2. 甘肃省肉羊繁育生物技术工程实验室, 民勤 733300
2. Engineering Laboratory of Mutton Sheep Breeding And Reproduction Biotechnology in Gansu Province, Minqin 733300, China
目前,高能、高淀粉饲粮已成为奶牛泌乳阶段或肉用反刍动物育肥阶段典型的营养特征[1-3],这一营养策略在提高动物生产性能的同时,也易引起瘤胃稳态失衡,导致以亚急性瘤胃酸中毒(SARA)为主的一系列瘤胃健康问题。Malekkhahi等[4]研究表明,奶牛采食高谷物饲粮所诱发的SARA对干物质采食量、产奶量、瘤胃微生物区系、血液代谢物等具有严重负面影响,其对动物健康及生产性能的危害是现代反刍动物生产面临的突出问题[5-6]。诸多研究发现,反刍动物过量采食高能、高淀粉饲粮时[7],可导致瘤胃液pH长时间低于正常生理范围,这是引起SARA的主要发病途径[8]和营养因素[9-10]。研究表明,尽管饲养管理条件一致,饲喂相同饲粮的奶牛[11]、肉牛[12]及绵羊[13],其个体间瘤胃液pH、发生SARA的风险存在显著差异,这种差异使得反刍动物生产管理和SARA的预防工作存在一定难度[11],其主要影响反刍动物营养供应策略、精准饲养实现、分群饲养依据和群体瘤胃健康程度的评价等。个体间SARA的变异,尤其是反刍动物本身的生理原因却鲜有报道,包括营养和生理途径导致个体SARA易感性的变异、瘤胃微生物多样性的改变等差异尚不清楚,因此,研究“反刍动物个体间SARA变异性来源及机制”是重要的科学问题,本文从采食行为、瘤胃上皮吸收功能以及微生物区系3个方面对影响反刍动物SARA易感性潜在营养生理因素进行综述,旨在为相关研究提供科学依据。
1 SARA易感性识别的必要性与变异性 1.1 必要性分阶段、分群饲养是反刍动物生产中实现精准饲养的主要营养管理手段[14],该方式基于动物群体在特定预期生产潜力或生产阶段的营养需要具有相似性,但实质上此营养供给方式并未充分考虑高产群体中个体间SARA耐受性的差异。科学研究中,由于试验动物个体在遗传及生活史等方面存在不同程度的异质性,这种异质性是导致试验结果产生变异的重要来源[15],生物统计角度认为这种变异称为随机误差,尽管增加动物数量、严格筛选试验动物等方法可减小该误差,但反刍动物实际生产过程中个体间SARA易感性变异却普遍存在,因此合理的饲养管理在集约化、规模化生产中至关重要。目前,基于随机抽样的瘤胃穿刺技术已应用于群体SARA风险的评估[5, 16-17],但该方法并不能有效检测出个体SARA易感性的差异,这意味着即使对高SARA风险的牛群改变饲粮策略(降低易发酵碳水化合物含量或增加粗饲料比例)以此降低群体SARA的风险,但同样会损失部分SARA耐受性个体发挥其最大生产性能,因此降低或消除个体SARA易感性差异是保证群体高效和健康生产的关键,未来反刍动物生产中可以考虑根据个体SARA耐受性的差异作为分群饲养的依据之一,结合特定生产阶段和预期生产潜力施加针对性的营养策略,保障动物瘤胃健康及高效生产,完善精准饲养手段。
1.2 变异性生产实践中,多种营养调控与饲养管理手段被用于预防SARA,如保证饲粮物理有效中性洗涤纤维(peNDF)与淀粉比例、分群饲养[18-19]、降低热应激[20]、增加饲喂频率[21]等。研究发现,牛羊采食相同饲粮时,其个体间发生SARA风险存在较大差异[11, 13]。Macmillan等[21]研究发现,泌乳期荷斯坦奶牛(n=16)饲喂精粗比为70 : 30饲粮后,其中7头奶牛出现SARA症状(瘤胃液pH < 5.8为535 min/d vs. 18.4 min/d)。Castillo-Lopez等[22]以肉牛为研究对象,表明育肥后期饲喂高谷物饲粮(含大麦谷物81.2%)肉牛(n=28)中平均有37.8%的个体发生SARA(瘤胃液pH < 5.5超过180 min/d),且个体间SARA发生概率在0~96.9%。Nasrollahi等[11]同样研究发现,泌乳期奶牛(n=78)饲喂精粗比为65 : 35饲粮后,31头奶牛出现SARA症状(瘤胃液pH < 5.8超过330 min/d);类似结果在奶山羊上也有报道[23-24]。此外,泌乳奶牛分娩后个体间瘤胃酸中毒严重程度存在极大变异,且这种变异与采食量、饲粮组成、瘤胃挥发性脂肪酸(VFA)浓度及比例无关[25]。以上研究证明,相同饲粮条件下,动物个体间SARA风险存在明显的差异,且这种差异可能与营养素的供给无关,受动物自身生理因素的调控。
