozambique tilapia intestine[J]. General and Comparative Endocrinology,2014,206:146-154. ( 3) 3)
|
| [34] | 吴庆元,蒋玫,李磊,等.低盐度胁迫对鲻鱼(Mugil cephalus)幼鱼鳃丝、肌肉、肠Na+-K+-ATP酶活性和MDA含量的影响[J]. 生态与农村环境学报,2014,30(4):481-487. ( 1) 1)
|
| [35] | GROSELL M.Intestinal anion exchange in marine fish osmoregulation[J]. Journal of Experimental Biology,2006,209(15):2813-2827. ( 1) 1)
|
| [36] | 张春晓,周磊,叶继丹,等.急性盐度胁迫对摄食不同镁水平饲料鲈血清渗透压和离子水平以及鳃丝ATP酶活力的影响[J]. 水产学报,2012,36(9):1425-1434. ( 1) 1)
|
| [37] | MCCORMICK S D.Endocrine control of osmoregulation in teleost fish[J]. American Zoologist,2001,41(4):781-794. ( 1) 1)
|
| [38] | GROSELL M,GILMOUR K M,PERRY S F.Intestinal carbonic anhydrase,bicarbonate,and proton carriers play a role in the acclimation of rainbow trout to seawater[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2007,293(5):R2099-R2111. ( 2) 2)
|
| [39] | RUSSELL J M.Sodium-potassium-chloride cotransport[J]. Physiological Reviews,2000,80(1):211-276. ( 1) 1)
|
| [40] | HEBERT S C,MOUNT D B,GAMBA G.Molecular physiology of cation-coupled Cl- cotransport:the SLC12 family[J]. Pflügers Archiv,2004,447(5):580-593. ( 1) 1)
|
| [41] | GAMBA G.Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters[J]. Physiological Reviews,2005,85(2):423-493. ( 1) 1)
|
| [42] | 廖雅丽,张晨捷,高权新,等.鱼类离子细胞及离子通道的研究进展[J]. 海洋渔业,2015,37(1):77-86. ( 1) 1)
|
| [43] | CUTLER C P,CRAMB G.Two isoforms of the Na+/K+/2Cl- cotransporter are expressed in the European eel (Anguilla anguilla)[J]. Biochimica et Biophysica Acta:Biomembranes,2002,1566(1/2):92-103. ( 3) 3)
|
| [44] | HIROI J,YASUMASU S,MCCORMICK S D,et al.Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish[J]. Journal of Experimental Biology,2008,211(16):2584-2599. ( 5) 5)
|
| [45] | KANG C K,TSAI H J,LIU C C,et al.Salinity-dependent expression of a Na+,K+,2Cl- cotransporter in gills of the brackish medaka Oryzias dancena:a molecular correlate for hyposmoregulatory endurance[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2010,157(1):7-18. ( 1) 1)
|
| [46] | CUTLER C P,CRAMB G.Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla)[J]. Comparative Biochemistry and Physiology Part B:Biochemistry & Molecular Biology,2008,149(1):63-73. ( 1) 1)
|
| [47] | TRESGUERRES M,LEVIN L R,BUCK J,et al.Modulation of NaCl absorption by HCO-3 in the marine teleost intestine is mediated by soluble adenylyl cyclase[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2010,299(1):R62-R71. ( 1) 1)
|
| [48] | SCOTT G R,KEIR K R,SCHULTE P M.Effects of spironolactone and RU486 on gene expression and cell proliferation after freshwater transfer in the euryhaline killifish[J]. Journal of Comparative Physiology B,2005,175(7):499-510. ( 1) 1)
|
| [49] | 范武江,李思发.萨罗罗非鱼NKCC1α基因cDNA克隆及mRNA组织表达差异[J]. 动物学研究,2010,31(6):601-609. ( 2) 2)
|
| [50] | HIROI J,MCCORMICK S D.Variation in salinity tolerance,gill Na+/K+-ATPase,Na+/K+/2Cl- cotransporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush,Salvelinus fontinalis and Salmo salar[J]. Journal of Experimental Biology,2007,210(6):1015-1024. ( 1) 1)
|
| [51] | LORIN-NEBEL C,BOULO V,BODINIER C,et al.The Na+/K+/2Cl- cotransporter in the sea bass Dicentrarchus labrax during ontogeny:involvement in osmoregulation[J]. Journal of Experimental Biology,2006,209(24):4908-4922. ( 1) 1)
|
| [52] | 邵占涛.鲈鱼头肾cDNA文库的构建与免疫相关基因的筛选及中肾NCC基因的研究[D]. 博士学位论文.济南:山东师范大学,2009. ( 1) 1)
|
| [53] | BREVES J P,SERIZIER S B,GOFFIN V,et al.Prolactin regulates transcription of the ion uptake Na+/Cl- cotransporter (NCC) gene in zebrafish gill[J]. Molecular and Cellular Endocrinology,2013,369(1/2):98-106. ( 1) 1)
|
| [54] | HSU H H,LIN L Y,TSENG Y C,et al.A new model for fish ion regulation:identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes)[J]. Cell and Tissue Research,2014,357(1):225-243. ( 1) 1)
|
| [55] | INOKUCHI M,HIROI J,WATANABE S,et al.Morphological and functional classification of ion-absorbing mitochondria-rich cells in the gills of Mozambique tilapia[J]. Journal of Experimental Biology,2009,212(7):1003-1010. ( 2) 2)
|
