2. 天津农学院水产学院, 天津市水产生态及养殖重点实验室, 天津 300384
2. Tianjin Key Laboratory of Aqua-Ecology and Aquaculture, Fisheries Science Department, Tianjin Agricultural University, Tianjin 300384, China
糖(碳水化合物)是动物饲料中最廉价的能源物质,若能利用价格低廉的糖作为能源物质替代部分蛋白质,起到“蛋白质节约作用(protein-sparing effects)”,则不仅可以降低饲料蛋白质含量和成本,同时还可以保护渔业资源,减少养殖过程中氮排泄对水体的污染[1]。然而,与陆生动物相比,鱼类对糖的利用能力差[2-3]。饲料中糖水平过高会导致鱼类生长迟缓,饲料利用率降低,抗病力减弱,甚至是死亡[4-5]。
三价铬离子(Cr3+)作为动物必需的微量元素形式,在机体糖、蛋白质、脂肪代谢中起重要作用[6-7]。研究发现,Cr3+通过增强胰岛素(insulin, INS)的作用,进而提高机体葡萄糖耐量[8-9]。已有研究证实,Cr3+能有效改善草鱼(Ctenopharyngodon idellus)[10]、虹鳟(Oncorhynchus mykiss)[11]、大黄鱼(Larmichthys crocea)[12]以及罗非鱼(Oreochromis niloticus ×Oreochromis aureus)[13]的生长性能,增强莫桑比克罗非鱼[14]、虹鳟[15]的免疫机能,还可提高条纹鲈(Morone saxatilis)[16]的糖利用能力。此外,Cr3+还能有效降低血浆中皮质醇(cortisol,COR)的含量,改善机体应激状态[17-18]。
目前,已有一些学者针对Cr3+在鲤生长、饲料利用方面的影响做了一些研究[19-20],但这些研究所使用的铬源多是无机铬,而研究证实,与无机铬相比,有机铬具有更高的吸收率、生物活性及稳定性[21],如畜禽动物中应用最为广泛的铬源——吡啶羧酸铬(CrPic)以及氨基酸螯合铬——蛋氨酸铬(CrMet),这2种铬源分别是吡啶甲酸和蛋氨酸与Cr3+的螯合物,能够有效缓解矿物元素之间的拮抗竞争作用,有利于铬的吸收。关于有机铬作为鲤饲料添加剂的安全剂量及对其机体生理的作用的报道较少。本文以鲤利用较差的葡萄糖为糖源,比较3种不同形式的铬——无机铬、有机酸铬、氨基酸铬对喂食高葡萄糖饲料鲤生长性能、血清生化指标及肝胰脏糖代谢酶活性的影响,为铬在鲤配合饲料中的应用提供理论依据。
1 材料与方法 1.1 试验饲料配制以酪蛋白为蛋白质源、大豆油为脂肪源、葡萄糖为糖源配制4种纯化饲料,分别为不添加铬的基础饲料(对照组)及基础饲料中分别添加Cr2O3、CrPic和CrMet的试验饲料,试验饲料中铬添加水平在2.60 mg/kg(以Cr3+计)左右。其中,CrPic购自国药集团化学试剂有限公司,有效含量98%,Cr含量为12.4%。CrMet购自湖北拓楚慷元医药化工有限公司有效含量为99%,Cr含量为80%。所有固体饲料原料均过80目筛,原料逐级放大混匀后,用双螺杆制粒机(华南理工大学机械工程研究所制造)制成颗粒饲料,每种饲料2种规格(直径为1.5和3.2 mm),在烘箱(DK400,日本Yamato)中40 ℃烘干2 h,取出后自然风干,保存于-20 ℃冰箱中。试验饲料组成及营养水平见表 1。
|
|
表 1 试验饲料组成及营养水平(风干基础) Table 1 Composition and nutrient levels of experimental diets (air-dry basis) |
试验鱼由天津市晨辉饲料有限公司养殖基地提供,运送到晨辉饲料有限公司养殖实验室后,暂养2周,期间投喂基础饲料(不添加Cr3+),待试验鱼适应实验室环境后,选取健康、规格基本一致的试验鱼[初始体重为(40.95±4.80) g],随机分配到12只800 L蓝色圆形塑料水箱中,每组设3个重复,每个重复投放试验鱼60尾。养殖模式为静水养殖。试验用水为曝气24 h以上的自来水,每天投喂2次(07:00、16:00),日投喂量为体重的4%~6%。每2周调整1次投饲量。每天换水1次,同时吸底并收取粪便,换水量为总水量的1/3,饲养试验期间水温为15~25 ℃,pH 7.6~7.8,氨氮浓度≤0.05 mg/L,溶解氧浓度≥6.0 mg/L。养殖周期为60 d。