2 影响SARA易感性的生理因素 2.1 采食行为瘤胃缓冲盐主要源于动物咀嚼产生的唾液、瘤胃上皮分泌的碳酸氢盐及饲粮在瘤胃降解产生的氨[26],其对稳定瘤胃液pH、微生物生长与养分消化有重要意义。其中唾液是瘤胃缓冲盐的主要来源,对于高产奶牛唾液中的磷酸盐和碳酸氢盐缓冲系统可中和瘤胃中约37%的氢离子[27],而反刍动物唾液分泌量主要取决于其咀嚼时间(采食时间与反刍时间之和)。研究表明,反刍动物的采食行为包括采食速度、反刍活动、挑食程度等方面存在较大的个体间变异[28-29]。Giger-Reverdin等[24]报道表明,奶山羊采食精粗比为60 : 40的饲粮,其平均咀嚼指数(咀嚼时间/干物质采食量)为4.05 h/kg,但个体间咀嚼指数在1.83~6.30 h/kg变化。同时对奶山羊(n=12)饲喂精粗比为65 : 35的饲粮,发现采食速度快的个体饲喂13 h后其瘤胃液pH在5.25~6.25波动,采食速度慢的个体瘤胃液pH在6.25~6.50变化,表明尽管饲粮组成相同,由于个体采食速度过快,导致其SARA风险的提高[30]。挑食行为是反刍动物饲养过程中不可避免的问题之一,长期的挑食行为使得反刍动物营养摄入不均衡、消化代谢紊乱,导致动物发生腹泻、蹄叶炎和肝脓肿等营养代谢疾病[31],对畜牧业发展造成巨大损失。因此,一些研究通过改变饲粮结构、饲喂策略等措施降低或消除其挑食行为[32]。Muhammad等[33]研究发现,断奶犊牛(n=28)饲喂长、短粒径苜蓿干草后,犊牛对其长粒径饲粮的挑食行为显著高于短粒径饲粮;Macmillan等[21]以哺乳期荷斯坦奶牛(n=8)为研究对象发现,饲喂3次/d相对于饲喂1次/d,在降低挑食行为方面前者优于后者,并且降低了高产奶牛患SARA的风险。上述研究表明,动物采食相同饲粮其个体间采食行为存在较大变异,由于采食行为影响唾液分泌量(咀嚼时间)、进入瘤胃营养素的速度(采食速度)及组成(挑食行为),这些行为差异均会影响瘤胃液pH(图 1)[34],采食行为可能是导致动物个体间SARA易感性差异的原因之一,但准确结论还有待进一步研究。
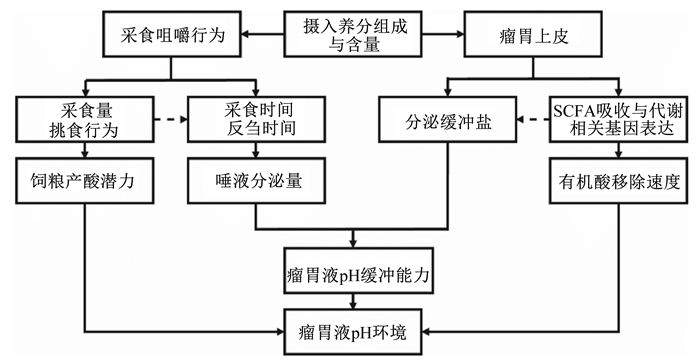
|
图 1 采食行为和瘤胃上皮功能作用瘤胃液pH的主要途径 Figure 1 Main pathways of feeding behavior and rumen epithelial functions affecting rumen fluid pH[34] |
瘤胃液pH波动是瘤胃内有机酸(VFA和乳酸)的产生、吸收、外流及缓冲盐中和等因素共同作用的结果(图 1),其中VFA移除的主要途径是经瘤胃上皮吸收,可占总VFA产量的50%~85%[26]。反刍动物在适应高谷物饲粮过程中其瘤胃上皮形态、瘤胃上皮生理功能(细胞增殖分化、吸收转运功能、代谢功能等[35])及电生理特性均发生改变,表现为基底层、棘层、颗粒层厚度降低[36-37],上皮通透性提高[38],内毒性物质增加等现象[39]。Penner等[40]发现,随饲粮精料水平提高,奶牛瘤胃上皮对丙酸和丁酸的吸收速率分别提高65.7%(0.35 mol/h vs. 0.58 mol/h)和77.8%(0.18 mol/h vs. 0.32 mol/h);同时,体外和体内研究也证明,VFA浓度提高及pH下降会影响瘤胃上皮细胞VFA吸收[41-42]、细胞内pH调节与质子转运相关基因表达水平[43-44],如Yan等[42]报道,适当增加饲粮精料(10% vs. 35%)可提高山羊瘤胃上皮细胞VFA转运与胞内pH调节相关基因表达,包括一元羧酸转运载体(MCT)(MCT1和MCT4)、阴离子转运载体[DRA、PAT1及阴离子交换蛋白2(AE2)]及Na+/H+交换载体(NHE)(NHE1、NHE2及NHE3),促进VFA吸收;与该结果类似,刘军花等[45]发现高谷物饲粮(65 : 35)显著升高了MCT1、Na+/K+-ATP酶的表达量,同时显著降低了MCT4的表达量。以上结果表明,瘤胃VFA吸收能力通过瘤胃上皮转运载体调控,随着饲粮精料比例增加,瘤胃上皮基因NHE1、NHE2、NHE3和Na+/K+-ATP酶等的表达量增加,有可能导致VFA的吸收能力增强[46]。与之结果类似,Penner等[13]通过一次性灌注葡萄糖诱导绵羊SARA发现,相对于SARA易感(acidosis-susceptible, AS)组,SARA耐受(acidosis-resistant, AR)组绵羊瘤胃上皮对乙酸和丁酸的吸收更迅速,加速酸从瘤胃中的移除,提高瘤胃液pH;同时,Schlau等[12]针对肉牛的研究表明,AR组pH与AS组瘤胃液pH(6.05 vs. 5.59)的差异主要与瘤胃液VFA浓度有关(122 mmol/L vs. 164 mmol/L),但该研究发现AR组瘤胃上皮细胞NHE3表达量显著提高,而其他VFA吸收相关基因(MCT1和DRA)表达量无显著差异。上述结果提示,反刍动物应对高精料饲粮过程中个体间瘤胃上皮转运载体基因的表达量存在差异,而瘤胃上皮转运载体基因的表达量同时受瘤胃液pH、VFA组成与浓度调控,瘤胃上皮转运基因表达量差异使得相同处理不同个体瘤胃上皮对VFA吸收能力存在较大变异,这种差异可能是导致动物SARA易感性不同的潜在生理因素。未来应针对个体瘤胃液pH、有机酸组成与瘤胃上皮转运载体三者结合,系统评估SARA变异的潜在机制。