| [56] | HAYASHI H,SZÁSZI K,GRINSTEIN S.Multiple modes of regulation of Na+/H+ exchangers[J]. Annals of the New York Academy of Sciences,2002,976:248-258. ( 1) 1)
|
| [57] | ORLOWSKI J,GRINSTEIN S.Na+/H+ exchangers of mammalian cells[J]. The Journal of Biological Chemistry,1997,272(36):22373-22376. ( 1) 1)
|
| [58] | PARKS S K,TRESGUERRES M,GOSS G G.Theoretical considerations underlying Na+ uptake mechanisms in freshwater fishes[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2008,148(4):411-418. ( 2) 2)
|
| [59] | KROGH A.The active absorption of ions in some freshwater animals[J]. Journal of Comparative Physiology,1938,25(3):335-350. ( 1) 1)
|
| [60] | YAN J J,CHOU M Y,KANEKO T,et al.Gene expression of Na+/H+ exchanger in zebrafish H+ -ATPase-rich cells during acclimation to low-Na+ and acidic environments[J]. The American Journal of Physiology:Cell Physiology,2007,293(6):C1814-C1823. ( 1) 1)
|
| [61] | ESAKI M,HOSHIJIMA K,KOBAYASHI S,et al.Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2007,292(1):R470-R480. ( 1) 1)
|
| [62] | CHOE K P,KATO A,HIROSE S,et al.NHE3 in an ancestral vertebrate:primary sequence,distribution,localization,and function in gills[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2005,289(5):R1520-R1534. ( 1) 1)
|
| [63] | WATANABE S,NIIDA M,MARUYAMA T,et al.Na+/H+ exchanger isoform 3 expressed in apical membrane of gill mitochondrion-rich cells in Mozambique tilapia Oreochromis mossambicus[J]. Fisheries Science,2008,74(4):813-821. ( 2) 2)
|
| [64] | RIMOLDI S,TEROVA G,BRAMBILLA F,et al.Molecular characterization and expression analysis of Na+/H+ exchanger (NHE)-1 and c-Fos genes in sea bass (Dicentrarchus labrax,L) exposed to acute and chronic hypercapnia[J]. Journal of Experimental Marine Biology and Ecology,2009,375(1/2):32-40. ( 1) 1)
|
| [65] | IVANIS G,BRAUN M,PERRY S F.Renal expression and localization of SLC9A3 sodium/hydrogen exchanger and its possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss)[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2008,295(3):R971-R978. ( 2) 2)
|
| [66] | HIRATA T,KANEKO T,ONO T,et al.Mechanism of acid adaptation of a fish living in a pH 3.5 lake[J]. American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2003,53(5):R1199-R1212. ( 1) 1)
|
| [67] | SCOTT G R,CLAIBORNE J B,EDWARDS S L,et al.Gene expression after freshwater transfer in gills and opercular epithelia of killifish:insight into divergent mechanisms of ion transport[J]. Journal of Experimental Biology,2005,208(14):2719-2729. ( 1) 1)
|
| [68] | WALL B,MORRISON-SHETLAR A I,CLAIBORNE J B.Effects of environment salinity and hypercapnia on NHE2-like and NHE3-like protein expression in the gill of the mumichog (Fundulus heteroclitus)[J]. Bulletin Mount Desert Island Biological Laboratory,2001,40:58-59. ( 1) 1)
|
| [69] | EDWARDS S L,CLAIBORNE J B,MORRISON-SHETLAR A I,et al.Expression of Na+/H+ exchanger mRNA in the gills of Atlantic hagfish (Myxine glutinosa) in response to metabolic acidosis[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2001,130(1):81-91. ( 1) 1)
|
| [70] | MARSHALL W S,WATTERS K D,HOVDESTAD L R,et al.CFTR Cl- channel functional regulation by phosphorylation of focal adhesion kinase at tyrosine 407 in osmosensitive ion transporting mitochondria rich cells of euryhaline killifish[J]. Journal of Experimental Biology,2009,212(15):2365-2377. ( 1) 1)
|
| [71] | SINGER T D,TUCKER S J,MARSHALL W S,et al.A divergent CFTR homologue:highly regulated salt transport in the euryhaline teleost F.heteroclitus[J]. American Journal of Physiology:Cell Physiology Published,1998,274(3 Pt 1):C715-C723. ( 1) 1)
|
| [72] | HIROI J,MCCORMICK S D,OHTANI-KANEKO R,et al.Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos,by means of triple immunofluorescence staining for Na+/K+-ATPase,Na+/K+/2Cl- cotransporter and CFTR anion channel[J]. Journal of Experimental Biology,2005,208(11):2023-2036. ( 1) 1)
|
| [73] | SINGER T D,CLEMENTS K M,SEMPLE J W,et al.Seawater tolerance and gene expression in two strains of Atlantic salmon smolts[J]. Canadian Journal of Fisheries and Aquatic Sciences,2002,59(1):125-135. ( 1) 1)
|




