2. 中国水产科学研究院长江水产研究所, 农业部淡水生物多样性保护与利用重点开放实验室, 武汉 430223
2. Key Laboratory of Freshwater Biodiversity Conservation and Utilization of Ministry of Agriculture, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences Wuhan 430223, China
渔业养殖的最终目的是以最少的投入生产出优质的渔获物,以获得最大的经济效益,并实现渔业的可持续发展。鱼类养殖经济效益的实现很大部分依赖于鱼类的生长,鱼类的生长与其摄食量密切相关,而食欲是取决动物摄食量的一个重要因素,因此深入了解鱼类的摄食机理,对促进水产养殖业的发展具有重要意义。目前对食欲的研究主要集中在哺乳动物上,关于鱼类食欲的研究相对少且零散,宏观方面的研究仅停留在摄食率或消化率方面,分子水平的研究也主要是对几种食欲调节因子的基因克隆,而没有深入的研究食欲调节的信号通路。因此,本文主要综述动物常见食欲调节因子和2条信号通路的研究进展,以期为鱼类食欲调控研究提供参考。
1 食欲调节因子食欲调节因子包括促进食欲因子和抑制食欲因子,由中枢神经系统或外周调节系统分泌(表 1)。食欲调节因子对食欲的调节虽为短期作用,却非常重要。胃肠道、胰岛、肝脏门脉系统及内脏脂肪等器官可感知机体能量状态,所产生的食欲相关肽通过神经和内分泌途径向中枢传递,由下丘脑整合多种信号后对摄食进行动态调节。在鱼类上研究的食欲调节因子目前主要有瘦素(leptin)、神经肽Y(NPY)、食欲肽(orexin)、可卡因及安非他明调节的转录因子(CART)和胆囊收缩素(CCK)[1, 2]。
| 表1 食欲调节因子 Table 1 Appetite regulation factors |
leptin是由瘦素基因(又称肥胖基因,obese)编码,由脂肪细胞分泌的一种调节机体能量平衡的蛋白质类激素,参与能量代谢、神经-内分泌、血管新生、生殖、免疫应答等的调节,能够促进细胞损伤的修复,有助于内环境紊乱的恢复[3]。leptin为一种饱食信号,主要作用于大脑的摄食和饱食中枢[4]。leptin对机体内各种生理功能的调节作用均通过与瘦素受体(leptin receptor,LEPR)的结合来实现,且leptin与LEPR间结合的比例为1:1。LEPR是糖尿病基因的产物,属于Ⅰ型细胞因子受体家族,存在6种亚型,即LEPRa、LEPRb、LEPRc、LEPRd、LEPRe和LEPRf,它们是由LEPR基因转录后通过不同剪切而生成的[5]。近年的研究发现leptin和LEPR在机体多个组织中有分布,leptin除了存在于常见的脂肪组织中,还存在于消化系统中;而LEPR分布更为广泛,如脑、心脏、胎盘、肝脏、胃、肠道、味蕾等[6]。研究还发现leptin通过与 味觉细胞的LEPR结合,从而抑制对甜味物质的反应[7]。
由leptin介导的食欲调节过程如图 1所示:当机体脂肪含量增加时,leptin在脂肪组织中合成分泌增多,后分泌到血液中与LEPRe结合形成leptin-Re;leptin-Re将leptin带入眼周围的脉络膜,在此处与LEPRa结合,生成leptin-Ra;leptin-Ra将leptin输送到脑脊液中与广泛分布在下丘脑的LEPRb结合,生成leptin-Rb;leptin-Rb在下丘脑诱发神经细胞阿片黑素促皮质素原(POMC)基因表达加强;POMC基因的强表达导致其分解产物α-促黑素细胞激素(α-MSH)的浓度升高;α-MSH进而与其受体黑素皮质素受体-4(MC4R)结合,产生抑制食欲的生理效应。食欲被抑制导致机体脂肪含量减少,leptin合成分泌下降,通过减少与LEPR的结合而导致下丘脑神经细胞POMC基因表达下降;POMC分泌下降导致α-MSH浓度降低,进而由于MC4R不被结合增加而产生食欲升高的生理效应[8, 9, 10]。通过leptin介导的食欲调节使机体的体重在正常生理条件下稳定在一定范围内,当以上任何基因发生突变而导致上述反馈过程被打破时,机体表现为嗜食且导致肥胖[11]。
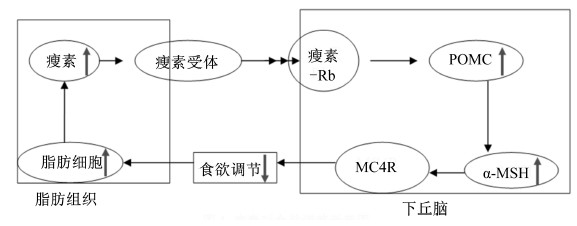 | 图1 瘦素对食欲调节示意图 Fig.1 Model of leptin regulate appetite |
以前的研究多集中在leptin对陆生动物脂肪沉积影响的研究,而近年关于leptin调节蛋白质代谢的作用逐渐受到学者们的关注[12]。长期高蛋白质饮食会导致人体能量摄入的减少和血清中leptin浓度的升高[13]。Carbó等[14]以生长雌性大鼠为试验动物,通过一次性静脉注射10 μg/kg体重的人leptin,发现短期大剂量leptin给予降低了骨骼肌内蛋白质的合成,但对骨骼肌内蛋白质分解没有显著影响。毛湘冰等[15]的研究发现,长期饲喂添加L-亮氨酸的饲粮可提高生长大鼠血浆中leptin浓度,并可显著调节生长大鼠骨骼肌蛋白质代谢。另外有研究者发现亮氨酸可以显著刺激C2C12肌管LEPR的表达,这种作用呈现剂量依赖性,且亮氨酸对哺乳动物雷帕霉素靶蛋白(mTOR)磷酸化水平的影响与对LEPR表达的影响呈现了类似的趋势;用mTOR磷酸化的特异性抑制剂(雷帕霉素,20 ng/mL)进行处理后,亮氨酸诱导的mTOR磷酸化水平被完全抑制,诱导的LEPR的表达被显著抑制。这表明亮氨酸可以诱导C2C12肌管内LEPR的表达,且这一过程是通过调节mTOR信号通路和LEPR mRNA表达丰度来完成的[16]。
目前关于鱼类leptin的研究主要是对leptin和LEPR基因的克隆,如斑马鱼、草鱼、大西洋鲑、罗非鱼、大麻哈鱼、金鱼、河豚、虹鳟、鲫鱼、鲤鱼、条纹鲈等[1, 17],也有关于leptin基因结构、重组表达和功能的少量研究[18]。与哺乳类不同的是,某些鱼类存在2种亚型的leptin,如青鳉、斑马鱼、石斑鱼等。鱼类LEPR同样与哺乳类存在差异,其亚型较少,目前发现在鲫鱼上存在3种亚型[19],在大西洋鲑上存在5种亚型[20]。但在功能上,鱼类和哺乳类相似,leptin可降低金鱼[21]、虹鳟[22]和草鱼[23]等的摄食量,促进草鱼脂肪分解和β氧化并抑制脂肪合成[24],降低罗非鱼肝脏糖原水平[25]。此外,鱼类leptin浓度与营养状况有关,并且对能量平衡具有长期调控作用[20, 26, 27]。未见关于leptin对鱼类蛋白质和氨基酸吸收代谢的调节的报道。
1.2 饥饿素(ghrelin)ghrelin是生长激素促分泌剂受体(GHS-R)的内源性配体,在哺乳动物上主要由胃组织泌酸腺的A样细胞分泌,小肠也有少量的分泌,在肺脏、胰腺、下丘脑、脑垂体、肾脏、肝脏、肌肉等组织中也有检测到,但ghrelin受体即GHS-R主要分布于中枢神经组织如下丘脑和脑垂体[28]。ghrelin与机体的免疫、水平衡、胃排空、胃酸分泌、细胞增殖、记忆、焦虑、睡眠、能量支出、骨骼代谢、繁殖和心血管的功能相关,其中最重要的功能是调控能量平衡和生长激素的分泌[29]。ghrelin基因一般有4个外显子和3个内含子,其蛋白含28个氨基酸,在第3个丝氨酸残基上有1个乙酰化的中长脂肪酸侧链,乙酰化的ghrelin才能结合ghrelin受体,并发挥生物学效应;去乙酰化的ghrelin不能结合GHS-R,不能增加摄食量,但却能促进胰岛素(insulin)的分泌,不影响胃酸的分泌,并导致人内脏脂肪蓄积增多[30, 31, 32]。去乙酰化的ghrelin通过增加下丘脑CART和尿皮质素(urocortin)的分泌来抑制食欲从而降低摄食[33]。ghrelin的乙酰化取决于饥饿素-O-乙酰基转移酶(ghrelin O-acyltransferase,GOAT)的活力,GOAT基因的长度约13.02 kb,同ghrelin一样广泛存在于各组织中,其蛋白含435个氨基酸,作用于细胞膜表面,不同物种间GOAT结构和功能均具有高度的保守型,该酶的适宜外界条件为:37~50 ℃,pH 7.0~7.5。该酶基因的突变会导致人的神经性厌食症[29, 34]。
人在空腹时体内ghrelin水平升高,摄食后降低,但其饮水后并不降低,说明胃的扩张并不抑制ghrelin的分泌[35]。无论外周还是中枢注射乙酰化的ghrelin均可刺激啮齿类动物快速摄食,且长期注射导致肥胖。ghrelin的分泌与饥饿度和体重有关,ghrelin与饥饿时间呈正相关,与体重呈负相关。饥饿时ghrelin乙酰化程度低,去乙酰化程度高[36]。肥胖者体内ghrelin水平下降,而饮食诱导体重减轻时,ghrelin的水平升高,并不存在ghrelin的抵抗[37]。但也有研究发肥胖者餐后ghrelin水平不降低或是只有轻微降低,这可能是肥胖者继续摄食的原因,也可能是脑肠肽参与肥胖的病理机制[38]。蛋白质、脂肪和碳水化合物均能影响ghrelin的分泌,摄食高水平的碳水化合物和脂肪均能使ghrelin水平降低,但高蛋白质食物反而使其水平升高[39]。但也有研究发现高蛋白质饮食并不影响血浆中ghrelin的水平[40]。ghrelin的水平受中链脂肪酸的调控最为明显,增加中链脂肪酸的摄入会导致其水平明显增加,并提高ghrelin乙酰化程度[41]。这些结果表明ghrelin乙酰化程度受到营养状况(是否摄食)和中链脂肪酸含量的调控。
关于鱼类ghrelin的研究进展,马细兰等[42]从结构、分布、功能等方面进行了比较详细的综述,鱼类的ghrelin结构(外显子数目、内含子长度、乙酰化等)和分布(鱼类主要是集中在消化道内,而在脑内的表达较弱)与哺乳类存在差异,ghrelin同样参与鱼类的摄食调节,其主要是通过促进生长激素的分泌来实现促生长作用。在罗非鱼[43]和金鱼[44]上发现外源性注射ghrelin均可提高摄食量,但是在虹鳟上发现,外源性向脑部和腹腔注射ghrelin并没有提高虹鳟的食欲和生长,反而起到抑制作用[45],ghrelin在鱼类上可能反而是一种抑制食欲相关肽,同时在罗非鱼上也发现饥饿时血浆中的ghrelin水平并未出现明显变化[46]。这表明ghrelin的功能可能存在物种差异,在鱼类上是否能促进摄食和生长值得进一步探讨。
因ghrelin本身存在具有活性的乙酰化和无活性的去乙酰化2种状态,且其功能的发挥主要在脑部,通过对ghrelin的调控来实现对鱼类食欲的改善显得较为困难,反而通过提高GOAT的活性来增加ghrelin的乙酰化,从而促进鱼类的食欲相对更可靠。
1.3 CCKCCK是一种由胃肠道黏膜细胞分泌的多肽类激素,在体内分布非常广泛,具有多种生物学功能。在消化方面,具有刺激胰液分泌和胆囊收缩、延缓胃排空等作用;在中枢及外周神经系统方面,具有抑制摄食、降低体温和对抗吗啡和内啡肽的镇痛效应[47]。CCK所有的生物学效应均是通过作用于相应受体来实现的。CCK受体分为CCK-A和CCK-B受体2种亚型,两者都属于G蛋白偶联受体,具有50%的同源性。CCK-A受体主要分布在胰腺腺泡、胆囊、幽门平滑肌、迷走神经传入纤维等处,脑中也有少量分布,且硫化CCK对CCK-A受体的亲和力比非硫化CCK大1 000倍;CCK-B受体主要分布在脑和胃中,且非硫化CCK和胃泌素对CCK-B受体有较大的亲和力。CCK主要通过与CCK-A受体作用发挥饱感信号功能,而且只有硫化的CCK才能起到食欲抑制作用[48]。CCK受体神经元集中于孤束核、脑桥中部、下丘脑等处,向这些部位注射CCK能够引起明显的食欲抑制,表明存在于这些部位的CCK可能作为神经递质或神经调质参与中枢神经系统的摄食调节[49, 50]。研究发现,相对酪蛋白和大豆蛋白,土豆蛋白提取物能抑制大鼠的食欲,并提高血浆CCK水平[51]。大豆蛋白提取物在小鼠上的研究也得出类似的结果[52]。