3 瘤胃微生物及代谢物与SARA易感性众所周知,高精料饲粮诱导的SARA可导致瘤胃微生物区系的改变,对其进行焦磷酸测序结果发现,SARA易感组瘤胃菌群丰度指数(Chao1和Ace指数)和多样性指数(Shannon指数)显著低于耐受组[47],其在细菌水平上表现为淀粉利用菌、产乳酸菌和乳酸利用菌数量升高,而纤维分解菌数量下降[48-50],瘤胃原虫水平主要表现为原虫数量的降低[51]。由于不同SARA易感性个体其瘤胃液pH环境存在差异,而pH直接影响瘤胃微生物的生长与增殖,故推测反刍动物SARA易感性变异会引起瘤胃微生物区系及发酵产物的差异。基于PCR-变性梯度凝胶电泳(DGGE)技术的研究证明,肉牛中AR(瘤胃液平均pH=6.02)与AS(瘤胃液平均pH=5.55)个体在瘤胃微生物多样性方面存在明显差异,且AS个体瘤胃更倾向于丙酸发酵型[52];据张瑞阳[47]研究发现高谷物诱导的SARA中,在门水平上,SARA组瘤胃拟杆菌门(Bacteroidetes)和蛋白菌门(Proteobacteria)的相对丰度显著降低,硬壁菌门(Firmicutes)和放线菌门(Actinobacteria)的相对丰度显著增加。在属水平上,SARA组奶牛瘤胃中普氏菌(Prevotella)、密螺旋体属(Treponema)、厌氧支原体属(Anaeroplasma)、不动杆菌属(Acinetobacter)、帕匹杆菌属(Papillibacter)的相对丰度显著降低,而瘤胃球菌属(Ruminococcus)、奇异菌属(Atopobium)、双歧杆菌(Bifidobacteria)、未分类的梭菌属(unclassified Clostridiales)的相对丰度显著升高。关于瘤胃代谢产物与SARA易感性的关系尚不明确,但基于代谢组学技术研究发现,奶牛饲喂同一种高精料饲粮(精粗比45 : 55)个体间部分瘤胃代谢产物乙酰乙酸、麦芽糖、甲酸等浓度差异在2~4倍[53],表明采食相同饲粮条件下奶牛瘤胃代谢产物也存在差异。目前,宏基因组学(metagenomics)和代谢组学(metabolomics)技术已被广泛应用于挖掘瘤胃微生物信息,包括物种多样性、种群结构、代谢规律及功能活性等方面。Mao等[50]以山羊为研究模型,采用454焦磷酸测序技术及代谢组学方法揭示了SARA瘤胃微生物多样性与瘤胃代谢组的关系,但不同SARA易感性是否引起瘤胃微生物在属、种水平的变化尚不清楚,有必要从微生物基因组和代谢组角度揭示其变异性机制。瘤胃微生物系统的建立与稳定取决于影响瘤胃环境的营养与生理要素,采食行为和瘤胃上皮功能分别决定瘤胃的缓冲能力与VFA的移除速度,二者共同影响瘤胃液pH、渗透压、氧化还原电势等瘤胃内环境参数,而上述参数即是影响瘤胃微生物区系的重要因素,也是判断个体SARA是否发生与严重程度的主要依据。因此,阐明采食行为-瘤胃上皮功能-微生物区系三者之间的关系对揭示个体间SARA易感性的变异机制有重要意义。
4 小结反刍动物个体间SARA的易感性存在差异,且这种差异主要受自身生理因素的调控,其中采食行为和瘤胃上皮的吸收能力的变异可能是导致个体SARA易感性差异的主要营养生理因素,在未来的生产与科研中需明确动物个体间SARA易感性变异的来源及机制、并针对不同个体采取相应的营养干预措施,保障动物的高效生产与瘤胃健康。
| [1] |
HATEW B, PODESTA S C, VAN LAAR H, et al. Effects of dietary starch content and rate of fermentation on methane production in lactating dairy cows[J]. Journal of Dairy Science, 2015, 98(1): 486-499. |
| [2] |
BOERMAN J P, POTTS S B, VAN DE HAAR M J, et al. Milk production responses to a change in dietary starch concentration vary by production level in dairy cattle[J]. Journal of Dairy Science, 2015, 98(7): 4698-4706. |
| [3] |
MORALES R, PARGA J, SUBIABRE I, et al. Finishing strategies for steers based on pasture or silage plus grain and time on feed and their effects on beef quality[J]. Ciencia E Investigacio'n Agraria, 2015, 42(1): 1-2. DOI:10.4067/S0718-16202015000100001 |
| [4] |
MALEKKHAHI M, TAHMASBI A M, NASERIAN A A, et al. Effects of supplementation of active dried yeast and malate during sub-acute ruminal acidosis on rumen fermentation, microbial population, selected blood metabolites, and milk production in dairy cows[J]. Animal Feed Science and Technology, 2016, 213: 29-43. DOI:10.1016/j.anifeedsci.2015.12.018 |