1.3 样品采集经60 d饲养后,试验鱼禁食24 h,经丁香酚(1 : 10 000)麻醉后,称重并记录尾数。每只水槽随机取6尾保存在-20 ℃冰箱中,用于体成分分析;另取10尾试验鱼,尾部采血,离心5 min(4 ℃,3 500 r/min),取上清后保存,备用,用于检测血清生化指标;采血后的试验鱼于冰盘上解剖取肝胰脏和肌肉组织,用于检测糖代谢酶活性、糖原含量活性,所有样品于-80 ℃冰箱中保存,备用。
1.4 测定方法试验饲料和鱼体常规营养成分的测定参照AOAC(1995)[22]进行:水分含量测定采用恒重恒压干燥法(GB/T 5009.3—2010);粗蛋白质、粗脂肪与粗灰分含量分别采用杜马斯燃烧法(GB/T 24318—2009,美国Thermo公司的Thermo Fisher scientific FLASH2000全自动蛋白质测定仪)、索氏抽提法(GB/T 5009.6—2010,德国Gerhardt公司的Gerhardt SOXTHERM索氏抽提仪)、灼烧重量法(GB/T 5009.4—2010)进行测定。饲料中铬含量采用原子吸收光谱法(石墨炉原子化法)测定。
肝胰脏、肌肉糖原含量的测定参考Hassid等[23]的方法,采用南京建成生物工程研究所生产的试剂盒。将肝胰脏或肌肉与强碱以1 : 3(质量体积比)混合,沸水浴20 min溶解形成溶液,分别稀释为1%和5%的检测液,然后将其与浓硫酸反应形成乙醛,最后加入蒽酮,然后染色剂染色,使用分光光度计在620 nm下测定吸光度值。
血清葡萄糖(glucose, GLU)、甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)、高密度脂蛋白(high-density lipoprotein, HDL)、低密度脂蛋白(low-density lipoprotein, LDL)含量及肌酸激酶(creatine kinase, CK)、乳酸脱氢酶(lactic dehydrogenase, LDH)活性由天津市金域医学检验所检验,仪器为德国Roche公司的Roche C311全自动生化分析仪。血清INS、胰高血糖素(glycogen, GC)、胰岛素受体(insulin receptor, ISR)、生长激素(growth hormone, GH)、COR含量均采用酶联免疫吸附试验(ELISA)试剂盒测定,其中INS、GC试剂盒购自美国Assay Designs公司,ISR、GH试剂盒购自上海酶联生物科技有限公司。
肝胰脏已糖激酶(hexokinase, HK)、丙酮酸激酶(pyruvate kinase, PK)、琥珀酸脱氢酶(succinic acid dehydrogenase, SDH)活性采用南京建成生物工程研究所试剂盒测定,肝胰脏磷酸果糖激酶(phosphofructokinase, 6-PFK1)、磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase, PEPCK)、葡萄糖-6-磷酸脱氢酶(glucose 6-phosphatase dehydrogenase, G6PDH)、糖原合成酶(glycogen synthase, GS)以及脂肪酸合成酶(fatty acid synthetase, FAS)活性采用双抗体夹心法测定,采用ELISA试剂盒(美国Assay Designs)测定。测定方法严格按照试剂盒说明书进行,其中,所有ELISA试剂盒测定方法为在微孔中加入样品,然后加入相应的抗体,37 ℃温浴60 min,洗板5次后加入相应显色剂反应,最后加入终止液终止反应,测定各微孔吸光度值,计算活性。
1.5 计算公式
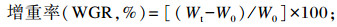
|
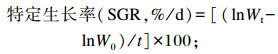
|
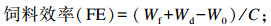
|
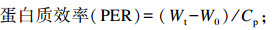
|
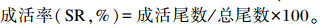
|
式中:W0为试验鱼初始体重;Wt为试验鱼终末体重;t为试验天数;C为摄饵采食量;Wf为试验鱼终末总重;Wd为死亡试验鱼总重;Wi为试验鱼初始总重;Cp为蛋白质摄入量。
1.6 数据统计分析采用SPSS 19.0软件对数据进行单因素方差分析(one-way ANOVA),若差异显著(P<0.05),则用Tukey’s法进行多重比较。所有数据均以平均值±标准差(mean±SD)表示。