鱼类的CCK免疫阳性反应以及mRNA在其胃肠道、神经系统和肝脏等组织中均被检测到。目前已经公布了斑马鱼、大西洋鲑、大西洋鳕、美国红鱼、虹鳟、罗非鱼、草鱼、白斑角鲨等的CCK mRNA序列,且主要研究了营养状况(饥饿或投喂)对某些鱼类CCK表达的影响[27, 53]。CCK是控制鱼类食欲的关键调控因子,研究发现给欧洲鲈口服CCK后,可诱发抑制食欲的效果,能调控欧洲鲈总的食物和单一营养素的摄入量,且这种作用可以被CCK受体拮抗剂丙谷胺的效果抵消[54]。
1.4 NPYNPY是由36个氨基酸组成的高度保守的活性单链多肽,该肽链折叠成发夹结构,Y是指分子两端的酪氨酸残基,它的结构与36个氨基酸的胰多肽和肽YY(PYY)极其相似,故认为同属胰多肽家族。其作用主要有促进动物采食、影响激素分泌、调节体温、生物节律、性行为及情绪等作用[55]。NPY至少有6种受体亚型(Y1~Y6受体),Y1和Y5受体在食欲控制方面起作用,Y1和T5受体的拮抗剂能抑制采食。将NPY直接注射到小鼠下丘脑和脑心室中,可以提高动物的采食量,增加体重,并减少体热产生[56, 57]。
对鱼类NPY的研究不如对哺乳类研究的清楚,但目前也有了相当的进展。现已克隆了斑马鱼、虹鳟、大西洋鲑、金鱼、大西洋鳕、斜带石斑鱼、半斑点叉尾 、南方鲇、日本鳗等的NPY基因[1]。与哺乳类相类似,鱼类NPY同样通过与特殊受体结合而对摄食活动起促进作用,这在金鱼和斑马鱼上进行了比较系统的研究,同时作为一种促进食欲因子能增加鱼类的摄食量和降低活动力,并影响鱼类的情绪、血管收缩、昼夜节律和垂体激素的分泌等[58]。将Y1受体激动剂注射入金鱼脑室可促进由NPY诱导的摄食活动,但供给金鱼脑室Y2受体激动剂2 h后并不能明显促进金鱼的摄食活动。由此推测,在金鱼中,NPY通过与Y1样受体(Y1和Y5受体)而不是Y2受体对摄食起调控作用[59]。斑马鱼在饥饿7 d后,下丘脑NPY mRNA水平较正常鱼显著升高;将NPY注入斑马鱼内脑室,其摄食显著增加;注入Y1受体抑制剂后其摄食显著下降[60]。
1.5 食欲肽食欲肽是下丘脑神经元分泌的一种神经递质或神经调质,主要作用是促进摄食,增加体重。食欲肽分为2个型:食欲肽A和食欲肽B,食欲肽A的作用大于食欲肽B。向脑室内注射或者直接向外侧下丘脑中注射食欲肽都可以增加啮齿类动物的摄入量,并且有一定的剂量-反应关系[61]。食欲肽与血液中葡萄糖、甘油三酯含量密切相关。动物试验结果显示食欲肽不仅可以抑制肠内葡萄糖的吸收,还可以影响胃排空时间[62]。Wortley等[63]通过动物试验研究发现喂饲高脂膳食后,食欲肽基因表达增加,并与甘油三酯含量的增加密切相关,提示食欲肽是一种肥胖易感肽,可对周围血脂的增加作出应答。
目前在爪蟾蜍、斑马鱼、河豚、罗非鱼、大西洋鲑、金鱼、大西洋鳕、青鳉、石斑鱼等鱼类上报道了食欲肽基因序列,其主要分布于脑部[64]。在金鱼上发现将食欲肽A激动剂注射入金鱼脑室可促进摄食活动,且提高脑内食欲肽A mRNA的表达丰度[65],并且在斑马鱼上食欲肽A的作用效果优于食欲肽B[66]。对布氏海猪鱼脑部注射28 pmol/g体重的食欲肽A,其食欲显著提高[67];饥饿能显著提高石斑鱼脑垂体食欲肽前体mRNA的表达[68]。以上结果表明食欲肽在鱼类上亦能促进食欲。
1.6 PYYPYY,又名酪酪肽,由胃肠道的L细胞分泌,其免疫活性在直肠最高,小肠较低,在人类下丘脑髓质及大鼠中枢神经包括下丘脑、脊髓、髓鞘也有表达。PYY为肽类物质,具有类激素作用,动物摄食后,PYY释放随血液循环进入效应器官,抑制胃酸的分泌,延迟胃排空以及食糜在小肠内转动,从而起到抑制食欲的作用。PYY在循环中有2种形式,包括PYY3-36和PYY3-37,两者具有相同的生物学作用,其中循环中的主要形式是PYY3-36[69]。PYY被认为是饱食的标志性信号,在正常人的试验中发现,高蛋白质饮食使血浆和机体中PYY水平均显著升高,且PYY水平和饱食感表现出一致性;在小鼠上的长期高蛋白质饮食试验亦证明高蛋白质饮食可以减轻体重并促进PYY的合成,可通过外源注射或摄入PYY来抑制食欲进而达到减肥的目的[70]。研究发现,肥胖者餐后体内PYY水平升高平缓,餐后2 h没有明显的高峰出现[71];高蛋白质饮食会导致人餐后体内PYY水平显著升高[72]。
PYY在海鲈脑部大量表达[73];草鱼的前肠PYY mRNA表达量在摄食3 h后达到最高[74];在西伯利亚鲟上发现饥饿导致PYY mRNA表达量显著降低,注射PYY到鱼体后其食欲显著下降[75]。这表明PYY在鱼类上主要起抑制食欲的作用。
1.7 胰高血糖素样肽-Ⅰ(GLP-Ⅰ)GLP-Ⅰ是由肠道L细胞分泌的肠促胰岛素,其受体存在于下丘脑,其中在弓状核(ARC)、室旁核(PVN)及视上核高表达。GLP-Ⅰ与其受体结合后,促进葡萄糖依赖的胰岛素分泌、胰岛β细胞增殖和分化并抑制其凋亡、延迟胃排空,但不引起体重增加和低血糖,从而保护了胰岛β细胞功能[76]。研究发现,高蛋白质饮食会导致人血浆GLP-Ⅰ水平上升[77],但也有人认为蛋白质饮食不影响人血浆GLP-Ⅰ水平[40]。这可能是套餐饮食中碳水化合物含量不同所导致,GLP-Ⅰ对碳水化合物的调节作用更为明显[78]。
与其他脊椎动物不同,鱼类GLP-Ⅰ并不能当作一种肠降血糖素,且其主要效应部位是肝脏,其先调节肝脏的糖原合成、糖异生和脂肪代谢等,然后才在肠道和脑发挥调节作用[79]。在斑点叉尾 的脑室内注入0.25 ng/g体重的GLP-Ⅰ,其摄食量显著降低50%,但在腹膜和静脉中注射GLP-Ⅰ却无显著影响[80]。目前,在鱼类上对GLP-Ⅰ调节机制的研究仅在虹鳟上较系统地开展,研究发现外周组织中注射GLP-Ⅰ可导致虹鳟持续的高血糖症,中枢组织注射GLP-Ⅰ可导致血浆中葡萄糖含量的增加,结果表明对血液中葡萄糖的调节部分取决于迷走神经和内脏的GLP-Ⅰ分泌[81]。在鱼类上GLP-Ⅰ的调节模型与哺乳动物不同,在哺乳动物上是通过肠-胰-脑轴来实现对葡萄糖代谢的调节,而鱼类可能是更低级的肠脑轴[82]。GLP-Ⅰ引起的高血糖症和缺乏肠促胰岛素功能,可能与肉食性鱼类葡萄糖耐受力不良有关[83]。
1.8 CARTCART是一种下丘脑神经肽食欲抑制因子,它可能作用于外侧下丘脑食欲肽系统,在人类上被认为是一种重要的抑制食欲相关肽[84]。研究发现,在小鼠下丘脑注入CART并不能促使5-羟色胺的分泌,且饥饿使其mRNA表达量显著下降,恢复投喂后表达量升高,这也表明CART是一种影响摄食行为的抑制因子[85]。下丘脑CART系统同样受到leptin和胰岛素的调节,CART与leptin出现同步趋势变化[86]。大西洋鲑的CART含118个氨基酸,mRNA全长为742 bp,主要在脑和眼睛中表达,且存在3~6个亚型,但表达量很低[87]。在大西洋鲑上发现,水温对其摄食的影响中,CART是关键的调控因子,CART mRNA表达量与摄食率呈负相关[88]。斑点叉尾 后脑的CART水平受到饥饿、2-脱氧-D-葡萄糖(葡萄糖代谢拮抗剂)、葡萄糖、leptin和胰岛素水平的调节,饥饿48 h和饲喂2-脱氧-D-葡萄糖使CART免疫反应显著减弱;葡萄糖使其免疫反应显著增强;leptin和胰岛素对斑点叉尾 的CART均有正调节作用,且体外切片孵育试验结果与之一致[86]。
1.9 胰岛素胰岛素是由胰岛β细胞受内源性或外源性物质如葡萄糖、乳糖、核糖、精氨酸、胰高血糖素等的刺激而分泌的一种蛋白质激素,由A、B链组成,共含51个氨基酸残基,能增强细胞对葡萄糖的摄取利用,对蛋白质及脂质代谢有促进合成的作用。胰岛素作为一个肥胖症信号可能和leptin具有相似的功能[89]。虽然胰岛素不是从脂肪细胞中释放,但是胰岛素的基础循环水平和体脂水平有相关关系。进食含蛋白质较多的食物后,血液中氨基酸浓度升高,胰岛素分泌也增加[90]。血浆胰岛素的水平随摄入的碳水化合物含量而变化,高碳水化合物会导致血浆胰岛素水平升高[70]。不同类型蛋白质对胰岛素分泌产生的影响有差异。与鱼肉蛋白或大豆蛋白相比较,牛奶蛋白能更有效地促使人胰岛素的分泌[91],这表明容易消化的蛋白质更能刺激胰岛素的分泌。此外,研究发现亮氨酸、酪氨酸、谷氨酸、甜菜碱以及支链氨基酸等对胰岛素的分泌也有刺激作用[90, 92, 93, 94, 95]。
目前,对于鱼类胰岛素的研究主要集中在胰岛素与糖代谢关系上,认为鱼类血浆胰岛素水平不足是造成其高血糖及影响鱼类糖利用能力的主要因素,同样鱼类胰岛素受到营养状况、碳水化合物水平、糖源、脂肪水平、精氨酸水平等的影响[96]。在胰岛素对鱼类食欲调节方面,投饲频率增加会促进欧洲鲈鱼胰岛素的分泌[97];蛋白质源和脂肪源均对金头鲷血浆胰岛素水平无显著影响[98];向南美白对虾饲料中添加微囊牛胰岛素后发现并不能显著提高其体增重,但能提高蛋白质合成量和免疫力[99]。这表明胰岛素对鱼类食欲的调节因物种或营养状况并未表现出一致的结论,或许胰岛素并不显著调节鱼类的食欲。
2 食欲调节的中枢信号通路动物食欲的生理调节是个非常复杂的神经-体液调节过程,涉及外周食欲感受装置和中枢神经系统之间的一系列相互作用机制,即各种外周的食欲相关物理或化学信号(包括神经冲动如视觉、嗅觉、味觉及触觉等,以及来自胃肠道各种感受器如机械、温度、化学和渗透压感受器等的信号,或体液信号如leptin、ghrelin、血糖水平等)通过神经系统或体液传递途径到达相应的中枢神经系统,然后汇聚在大脑皮层的特定区域形成食欲的感觉,并最终影响动物的采食行为[100],具体见图 2。下丘脑是食欲调控中一个极为重要的关键区域,下丘脑各神经区域通过接受、整合、发放食欲信号相互联系,相互影响,从而达到对食欲的调节。有人提出经典的“双重中心理论”,即:“外侧下丘脑”饥饿中心和“腹内侧核”饱感中心。下丘脑基底部的一些神经核被看作是调控能量平衡的关键部位,尤其是那些与食欲调节相关的神经区域更引起研究人员的广泛关注。这些下丘脑区域包括孤束核(NTS)、ARC、外侧下丘脑(LH)、PVN、视交叉上核(SCN)、腹内侧核(VMN)、背中核(DMN)等。其中NTS的头端接受味觉纤维,并对进食有关的传入信息进行整合,ARC、LH是食欲信号合成和释放的区域,PVN是食欲信号相互作用的区域,而SCN、VMN、DMN是调控食欲信号的区域[101, 102, 103]。中枢信号通路对食欲的调节为长期作用,下丘脑是中枢信号通路中一个极为重要的关键区域,现在研究较多的是5′-磷酸腺苷激活蛋白激酶(AMPK)信号通路和雷帕霉素靶蛋白(TOR)信号通路。
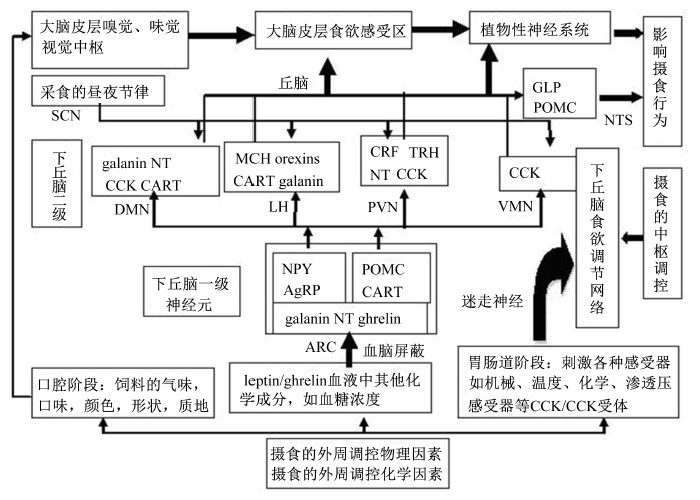 | 图2 动物食欲调节机制以及摄食调控示意图 Fig.2 Schematic diagram of appetite-regulating mechanism and feeding regulation |