| [5] |
KLEEN J L, CANNIZZO C. Incidence, prevalence and impact of SARA in dairy herds[J]. Animal Feed Science and Technology, 2012, 172(1/2): 4-8. |
| [6] |
王洪荣. 反刍动物瘤胃酸中毒机制解析及其营养调控措施[J]. 动物营养学报, 2014, 26(10): 3140-3148. DOI:10.3969/j.issn.1006-267x.2014.10.028 |
| [7] |
GOLDER H M, CELI P, RABIEE A R, et al. Effects of grain, fructose, and histidine on ruminal pH and fermentation products during an induced subacute acidosis protocol[J]. Journal of Dairy Science, 2012, 95(4): 1971-1982. |
| [8] |
VALENTE T N P, SAMPAIO C B, LIMA E D S, et al. Aspects of acidosis in ruminants with a focus on nutrition:a Review[J]. Journal of Agricultural Science, 2017, 9(3): 90-97. DOI:10.5539/jas.v9n3p90 |
| [9] |
ZEBELI Q, ASCHENBACH J R, TAFAJ M, et al. Invited review:role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle[J]. Journal of Dairy Science, 2012, 95(3): 1041-1056. |
| [10] |
姚军虎, 李飞, 李发弟, 等. 反刍动物有效纤维评价体系及需要量[J]. 动物营养学报, 2014, 26(10): 3168-3174. DOI:10.3969/j.issn.1006-267x.2014.10.031 |
| [11] |
NASROLLAHI S M, ZALI A, GHORBANI G R, et al. Variability in susceptibility to acidosis among high producing mid-lactation dairy cows is associated with rumen pH, fermentation, feed intake, sorting activity, and milk fat percentage[J]. Animal Feed Science and Technology, 2017, 228: 72-82. |
| [12] |
SCHLAU N, GUAN L L, OBA M. The relationship between rumen acidosis resistance and expression of genes involved in regulation of intracellular pH and butyrate metabolism of ruminal epithelial cells in steers[J]. Journal of Dairy Science, 2012, 95(10): 5866-5875. |
| [13] |
PENNER G B, ASCHENBACH J R, GÄBEL G, et al. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep[J]. The Journal of Nutrition, 2009, 139(9): 1714-1720. DOI:10.3945/jn.109.108506 |
| [14] |
SALIM J K, DILLON C R, SAGHAIAN S H, et al. Profitability of dairy cattle through precision livestock farming management practices[C]//Southern Agricultural Economics Association. [S. l. ]: [s. n. ], 2005: 1-15.
|
| [15] |
张元跃. 营养试验中消除动物异质性的研究[J]. 动物营养学报, 1998, 10(2): 59-63. |
| [16] |