2 结果 2.1 不同铬源对喂食高葡萄糖饲料鲤生长性能的影响不同铬源对喂食高葡萄糖饲料鲤生长性能的影响见表 2。由表可知,各组试验鱼的SR在91.67%~95.00%,组间无显著差异(P>0.05)。铬源添加组WGR、SGR显著高于对照组(P < 0.05),最高值均出现在CrMet组,但与CrPic组相比,差异均不显著(P>0.05)。与对照组相比,CrPic和CrMet组FE和PER显著提高(P < 0.05),Cr2O3组无显著变化(P>0.05)。
|
|
表 2 不同铬源对喂食高葡萄糖饲料鲤生长性能的影响 Table 2 Effects of different chromium sources on growth performance of common carp fed high glucose diets |
不同铬源对喂食高葡萄糖饲料鲤常规营养成分的影响见表 3。由表可知,CrPic、CrMet组全鱼脂肪含量显著高于对照组和Cr2O3组(P < 0.05)。CrMet组全鱼肝糖原含量显著高于对照组和Cr2O3组(P < 0.05)。铬源添加组全鱼肌糖原含量显著提高(P < 0.05)。各组全鱼水分、蛋白质、灰分无显著差异(P>0.05)。
|
|
表 3 不同铬源对喂食高葡萄糖饲料鲤全鱼常规营养成分的影响 Table 3 Effects of different chromium sources on common nutritional components of whole body of common carp fed high glucose diets |
不同铬源对喂食高葡萄糖饲料鲤血清生化指标的影响见表 4。由表可知,与对照组相比,CrMet组血清TG和TC含量显著降低(P < 0.05),Cr2O3和CrPic组无显著变化(P>0.05)。各组血清HDL和LDL含量无显著差异(P>0.05)。与对照组相比,CrMet组血清INS、ISR含量显著提高(P < 0.05),而Cr2O3、CrPic组无显著变化(P>0.05)。与对照组相比,铬源添加组鲤血清GH含量和LDH活性均显著提高(P < 0.05),血清葡萄糖及COR含量显著降低(P < 0.05),且铬源添加组间均无显著差异(P>0.05)。与对照组相比,Cr2O3、CrMet组血清CK活性均显著提高(P < 0.05),而CrPic组无显著变化(P>0.05)。各组血清GC含量无显著差异(P>0.05)。
|
|
表 4 不同铬源对喂食高葡萄糖饲料鲤血清生化指标的影响 Table 4 Effects of different chromium sources on serum biochemical indices of common carp fed high glucose diets |
不同铬源对喂食高葡萄糖饲料鲤肝胰脏糖代谢酶活性的影响见表 5。由表可知,与对照组相比,铬源添加组肝胰脏HK、PK活性均显著提高(P < 0.05)。CrPic组肝胰脏SDH活性最高,显著高于其他各组(P < 0.05)。与对照组相比,CrPic、CrMet组肝胰脏PEPCK活性显著降低(P < 0.05),肝胰脏FAS活性显著提高(P < 0.05)。与对照组相比,铬源添加组肝胰脏GS活性显著提高(P < 0.05)。各组肝胰脏6-PFK1和G6PDH活性无显著差异(P>0.05)。
|
|
表 5 不同铬源对喂食高葡萄糖饲料鲤肝胰脏糖代谢酶活性的影响 Table 5 Effects of different chromium sources on hepatopancreas glycometabolism enzyme activities of common carp fed high glucose diets |
本试验结果显示,添加3种铬源均能提高鲤的生长性能,这与Ahmed等[19]用添加氯化铬(CrCl3)的饲料投喂鲤、潘庆等[24]用添加CrPic和烟酸铬(CrNic)的饲料投喂奥尼罗非鱼、Wang等[12]用添加CrNic的饲料投喂大黄鱼以及在以葡萄糖为糖源的饲料中添加Cr2O3、CrCl3投喂罗非鱼[25-28]的研究结果相一致。而在饲料中添加CrPic对虹鳟和奥尼罗非鱼的生长性能没有显著影响[11, 28-29],类似的结果也出现在用添加酵母铬的饲料投喂金头鲷(Sparus aurata)[30]和虹鳟[31]的试验中,其原因可能与试验鱼的种类、铬的添加形式及水平、饲养环境条件、试验设计等因素有关。同时,本试验结果显示添加有机铬后对鲤生长的促进效果的影响要优于无机铬。一般认为,在生理环境下有机铬比无机铬具有更高的生物活性和稳定性,而且机体对有机铬的吸收率要远高于无机铬,研究发现无机铬的吸收率仅为1%~3%[32],而有机铬的吸收率为10%~25%[33]。因而,本试验结果可能因为添加有机铬组的试验鱼吸收更多的铬,更有效地促进了鲤的生长,这与刘太亮[34]对草鱼的研究结果相类似。