下丘脑的各个区域(ARC、PVN、DMN、VMN和LH)都有AMPK的表达,AMPK是一个异源三聚体,包括催化亚基α、调节亚基β和γ亚基的丝氨酸/苏氨酸蛋白激酶,α、β亚基均存在2个亚型,γ亚基存在3个亚型。其中α亚基N端存在1个激酶激活域,C端是与β和γ亚基结合的部位;β亚基中心的保守区域则被认为是糖原结合域;γ亚基的N端后面有4个串联重复序列,每个串联重复序列由60个氨基酸构成,被命名为CBS,每1 对形成1个Bateman域,可以结合1分子的 AMP[104]。
在正常生理情况下,为了维持基本的代谢需要,细胞中保持着高水平的ATP。在多数真核细胞中,ATP与ADP的比值约为10:1,而且在很小的范围内变化,AMPK处于失活状态。只有当代谢性应激引起细胞内AMP与ATP的比值升高时,如当细胞缺血、缺氧、葡萄糖缺乏等[105],AMPK才被激活,激活后磷酸化下游的信号分子,关闭消耗ATP的合成代谢途径,开启产生ATP的分解代谢途径,即抑制糖类、脂质和胆固醇的合成等,促进脂肪酸氧化和葡萄糖的转运;反之,当AMP与ATP的比值降低时,AMPK则促进合成代谢。由此可见,AMP是调节AMPK的关键,其作用过程为AMP通过结合AMPK的γ亚基引起AMPK的构象改变,一方面可以直接增加酶的活性,另一方面可以使其构象改变,更有利于AMPK激酶对其磷酸化,同时拮抗蛋白磷酸酶的去磷酸化作用,进而增加AMPK活性,这个过程可被高水平的ATP抑制。因此,AMPK被称为细胞能量感受器,广泛参与细胞内的物质代谢。最开始认为,AMP与ATP的比值升高是激活AMPK的经典途径,随着研究的深入,后来发现许多激素、细胞因子以及某些胞外配体,都参与了AMPK信号途径[106]。leptin能够选择性地激活骨骼肌中的AMPK-α2亚基,包括直接对骨骼肌的快速激活作用和依赖于下丘脑-交感神经系统的长期激活作用,同时leptin对肌肉脂肪酸代谢的调控作用需要通过AMPK的调节来实现[107]。黑皮质素受体激动剂能抑制AMPK的活性,但是野鼠相关蛋白能提高其活性[108]。
AMPK对摄食的调节也起到非常关键的作用,其下丘脑和外周组织的主要调节途径是:AMPK→乙酰辅酶A羧化酶(ACC)→丙二酰辅酶A→肉碱棕榈酰基转移酶1(CPT1)[109]。激活下丘脑的AMPK可增加下丘脑ARC NPY的表达,增加食物的摄入,减少能量消耗;而抑制下丘脑的AMPK可以减少NPY的表达,减少食物的摄入,增加能量消耗[110]。leptin和ghrelin均可作用于下丘脑,通过AMPK调节食欲:leptin可抑制下丘脑的AMPK,使NPY释放减少,减少食物摄入;ghrelin则可激活下丘脑的AMPK,使NPY释放增加,增加食物的摄入[111, 112]。
目前在鱼类上也有关于AMPK信号通路的报道。在虹鳟上,AMPK信号通路是调节肝脏能量平衡的主要信号通路[113],尽管饲料脂肪水平对虹鳟摄食量无显著影响,但高脂组的虹鳟下丘脑AMPK蛋白磷酸化水平较低脂组显著升高,因此认为AMPK信号通路主要影响了虹鳟对脂肪酸的利用率[114];另外,高碳水化合物水平可显著抑制AMPK蛋白磷酸化水平[115]。在鳎上的研究发现,高脂组AMPK的活性较低脂组显著上升,但是否缺氧对其无显著影响[116];同样,金鱼各组织中AMPK蛋白磷酸化水平在低氧条件下无显著变化[117];在石斑鱼上,饥饿3周使AMPK蛋白磷酸化水平显著上升,短期投喂使其显著下降,但长期投喂对其反而无显著影响[118]。以上结果表明,鱼类AMPK信号通路受外界环境的影响与哺乳类存在差异,如缺氧并不能导致其被激活,但在对能量的调节上类似。关于AMPK信号通路对食欲的调节有待进一步研究。
2.2 TOR信号通路TOR是一种丝/苏氨酸蛋白激酶,是共有2 549个氨基酸的蛋白,从酵母到哺乳动物其广泛存在,且存在于各种细胞中,进化十分保守。因为在TOR的C末端有1个激酶结构域(kinase domain),约有234个氨基酸,因此TOR属于磷脂酰肌醇3-激酶相关激酶(phosphatidylinositol 3-kinase-related kinase,PIKK)蛋白家族[91]。TOR的N端有20个串联的HEAT重复序列(即huntignton、EF3、PP2A的1个亚基、TOR1),C末端的FATC结构域(focal adhesion targeting domain of C-ternimal)被认为与FAT结构域(FRAP-ATM-TRAP domain)相互作用,从而暴露出激酶结构域[119]。
TOR在营养物质感知、调节细胞生长和增殖、调控细胞周期等多个方面起到重要作用[120]。TOR与其他蛋白结合,形成了2种复合体TORC1和TORC2,前者对雷帕霉素敏感,后者不敏感[121]。TORC1能促进细胞的蛋白质合成与代谢以及核酸的合成与转录,并抑制自体吞噬,TORC2可能参与了细胞骨架的形成。目前对TORC1的研究较多,且认为TOR信号通路的上游刺激因子主要有生长因子与胰岛素、营养因子、能量以及压力[122]。
TOR信号通路其上游效应器主要有Rheb(Ras homolog enriched in brain)、结节性硬化症1(tuberous sclerosis complex 1,TSC1)和结节性硬化症2(tuberous sclerosis complex 2,TSC2),其中Rheb是具有GTP酶(GTPase)活性的蛋白,其活性状态为Rheb-GTP,可结合于TOR,并对TOR进行正调节,同时其活性受到TSC1和TSC2的调节[123]。TSC1和TSC2形成复合物对TOR信号通路的下游进行调节,TSC2是GTPase激活蛋白,可使Rheb-GTP水解形成Rheb-GDP状态,从而失活,对TOR进行负调节。TOR下游效应器主要有4E结合蛋白(4E-binding proteins,4E-BPs)和核糖体S6蛋白激酶(ribosomal protein S6 kinases,S6Ks),前者是真核细胞翻译起始因子,结合于mRNA的5′-cap的真核翻译起始因子4G(eIF4G),进而启动5′-cap mRNA的翻译,后者是核糖体蛋白激酶,哺乳动物中存在2种形式,其主要影响细胞的蛋白质合成和生长[123]。
TOR调控营养物质,如葡萄糖、氨基酸和脂肪酸等的摄入,并影响激素的分泌,TOR信号通路的激活能抑制机体的摄食量[124, 125]。TOR对食欲调节主要是通过磷脂酰肌醇3-激酶(PIK3)→ 3-磷酸肌醇依赖性蛋白激酶1(PDK1)→蛋白激酶B(PKB/Akt)→TSC1/TSC2→Rheb→TORC1→4E-BPs这条途径,最终通过抑制NPY/野鼠相关蛋白(AgRP)的活性来实现对食欲的抑制调节[126]。关于营养物质对TOR信号通路的调节,目前对氨基酸的研究较多,氨基酸主要作用于TORC1,且主要是抑制TORC1信号通路来降低食欲[127]。如亮氨酸可以直接激活下丘脑TOR信号通路发挥食欲调节作用,主要是通过抑制AgRP的活性来实现[124, 128]。
鱼类TOR基因与人类的同源性达到90%以上,鲤鱼与斑马鱼的TOR基因同源性达到97%以上[129, 130],目前鱼类TOR信号通路研究还处在初步阶段。在虹鳟上的研究发现,雷帕霉素抑制TOR信号通路中的TORC1及下游因子(S6、S6K1和4E-BP1)的蛋白磷酸化水平,但不影响上游的Akt及TORC2的蛋白磷酸化水平[131],同时碳水化合物水平显著激活了TOR磷酸化[113]。关于营养状况对其影响的研究发现,短期饥饿使石斑鱼TOR磷酸化被显著抑制,而恢复投喂后无论时间长短均使TOR被显著激活[118]。国内学者也开展了营养素对TOR信号通路转录水平的影响研究:对鲫鱼的研究发现,饲料中添加0.54%精氨酸显著降低了肝脏和肌肉中TOR和S6K1的mRNA表达量,但不影响4E-BP2 mRNA的表达量[132];随饲料亮氨酸水平的升高,团头鲂肝脏中TOR mRNA的表达量亦显著升高[133];色氨酸抑制了建鲤肌肉和肝脏中TOR mRNA的表达,促进了中肠和后肠TOR和4E-BP mRNA的表达[134];亮氨酸和精氨酸可提高饥饿处理后的中国对虾TOR和S6K1蛋白磷酸化水平及其mRNA的表达量[135]。这些研究均显示氨基酸对水产动物TOR信号通路的关键因子在转录水平上有显著影响,而在高等动物上的结果大多体现在蛋白水平上,因此需要进一步通过蛋白水平来确认鱼类与哺乳类的差异。
3 研究展望 3.1 鱼类食欲调节因子的功能研究综上所述,在鱼类上目前仅围绕个别食欲调节因子如leptin、NPY和ghrelin,开展了其对食欲调控研究,并且大多研究仅限于基因克隆,未进行进一步的功能研究,特别是缺乏对相关调节因子的受体的研究。因此,需要从食欲调节因子的功能及其受体进一步深入研究其在鱼类食欲调控中的作用,基于此方面的研究,开发出促进鱼类食欲的专用生理调控剂。
3.2 鱼类食欲调控信号通路目前关于鱼类食欲调节的中枢信号通路并未见完整的报道,同时由于在进化上鱼类远远低于哺乳类,因此鱼类的信号通路不可完全借鉴高等动物。如何在现有研究的基础上开展鱼类食欲调控信号通路的研究,找出关键的调控位点,理清各因子的作用途径及其交互作用,理顺鱼类特有的食欲调控信号通路,这是现今鱼类食欲调控研究的难点,也是有效调控鱼类摄食行为的前提和保障。
| [1] | MALCOLM J,ANDERS A,CHRIS N,et al.Appetite and feed intake[M]//FELICITY H,MALCOLM J,SUNIL K.Aquaculture and behavior.New York:Wiley-Blackwell,2012:183-219.( 3) 3) |
| [2] | VOLKOFF H.The role of neuropeptide Y,orexins,cocaine and amphetamine-related transcript,cholecystokinin,amylin and leptin in the regulation of feeding in fish[J].Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2006,144(3):325-331.( 1) 1) |
| [3] | ZHANG Y Y,PROENCA R,MAFFEI M,et al.Positional cloning of the mouse obese gene and its human homologue[J].Nature,1994,372(6505):425-432.( 1) 1) |
| [4] | ZHANG F M,BASINSKI M B,BEALS J M,et al.Crystal structure of the obese protein leptin-E100[J].Nature,1997,387(6629):206-209.( 1) 1) |
| [5] | DEVOS R,GUISEZ Y,VAN DER HEYDEN J,et al.Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding[J].Journal of Biological Chemistry,1997,272(29):18304-18310.( 1) 1) |
| [6] | 李海艳,陈萌,孔祥玉.瘦素及其受体与消化系统关系的研究进展[J].承德医学院学报,2012,29(2):187-189.( 1) 1) |