MORGANTE M, STELLETTA C, BERZAGHI P, et al. Subacute rumen acidosis in lactating cows:an investigation in intensive Italian dairy herds[J]. Journal of Animal Physiology and Animal Nutrition, 2007, 91(5/6): 226-234. |
| [17] |
KLEEN J L, HOOIJER G A, REHAGE J, et al. Subacute ruminal acidosis in Dutch dairy herds[J]. Veterinary Record, 2009, 164(22): 681-684. |
| [18] |
李飞. 奶山羊亚急性瘤胃酸中毒模型构建与奶牛日粮CBI的优化[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2014: 10-13. http://cdmd.cnki.com.cn/Article/CDMD-10712-1014421086.htm
|
| [19] |
LI F, YANG X J, CAO Y C, et al. Effects of dietary effective fiber to rumen degradable starch ratios on the risk of sub-acute ruminal acidosis and rumen content fatty acids composition in dairy goat[J]. Animal Feed Science and Technology, 2014, 189: 54-62. DOI:10.1016/j.anifeedsci.2013.12.011 |
| [20] |
YAZDI M H, MIRZAEI-ALAMOUTI H R, AMANLOU H, et al. Effects of heat stress on metabolism, digestibility, and rumen epithelial characteristics in growing Holstein calves[J]. Journal of Animal Science, 2016, 94(1): 77-89. |
| [21] |
MACMILLAN K, GAO X, OBA M. Increased feeding frequency increased milk fat yield and may reduce the severity of subacute ruminal acidosis in higher-risk cows[J]. Journal of Dairy Science, 2017, 100(2): 1045-1054. |
| [22] |
CASTILLO-LOPEZ E, WIESE B I, HENDRICK S, et al. Incidence, prevalence, severity, and risk factors for ruminal acidosis in feedlot steers during backgrounding, diet transition, and finishing[J]. Journal of Animal Science, 2014, 92(7): 3053-3063. |
| [23] |
DESNOYERS M, DUVAUX-PONTER C, RIGALMA K, et al. Effect of concentrate percentage on ruminal pH and time-budget in dairy goats[J]. Animal, 2008, 2(12): 1802-1808. |
| [24] |
GIGER-REVERDIN S, RIGALMA K, DESNOYERS M, et al. Effect of concentrate level on feeding behavior and rumen and blood parameters in dairy goats:relationships between behavioral and physiological parameters and effect of between-animal variability[J]. Journal of Dairy Science, 2014, 97(7): 4367-4378. |
| [25] |
MOHAMMED R, STEVENSON D M, WEIMER P J, et al. Individual animal variability in ruminal bacterial communities and ruminal acidosis in primiparous Holstein cows during the periparturient period[J]. Journal of Dairy Science, 2012, 95(11): 6716-6730. |