| [7] | SHIGEMURA N,OHTA R,KUSAKABE Y,et al.Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures[J].Endocrinology,2004,145(2):839-847.( 1) 1) |
| [8] | 张崇本.瘦蛋白介导的人食欲与体重调节机制[J].生命的化学,2004,24(3):274-275.( 1) 1) |
| [9] | SCHWARTZ M W,WOODS S C,PORTE D,et al.Central nervous system control of food intake[J].Nature,2000,404(6778):661-671.( 1) 1) |
| [10] | SCHWARTZ M W.Central nervous system regulation of food intake[J].Obesity,2006,14(Suppl.2):1S-8S.( 1) 1) |
| [11] | MORTON G J,CUMMINGS D E,BASKIN D G,et al.Central nervous system control of food intake and body weight[J].Nature,2006,443(7109):289-295.( 1) 1) |
| [12] | PÉREZ-PÉREZ A,GAMBINO Y,MAYMÓ J,et al.MAPK and PI3K activities are required for leptin stimulation of protein synthesis in human trophoblastic cells[J].Biochemical and Biophysical Research Communications,2010,396(4):956-960.( 1) 1) |
| [13] | WEIGLE D S,BREEN P A,MATTHYS C C,et al.A high-protein diet induces sustained reductions in appetite,ad libitum caloric intake,and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations[J].The American Journal of Clinical Nutrition,2005,82(1):41-48.( 1) 1) |
| [14] | CARBÓ N,RIBAS V,BUSQUETS S,et al.Short-term effects of leptin on skeletal muscle protein metabolism in the rat[J].The Journal of Nutritional Biochemistry,2000,11(9):431-435.( 1) 1) |
| [15] | 毛湘冰,曾祥芳,蔡传江,等.日粮中添加亮氨酸对生长大鼠血浆瘦素水平和骨骼肌蛋白质代谢的影响[J].中国畜牧杂志,2011,47(15):26-30.( 1) 1) |
| [16] | MAO X B,ZENG X F,WANG J J,et al.Leucine promotes leptin receptor expression in mouse C2C12 myotubes through the mTOR pathway[J].Molecular Biology Reports,2011,38(5):3201-3206.( 1) 1) |
| [17] | JOHNSON R M,JOHNSON T M,LONDRAVILLE R L.Evidence for leptin expression in fishes[J].The Journal of Experimental Zoology,2000,286(7):718-724.( 1) 1) |
| [18] | 李观贵.草鱼瘦素的基因结构、重组表达与功能研究[D].硕士学位论文.广州:暨南大学,2010.( 1) 1) |
| [19] | CAO Y B,XUE J L,WU L Y,et al.The detection of 3 leptin receptor isoforms in crucian carp gill and the influence of fasting and hypoxia on their expression[J].Domestic Animal Endocrinology,2011,41(2):74-80.( 1) 1) |
| [20] | RØNNESTAD I,NILSEN T O,MURASHITA K,et al.Leptin and leptin receptor genes in Atlantic salmon:cloning,phylogeny,tissue distribution and expression correlated to long-term feeding status[J].General and Comparative Endocrinology,2010,168(1):55-70.( 2) 2) |
| [21] | DE PEDRO N,MARTÍNEZ-ÁLVAREZ R,DELGADO M J.Acute and chronic leptin reduces food intake and body weight in goldfish(Carassius auratus)[J].Journal of Endocrinology,2006,188(3):513-520.( 1) 1) |
| [22] | MURASHITA K,UJI S,YAMAMOTO T,et al.Production of recombinant leptin and its effects on food intake in rainbow trout(Oncorhynchus mykiss)[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2008,150(4):377-384.( 1) 1) |
| [23] | LI G G,LIANG X F,XIE Q L,et al.Gene structure,recombinant expression and functional characterization of grass carp leptin[J].General and Comparative Endocrinology,2010,166(1):117-127.( 1) 1) |
| [24] | LU R H,LIANG X F,WANG M,et al.The role of leptin in lipid metabolism in fatty degenerated hepatocytes of the grass carp Ctenopharyngodon idellus[J].Fish Physiology and Biochemistry,2012,38(6):1759-1774.( 1) 1) |
| [25] | BALTZEGAR D A,READING B J,DOUROS J D,et al.Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes[J].Journal of Endocrinology,2014,220(1):61-72.( 1) 1) |
| [26] | FRANCIS D S,THANUTHONG T,SENADHEERA S P S D,et al.n-3 LC-PUFA deposition efficiency and appetite-regulating hormones are modulated by the dietary lipid source during rainbow trout grow-out and finishing periods[J].Fish Physiology and Biochemistry,2014,40(2):577-593.( 1) 1) |
| [27] | TIAN J,HE G,MAI K S,et al.Effects of postprandial starvation on mRNA expression of endocrine-,amino acid and peptide transporter-,and metabolic enzyme-related genes in zebrafish(Danio rerio)[J].Fish Physiology and Biochemistry,2015,41(3):773-787.( 2) 2) |
| [28] | GNANAPAVAN S,KOLA B,BUSTIN S A,et al.The Tissue distribution of the mRNA of ghrelin and subtypes of its receptor,GHS-R,in humans[J].The Journal of Clinical Endocrinology & Metabolism,2002,87(6):2988-2991.( 1) 1) |
| [29] | MASSADI O A,TSCHÖP M H,TONG J.Ghrelin acylation and metabolic control[J].Peptides,2011,32(11):2301-2308.( 2) 2) |
| [30] | GRANATA R,SETTANNI F,BIANCONE L,et al.Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic β-cells and human islets:involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A,extracellular signal-regulated kinase 1/2,and phosphatidyl inositol 3-kinase/akt signaling[J].Endocrinology,2007,148(2):512-529.( 1) 1) |
| [31] | BALDANZI G,FILIGHEDDU N,CUTRUPI S,et al.Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT[J].The Journal of Cell Biology,2002,159(6):1029-1037.( 1) 1) |
| [32] | RODRÍGUEZ A,GÓMEZ-AMBROSI J,CATALÁN V,et al.Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes[J].International Journal of Obesity,2009,33(5):541-552.( 1) 1) |
| [33] | ASAKAWA A,INUI A,FUJIMIYA M,et al.Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin[J].Gut,2005,54(1):18-24.( 1) 1) |
| [34] | GUTIERREZ J A,SOLENBERG P J,PERKINS D R,et al.Ghrelin octanoylation mediated by an orphan lipid transferase[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(17):6320-6325.( 1) 1) |
| [35] | SHⅡYA T,NAKAZATO M,MIZUTA M,et al.Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion[J].The Journal of Clinical Endocrinology & Metabolism,2002,87(1):240-244.( 1) 1) |
| [36] | LIU J H,PRUDOM C E,NASS R,et al.Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men[J].The Journal of Clinical Endocrinology & Metabolism,2008,93(5):1980-1987.( 1) 1) |
| [37] | TSCHÖP M,SMILEY D L,HEIMAN M L.Ghrelin induces adiposity in rodents[J].Nature,2000,407(6806):908-913.( 1) 1) |
| [38] | ENGLISH P J,GHATEI M A,MALIK I A,et al.Food fails to suppress ghrelin levels in obese humans[J].The Journal of Clinical Endocrinology & Metabolism,2002,87(6):2984-2987.( 1) 1) |
| [39] | ERDMANN J,LIPPL F,SCHUSDZIARRA V.Differential effect of protein and fat on plasma ghrelin levels in man[J].Regulatory Peptides,2003,116(1/2/3):101-107.( 1) 1) |
| [40] | SMEETS A J,SOENEN S,LUSCOMBE-MARSH N D,et al.Energy expenditure,satiety,and plasma ghrelin,glucagon-like peptide 1,and peptide tyrosine-tyrosine concentrations following a single high-protein lunch[J].The Journal of Nutrition,2008,138(4):698-702.( 2) 2) |
| [41] | KIRCHNER H,GUTIERREZ J A,SOLENBERG P J,et al.GOAT links dietary lipids with the endocrine control of energy balance[J].Nature Medicine,2009,15(7):741-745.( 1) 1) |
| [42] | 马细兰,刘晓春,周立斌,等.鱼类ghrelin研究进展[J].水生生物学报,2009,33(3):546-551.( 1) 1) |
| [43] | RILEY L G,FOX B K,KAIYA H,et al.Long-term treatment of ghrelin stimulates feeding,fat deposition,and alters the GH/IGF-I axis in the tilapia,Oreochromis mossambicus[J].General and Comparative Endocrinology,2005,142(1/2):234-240.( 1) 1) |
| [44] | MATSUDA K,MIURA T,KAIYA H,et al.Regulation of food intake by acyl and des-acyl ghrelins in the goldfish[J].Peptides,2006,27(9):2321-2325.( 1) 1) |
| [45] | JÖNSSON E,KAIYA H,BJÖRNSSON B T.Ghrelin decreases food intake in juvenile rainbow trout(Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system[J].General and Comparative Endocrinology,2010,166(1):39-46.( 1) 1) |
| [46] | RILEY L G,FOX B K,BREVES J P,et al.Absence of effects of short-term fasting on plasma ghrelin and brain expression of ghrelin receptors in the tilapia,Oreochromis mossambicus[J].Zoological Science,2008,25(8):821-827.( 1) 1) |
| [47] | LIDDLE R A.Cholecystokinin cells[J].Annual Review of Physiology,1997,59:221-242.( 1) 1) |
| [48] | REHFELD J F.Cholecystokinin[J].Best Practice & Research Clinical Endocrinology & Metabolism,2004,18(4):569-586.( 1) 1) |
| [49] | REHFELD J F,FRⅡS-HANSEN L,GOETZE J P,et al.The biology of cholecystokinin and gastrin peptides[J].Current Topics in Medicinal Chemistry,2007,7(12):1154-1165.( 1) 1) |
| [50] | WANK S A.Cholecystokinin receptors[J].The American Journal of Physiology,1995,269(5):G628-G646.( 1) 1) |
| [51] | CHEN W Y,HIRA T,NAKAJIMA S,et al.Suppressive effect on food intake of a potato extract(potein®) involving cholecystokinin release in rats[J].Bioscience,Biotechnology,and Biochemistry,2012,76(6):1104-1109.( 1) 1) |
| [52] | HIDAYAT M,LADI J E.Increasing of plasma cholecystokinin level and jejunum histological changes after treatment with soybean extracts protein[J].HAYATI Journal of Biosciences,2012,19(2):53-59.( 1) 1) |
| [53] | FENG K,ZHANG G R,WEI K J,et al.Molecular characterization of cholecystokinin in grass carp(Ctenopharyngodon idellus):cloning,localization,developmental pro le,and effect of fasting and refeeding on expression in the brain and intestine[J].Fish Physiology and Biochemistry,2012,38(6):1825-1834.( 1) 1) |
| [54] | RUBIO V C,SÁNCHEZ-VÁZQUEZ F J,MADRID J A.Role of cholecystokinin and its antagonist proglumide on macronutrient selection in European sea bass Dicentrarchus labrax,L[J].Physiology & Behavior,2008,93(4/5):862-869.( 1) 1) |
| [55] | TATEMOTO K,CARLQUIST M,MUTT V.Neuropeptide Y-a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide[J].Nature,1982,296(5858):659-660.( 1) 1) |
| [56] | RAPOSINHO P D,CASTILLO E,D'ALLEVES V,et al.Chronic blockade of the melanocortin 4 receptor subtype leads to obesity independently of neuropeptide y action,with no adverse effects on the gonadotropic and somatotropic axes[J].Endocrinology,2000,141(12):4419-4427.( 1) 1) |
| [57] | GRUNDEMAR L,JONAS S E,MÖRNER N,et al.Characterization of vascular neuropeptide Y receptors[J].British Journal of Pharmacology,1992,105(1):45-50.( 1) 1) |
| [58] | MATSUDA K,SAKASHITA A,YOKOBORI E,et al.Neuroendocrine control of feeding behavior and psychomotor activity by neuropeptide Y in fish[J].Neuropeptides,2012,46(6):275-283.( 1) 1) |