| [26] |
ASCHENBACH J R, PENNER G B, STUMPFF F, et al. Ruminant nutrition symposium:role of fermentation acid absorption in the regulation of ruminal pH[J]. Journal of Animal Science, 2011, 89(4): 1092-1107. |
| [27] |
ALLEN M S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber[J]. Journal of Dairy Science, 1997, 80(7): 1447-1462. |
| [28] |
BROWN M S, KREHBIEL C R, GALYEAN M L, et al. Evaluation of models of acute and subacute acidosis on dry matter intake, ruminal fermentation, blood chemistry, and endocrine profiles of beef steers[J]. Journal of Animal Science, 2000, 78(12): 3155-3168. |
| [29] |
GAO X, OBA M. Relationship of severity of subacute ruminal acidosis to rumen fermentation, chewing activities, sorting behavior, and milk production in lactating dairy cows fed a high-grain diet[J]. Journal of Dairy Science, 2014, 97(5): 3006-3016. |
| [30] |
GIGERR-EVERDIN S, DESNOYERS M, DUVAUX-PONTER C, et al. Modelling within-day variability in feeding behaviour in relation to rumen pH: application to dairy goats receiving an acidogenic diet[M]//SAUVANT D, VAN MILGEN J, FAVERDIN P, et al, eds. Modelling nutrient digestion and utilisation in farm animals. Wageningen: Wageningen Academic Publishers, 2015: 121-129.
|
| [31] |
MILLER-CUSHON E K, DEVRIES T J. Feed sorting in dairy cattle:causes, consequences, and management[J]. Journal of Dairy Science, 2017, 100(5): 4172-4183. |
| [32] |
GRETER A, DEVRIES T J. Effect of feeding amount on the feeding and sorting behaviour of lactating dairy cattle[J]. Canadian Journal of Animal Science, 2017, 91(1): 47-54. |
| [33] |
MUHAMMAD A U R, XIA C Q, CAO B H. Dietary forage concentration and particle size affect sorting, feeding behaviour, intake and growth of Chinese Holstein male calves[J]. Journal of Animal Physiology and Animal Nutrition, 2016, 100(2): 217-223. DOI:10.1111/jpn.2016.100.issue-2 |
| [34] |
GREGORYB P, KARENA B. Variation in the susceptibility to ruminal acidosis: challenge or opportunity?[C]//Advances in Dairy Technology: Proceedings of the Western Canadian Dairy Seminar. [S. l. ]: [s. n. ], 2010: 173-187.
|
| [35] |
刘军花. 亚急性瘤胃酸中毒对山羊瘤胃上皮屏障功能的影响及其机制[D]. 博士学位论文. 南京: 南京农业大学, 2014: 57-68.