| [59] | DE PEDRO N,LÓPEZ-PATIÑO M A,GUIJARRO A I,et al.NPY receptors and opioidergic system are involved in NPY-induced feeding in goldfish[J].Peptides,2000,21(10):1495-1502.( 1) 1) |
| [60] | YOKOBORI E,AZUMA M,NISHIGUCHI R,et al.Neuropeptide Y stimulates food intake in the zebrafish,Danio rerio[J].Journal of Neuroendocrinology,2012,24(5):766-773.( 1) 1) |
| [61] | DUBE M G,KALRA S P,KALRA P S.Food intake elicited by central administration of orexins/hypocretins:identification of hypothalamic sites of action[J].Brain Research,1999,842(2):473-477.( 1) 1) |
| [62] | EHRSTRÖM M,LEVIN F,KIRCHGESSNER A L,et al.Stimulatory effect of endogenous orexin A on gastric emptying and acid secretion independent of gastrin[J].Regulatory Peptides,2005,132(1/2/3):9-16.( 1) 1) |
| [63] | WORTLEY K E,CHANG G Q,DAVYDOVA Z,et al.Orexin gene expression is increased during states of hypertriglyceridemia[J].American Journal of Physiology:Regulatory,Integrative and Comparative Physiology,2003,284(6):R1454-R1465.( 1) 1) |
| [64] | MATSUDA K,AZUMA M,KANG K S. Chapter eighteen-orexin system in teleost fish[J].Vitamins & Hormones,2012,89:341-361.( 1) 1) |
| [65] | MIURA T,MARUYAMA K,SHIMAKURA S I,et al.Regulation of food intake in the goldfish by interaction between ghrelin and orexin[J].Peptides,2007,28(6):1207-1213.( 1) 1) |
| [66] | WONG K K Y,NG S Y L,LEE L T O,et al.Orexins and their receptors from fish to mammals:a comparative approach[J].General and Comparative Endocrinology,2011,171(2):124-130.( 1) 1) |
| [67] | FACCIOLO R M,CRUDO M,GIUSI G,et al.Light- and dark-dependent orexinergic neuronal signals promote neurodegenerative phenomena accounting for distinct behavioral responses in the teleost Thalassoma pavo[J].Journal of Neuroscience Research,2009,87(3):748-757.( 1) 1) |
| [68] | BUCKLEY C,MACDONALD E E,TUZIAK S M,et al.Molecular cloning and characterization of two putative appetite regulators in winter flounder(Pleuronectes americanus):preprothyrotropin-releasing hormone(TRH) and preproorexin(OX)[J].Peptides,2010,31(9):1737-1747.( 1) 1) |
| [69] | UENO H,YAMAGUCHI H,MIZUTA M,et al.The role of PYY in feeding regulation[J].Regulatory Peptides,2008,145(1/2/3):12-16.( 1) 1) |
| [70] | BATTERHAM R L,HEFFRON H,KAPOOR S,et al.Critical role for peptide YY in protein-mediated satiation and body-weight regulation[J].Cell Metabolism,2006,4(3):223-233.( 2) 2) |
| [71] | MITTELMAN S D,KLIER K,BRAUN S,et al.Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY[J].Obesity,2010,18(5):918-925.( 1) 1) |
| [72] | LEIDY H J,ARMSTRONG C L H,TANG M H,et al.The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men[J].Obesity,2010,18(9):1725-1732.( 1) 1) |
| [73] | CERDÁ-REVERTER J M,MARTÍNEZ-RODRÍGUEZ G,ANGLADE I,et al.Peptide YY(PYY) and fish pancreatic peptide Y(PY) expression in the brain of the sea bass(Dicentrarchus labrax) as revealed by in situ hybridization[J].Journal of Comparative Neurology,2000,426(2):197-208.( 1) 1) |
| [74] | CHEN Y,PANDIT N P,FU J J,et al.Identification,characterization and feeding response of peptide YYb(PYYb) gene in grass carp(Ctenopharyngodon idellus)[J].Fish Physiology and Biochemistry,2014,40(1):45-55.( 1) 1) |
| [75] | CHEN H,ZHANG X,HAO J,et al.Molecular cloning,expression analysis,and appetite regulatory effect of peptide YY in Siberian sturgeon(Acipenser baerii)[J].Gene,2015,563(2):172-179.( 1) 1) |
| [76] | TURTON M D,O'SHEA D,GUNN I,et al.A role for glucagon-like peptide-1 in the central regulation of feeding[J].Nature,1996,379(6560):69-72.( 1) 1) |
| [77] | LEJEUNE M P,WESTERTERP K R,ADAM T C M,et al.Ghrelin and glucagon-like peptide 1 concentrations,24-h satiety,and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber[J].The American Journal of Clinical Nutrition,2006,83(1):89-94.( 1) 1) |
| [78] | VELDHORST M,SMEETS A,SOENEN S,et al.Protein-induced satiety:effects and mechanisms of different proteins[J].Physiology & Behavior,2008,94(2):300-307.( 1) 1) |
| [79] | MOMMSEN T P.Glucagon-like peptide-1 in fishes:the liver and beyond[J].American Zoologist,2000,40(2):259-268.( 1) 1) |
| [80] | SILVERSTEIN J T,BONDAREVA V M,LEONARD J B K,et al.Neuropeptide regulation of feeding in catfish,Ictalurus punctatus:a role for glucagon-like peptide-1(GLP-1)?[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2001,129(2/3):623-631.( 1) 1) |
| [81] | POLAKOF S,MÍGUEZ J M,SOENGAS J L.Evidence for a gut-brain axis used by glucagon-like peptide-1 to elicit hyperglycaemia in fish[J].Journal of Neuroendocrinology,2011,23(6):508-518.( 1) 1) |
| [82] | BUSBY E R,MOMMSEN T P.Glucagon-like peptide-1 in fishes:The liver and beyond[J].Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,1999,124(Suppl.1):S89.( 1) 1) |
| [83] | SOENGAS J L.Contribution of glucose- and fatty acid sensing systems to the regulation of food intake in fish.a review[J].General and Comparative Endocrinology,2014,205:36-48.( 1) 1) |