|
| [36] |
胡红莲. 奶山羊亚急性瘤胃酸中毒营养生理机制的研究[D]. 博士学位论文. 呼和浩特: 内蒙古农业大学, 2008: 2-15. http://cdmd.cnki.com.cn/Article/CDMD-10129-2008131944.htm
|
| [37] |
STEELE M A, CROOM J, KAHLER M, et al. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis[J]. Regulatory, Integrative and Comparative Physiology, 2011, 300(6): 1515-1523. DOI:10.1152/ajpregu.00120.2010 |
| [38] |
KLEVENHUSEN F, HOLLMANN M, PODSTATZKY-LICHTENSTEIN L, et al. Feeding barley grain-rich diets altered electrophysiological properties and permeability of the ruminal wall in a goat model[J]. Journal of Dairy Science, 2013, 96(4): 2293-2302. |
| [39] |
LI S, KHAFIPOUR E, KRAUSE D O, et al. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows[J]. Journal of Dairy Science, 2012, 95(1): 294-303. |
| [40] |
PENNER G B, TANIGUCHI M, GUAN L L, et al. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue[J]. Journal of Dairy Science, 2009, 92(6): 2767-2781. |
| [41] |
METZLER-ZEBELI BU, HOLLMANN M, SABITZER S, et al. Epithelial response to high-grain diets involves alteration in nutrient transporters and Na+/K+-ATPase mRNA expression in rumen and colon of goats[J]. Journal of Animal Science, 2013, 91(9): 4256-4266. |
| [42] |
YAN L, ZHANG B, SHEN Z M. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats[J]. Journal of Dairy Science, 2014, 97(9): 5668-5675. |
| [43] |
ETSCHMANN B, SUPLIE A, MARTENS H. Change of ruminal sodium transport in sheep during dietary adaptation[J]. Archives of Animal Nutrition, 2009, 63(1): 26-38. DOI:10.1080/17450390802506885 |
| [44] |
MARTENS H, RABBANI I, SHEN Z M, et al. Changes in rumen absorption processes during transition[J]. Animal Feed Science and Technology, 2012, 172(1/2): 95-102. |
| [45] |
刘军花, 朱伟云, 毛胜勇. 高谷物日粮促进山羊瘤胃上皮单羧酸转运蛋白1及钠钾ATP酶mRNA的表达[J]. 草业学报, 2017, 26(2): 95-101. DOI:10.11686/cyxb2016121 |
| [46] |
艳城. 日粮对细毛羊瘤胃上皮的SCFA吸收相关基因及氮素转运的调控[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2015: 46-51.
|
| [47] |
张瑞阳. 组学技术研究亚急性瘤胃酸中毒对奶牛瘤胃微生物、代谢和上皮功能的影响[D]. 博士学位论文. 南京: 南京农业大学, 2015: 45-61.
|
| [48] |
LI F, CAO Y C, LIU N N, et al. Subacute ruminal acidosis challenge changed in situ degradability of feedstuffs in dairy goats[J]. Journal of Dairy Science, 2014, 97(8): 5101-5109. |
| [49] |
SUN Y Z, MAO S Y, ZHU W Y. Rumen chemical and bacterial changes during stepwise adaptation to a high-concentrate diet in goats[J]. Animal, 2010, 4(2): 210-217. |
| [50] |
MAO S Y, HUO W J, ZHU W Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model[J]. Environmental Microbiology, 2015, 18(2): 525-541. |
| [51] |
VALENTE T N P, DA SILVA LIMA E, DOS SANTOS W B R, et al. Ruminal microorganism consideration and protein used in the metabolism of the ruminants:a review[J]. African Journal of Microbiology Research, 2016, 10(14): 456-464. |
| [52] |
CHEN Y H, OBA M, GUAN L L. Variation of bacterial communities and expression of Toll-like receptor genes in the rumen of steers differing in susceptibility to subacute ruminal acidosis[J]. Veterinary Microbiology, 2012, 159(3/4): 451-459. |
| [53] |
SALEEM F, AMETAJ B N, BOUATRA S, et al. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows[J]. Journal of Dairy Science, 2012, 95(11): 6606-6623. |