| [84] | STANLEY S A,SMALL C J,MURPHY K G,et al.Actions of cocaine- and amphetamine-regulated transcript(CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats[J].Brain Research,2001,893(1/2):186-194.( 1) 1) |
| [85] | ORLANDO G,BRUNETTI L,NISIO C D,et al.Effects of cocaine- and amphetamine-regulated transcript peptide,leptin and orexins on hypothalamic serotonin release[J].European Journal of Pharmacology,2001,430(2/3):269-272.( 1) 1) |
| [86] | SUBHEDAR N,BARSAGADE V G,SINGRU P S,et al.Cocaine- and amphetamine-regulated transcript peptide(CART) in the telencephalon of the catfish,Clarias gariepinus:distribution and response to fasting,2-deoxy-D-glucose,glucose,insulin,and leptin treatments[J].Journal of Comparative Neurology,2011,519(7):1281-1300.( 2) 2) |
| [87] | MURASHITA K,KUROKAWA T,EBBESSON L O E,et al.Characterization,tissue distribution,and regulation of agouti-related protein(AgRP),cocaine- and amphetamine-regulated transcript(CART) and neuropeptide Y(NPY) in Atlantic salmon(Salmo salar)[J].General and Comparative Endocrinology,2009,162(2):160-171.( 1) 1) |
| [88] | KEHOE A S,VOLKOFF H.The effects of temperature on feeding and expression of two appetite-related factors,neuropeptide y and cocaine- and amphetamine-regulated transcript,in atlantic cod,Gadus Morhua[J].Journal of the World Aquaculture Society,2008,39(6):790-796.( 1) 1) |
| [89] | PORTE D,Jr,BASKIN D G,SCHWARTZ M W.Leptin and insulin action in the central nervous system[J].Nutrition Reviews,2002,60(Suppl.10):S20-S29.( 1) 1) |
| [90] | NEWGARD C B,MATSCHINSKY F M.Substrate control of insulin release[M]//POLLOCK D M.Comprehensive physiology.New York:John Wiley & Sons,Inc.,2010.( 2) 2) |
| [91] | VON POST-SKAGEGÅRD M,VESSBY B,KARLSTRÖM B.Glucose and insulin responses in healthy women after intake of composite meals containing cod-,milk-,and soy protein[J].European Journal of Clinical Nutrition,2006,60(8):949-954.( 2) 2) |
| [92] | DANGIN M,BOIRIE Y,GARCIA-RODENAS C,et al.The digestion rate of protein is an independent regulating factor of postprandial protein retention[J].American Journal of Physiology:Endocrinology and Metabolism,2001,280(2):E340-E348.( 1) 1) |
| [93] | ISHIYAMA N,RAVIER M A,HENQUIN J C.Dual mechanism of the potentiation by glucose of insulin secretion induced by arginine and tolbutamide in mouse islets[J].American Journal of Physiology:Endocrinology and Metabolism,2006,290(3):E540-E549.( 1) 1) |
| [94] | FLOYD J C,Jr,FAJANS S S,CONN J W,et al.Stimulation of insulin secretion by amino acids[J].The Journal of Clinical Investigation,1966,45(9):1487-1502.( 1) 1) |
| [95] | VAN LOON L J,SARIS W H M,VERHAGEN H,et al.Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate[J].The American Journal of Clinical Nutrition,2000,72(1):96-105.( 1) 1) |
| [96] | 高进,燕磊,艾庆辉.鱼类胰岛素及胰高血糖素类激素的研究进展[J].河北渔业,2010(12):41-44.( 1) 1) |
| [97] | MESSINA M,SÃNCHEZ-GURMACHES J,NAVARRO I,et al.Feeding frequency differently affects post prandial patterns of plasma glucose,insulin and insulin-like growth factor Ⅰ in European Sea bass(Dicentrarchus labrax)[J].Turkish Journal of Fisheries and Aquatic Sciences,2014,14:921-928.( 1) 1) |
| [98] | BOURAOUI L,SÁNCHEZ-GURMACHES J,CRUZ-GARCIA L,et al.Effect of dietary fish meal and fish oil replacement on lipogenic and lipoprotein lipase activities and plasma insulin in gilthead sea bream(Sparus aurata)[J].Aquaculture Nutrition,2011,17(1):54-63.( 1) 1) |
| [99] | GONZALEZ J P,FARNÉS O C,GAXIOLA G,et al.The effects of microencapsulated bovine insulin given to Litopenaeus vannamei juveniles as a feed additive on growth,metabolism,and digestive enzyme activities[J].Aquaculture,2010,306(1/2/3/4):252-258.( 1) 1) |
| [100] | 陈黎龙,肖世平,刘万平.促食欲饲用生理调控剂的研究与开发[J].饲料与畜牧,2009(3):41-44.( 1) 1) |
| [101] | 槐瑞托,牛丽静,管振龙.孤束核的结构与功能[J].河北师范大学学报:自然科学版,2003,27(2):185-188.( 1) 1) |
| [102] | 张云波,那晓琳.食欲调节机制的研究进展[J].国外医学卫生学分册,2008,35(2):97-100.( 1) 1) |
| [103] | BERTHOUD H R.Multiple neural systems controlling food intake and body weight[J].Neuroscience & Biobehavioral Reviews,2002,26(4):393-428.( 1) 1) |
| [104] | TOWLER M C,HARDIE D G.Amp-activated protein kinase in metabolic control and insulin signaling[J].Circulation Research,2007,100(3):328-341.( 1) 1) |
| [105] | KAHN B B,ALQUIER T,CARLING D,et al.AMP-activated protein kinase:ancient energy gauge provides clues to modern understanding of metabolism[J].Cell Metabolism,2005,1(1):15-25.( 1) 1) |
| [106] | LIM C T,KOLA B,KORBONITS M.AMPK as a mediator of hormonal signalling[J].Journal of Molecular Endocrinology,2010,44(2):87-97.( 1) 1) |
| [107] | MINOKOSHI Y,KIM Y B,PERONI O D,et al.Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase[J].Nature,2002,415(6869):339-343.( 1) 1) |
| [108] | MINOKOSHI Y,ALQUIER T,FURUKAWA N,et al.AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus[J].Nature,2004,428(6982):569-574.( 1) 1) |
| [109] | XUE B Z,KAHN B B.AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues[J].The Journal of Physiology,2006,574(1):73-83.( 1) 1) |
| [110] | CHAU-VAN C,GAMBA M,SALVI R,et al.Metformin inhibits adenosine 5'-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons[J].Endocrinology,2007,148(2):507-511.( 1) 1) |
| [111] | KOHNO D,SONE H,MINOKOSHI Y,et al.Ghrelin raises[Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons[J].Biochemical and Biophysical Research Communications,2008,366(2):388-392.( 1) 1) |
| [112] | MOUNTJOY P D,BAILEY S J,RUTTER G A.Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity[J].Diabetologia,2007,50(1):168-177.( 1) 1) |
| [113] | POLAKOF S,PANSERAT S,CRAIG P M,et al.The metabolic consequences of hepatic AMP-kinase phosphorylation in rainbow trout[J].PLoS One,2011,6(5):e20228.( 2) 2) |
| [114] | LIBRÁN-PÉREZ M,GEURDEN I,DIAS K,et al.Feeding rainbow trout with a lipid-enriched diet:effects on fatty acid sensing,regulation of food intake and cellular signaling pathways[J].Journal of Experimental Biology,2015,218(16):2610-2619.( 1) 1) |
| [115] | KAMALAM B S,MEDALE F,KAUSHIK S,et al.Regulation of metabolism by dietary carbohydrates in two lines of rainbow trout divergently selected for muscle fat content[J].Journal of Experimental Biology,2012,215(15):2567-2578.( 1) 1) |
| [116] | MAZURAIS D,FERRARESSO S,GATTA P P,et al.Identification of hypoxia-regulated genes in the liver of common sole(Solea solea) fed different dietary lipid contents[J].Marine Biotechnology,2014,16(3):277-288.( 1) 1) |
| [117] | JIBB L A,RICHARDS J G.AMP-activated protein kinase activity during metabolic rate depression in the hypoxic goldfish,Carassius auratus[J].Journal of Experimental Biology,2008,211(19):3111-3122.( 1) 1) |
| [118] | FUENTES E N,SAFIAN D,EINARSDOTTIR I E,et al.Nutritional status modulates plasma leptin,AMPK and TOR activation,and mitochondrial biogenesis:implications for cell metabolism and growth in skeletal muscle of the fine flounder[J].General and Comparative Endocrinology,2013,186:172-180.( 2) 2) |
| [119] | HAY N,SONENBERG N.Upstream and downstream of mTOR[J].Genes & Development,2004,18(16):1926-1945.( 1) 1) |
| [120] | SCHMELZLE T,HALL M N.TOR,a central controller of cell growth[J].Cell,2000,103(2):253-262.( 1) 1) |
| [121] | LOEWITH R,JACINTO E,WULLSCHLEGER S,et al.Two TOR complexes,only one of which is rapamycin sensitive,have distinct roles in cell growth control[J].Molecular Cell,2002,10(3):457-468.( 1) 1) |
| [122] | WULLSCHLEGER S,LOEWITH R,HALL M N.TOR Signaling in growth and metabolism[J].Cell,2006,124(3):471-484.( 1) 1) |
| [123] | 王志刚,吴应积,旭日干.mTOR信号通路与细胞生长调控[J].生物物理学报,2007,23(5):333-342.( 2) 2) |
| [124] | COTA D,PROULX K,SMITH K A B,et al.Hypothalamic mTOR signaling regulates food intake[J].Science,2006,312(5775):927-930.( 2) 2) |
| [125] | WICZER B M,THOMAS G.The role of the mTOR pathway in regulating food intake[J].Current Opinion in Drug Discovery & Development,2010,13(5):604-612.( 1) 1) |
| [126] | 刘磊,宋志刚.动物食欲调节的中枢信号通路[J].动物营养学报,2012,24(2):226-231.( 1) 1) |
| [127] | AVRUCH J,LONG X M,ORTIZ-VEGA S,et al.Amino acid regulation of TOR complex 1[J].American Journal of Physiology:Endocrinology and Metabolism,2009,296(4):E592-E602.( 1) 1) |
| [128] | MORRISON C D,XI X C,WHITE C L,et al.Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism[J].American Journal of Physiology:Endocrinology and Metabolism,2007,293(1):E165-E171.( 1) 1) |
| [129] | 邓会玲,刘国华,刘宁.氨基酸介导的TOR信号传导通路研究进展[J].动物营养学报,2011,23(4):529-535.( 1) 1) |
| [130] | 王嘉,薛敏,吴秀峰,等.鱼类对不同蛋白质源饲料选择性摄食调控机制的研究进展[J].动物营养学报,2014,26(4):833-842.( 1) 1) |
| [131] | DAI W W,PANSERAT S,MENNIGEN J A,et al.Post-prandial regulation of hepatic glucokinase and lipogenesis requires the activation of TORC1 signalling in rainbow trout(Oncorhynchus mykiss)[J].Journal of Experimental Biology,2013,216(23):4483-4492.( 1) 1) |
| [132] | TU Y Q,XIE S Q,HAN D,et al.Dietary arginine requirement for gibel carp(Carassis auratus gibelio var.CASⅢ) reduces with fish size from 50 g to 150 g associated with modulation of genes involved in TOR signaling pathway[J].Aquaculture,2015,449:37-47.( 1) 1) |
| [133] | REN M C,HABTE-TSION H M,LIU B,et al.Dietary leucine level affects growth performance,whole body composition,plasma parameters and relative expression of TOR and TNF-ɑ in juvenile blunt snout bream,Megalobrama amblycephala[J].Aquaculture,2015,448:162-168.( 1) 1) |
| [134] | TANG L,FENG L,SUN C Y,et al.Effect of tryptophan on growth,intestinal enzyme activities and TOR gene expression in juvenile Jian carp(Cyprinus carpio var.Jian):studies in vivo and in vitro[J].Aquaculture,2013,412-413:23-33.( 1) 1) |
| [135] | SUN S J,WANG B J,JIANG K Y,et al.Target of rapamycin(TOR) in Fenneropenaeus chinensis:cDNA cloning,characterization,tissue expression and response to amino acids[J].Aquaculture Nutrition,2015,21(1):1-9.( 1) 1) |