3.2 不同铬源对喂食高葡萄糖饲料鲤常规营养成分的影响本试验结果显示,不同铬源对试验鱼体水分、蛋白质、灰分含量均无显著影响,而添加有机铬能显著提高鱼体脂肪含量,与已报道的罗非鱼的研究结果一致[28, 35]。研究发现,Cr3+能够通过协同INS发挥生物学功能,提高相关代谢酶的活性,从而参与机体营养物质的代谢,进而改变营养物质的沉积方向[36-37]。本试验中有机铬组试验鱼体脂肪含量的升高可能是添加有机铬后提高了肝胰脏FAS的活性,从而对全鱼脂肪含量产生影响,在猪饲料中添加铬后也发现了相类似的情况[38-39]。
葡萄糖在GS的催化作用下,经糖异生作用合成糖原贮存在机体各组织中,其中肝脏和肌肉是最主要的贮存场所[40]。Steele等[41]报道了CrCl3能有效提高土耳其火鸡肝脏GS活性,促进机体糖异生作用,从而提高了肝糖原含量。本试验结果与上述报道相类似,在饲料中添加3种铬源均能显著提高试验鱼肌糖原含量,肝糖原含量也有所增加,添加CrMet的试验鱼肝糖原含量显著高于对照组。同时,对肝胰脏GS活性的检测结果发现3种形式铬均显著提高该酶的活性,表明3种铬源对于促进肝胰脏和肌糖原合成作用具有显著效果。类似的结果也出现在Campbell等[42]对小鼠的报道中。
3.3 不同铬源对喂食高葡萄糖饲料鲤血清生化指标的影响铬元素对动物机体脂类代谢的主要作用是维持血液中正常胆固醇水平。研究发现,在饲料中添加CrPic可有效降低罗非鱼血清TC含量[43]。本试验结果与上述结果相类似,添加CrMet能有效降低试验鱼血清TG、TC含量,改善试验鱼血脂水平。而刘太亮[34]在草鱼饲料中添加不同形式铬后未发现对其血清TC含量产生影响。其原因可能是研究试验动物种类、饲料营养成分、养殖条件有所不同。
研究证实,Cr3+能够有效加快血液中葡萄糖的消失速度,缩短葡萄糖的半衰期,从而降低血清中葡萄糖含量[44]。对罗非鱼的研究发现,添加不同铬源(Cr2O3、CrCl3、CrPic、CrNic)均延迟了血清葡萄糖含量高峰的到来,降低了血清葡萄糖含量,提高了罗非鱼对饲料中糖的利用[24, 25, 28, 43]。刘太亮[34]在草鱼饲料中分别添加了CrPic和CrNic,结果发现这2种铬源均能有效降低草鱼血清葡萄糖含量,提高肝糖原含量,证实CrPic和CrNic能够提高草鱼对饲料中糖的利用率。本试验结果显示,CrMet能显著降低试验鱼血清葡萄糖含量,与上述结果相类似,而Cr2O3和CrPic没有对血清葡萄糖含量产生影响。这说明以CrMet形式存在的铬源可能促进了铬的吸收,增加了INS的敏感性,增强了鱼体糖代谢,从而降低了血清葡萄糖含量。血清中LDH活性在添加CrMet后显著升高也表明了鱼体糖代谢的增强。
Liu等[10]的研究发现,添加适量CrPic能显著提高草鱼血清INS含量。本试验与上述结果相类似,添加CrMet后试验鱼血清INS含量显著升高。研究发现,Cr3+通过增加ISR含量或促进INS和细胞膜上的ISR结合,从而提高细胞对葡萄糖的敏感性[9, 33],本试验中CrMet组血清ISR含量显著升高这一结果也证实了此观点。同时,血清COR含量在添加3种铬源后均显著降低,这反映出典型的INS(合成代谢)与COR(分解代谢)间的代谢关系,即机体INS含量与COR含量呈反向相关,而不同形式铬的添加没有改变这种相关性。添加铬后试验鱼血清INS含量升高,证明了铬作为INS的辅因子来协同INS作用的生理学作用。类似的结果也出现在Sahin等[45]的研究中,该研究得出CrPic能够提高低温条件下产蛋鸡血浆INS含量,并显著降低血浆COR含量。
3.4 不同铬源对喂食高葡萄糖饲料鲤肝胰脏糖代谢酶活性的影响Cr3+通过调节葡萄糖代谢酶影响机体葡萄糖代谢。HK是肝胰脏利用葡萄糖的第1限速酶。在本试验中,添加3种铬源的肝胰脏HK活性均显著高于对照组,这可能是由于Cr3+的添加直接刺激组织糖酵解途径,加速葡萄糖氧化,从而使得葡萄糖含量降低,ATP生成增加。然而,Ahmed等[19]报道在饲料中添加CrCl3对鲤肝胰脏HK活性没有显著影响。这种差异可能是由于铬的不同添加形式以及水平造成的。除了HK,6-PFK1和PK也是控制糖酵解途径速率的关键酶。PK催化的反应是葡萄糖生成丙酮酸的最后一步反应,即催化磷酸烯醇式丙酮酸转化成丙酮酸。本试验结果显示,喂食添加3种铬源饲料的鲤肝胰脏中PK的活性均显著上升,说明3种铬源能够促进糖酵解途径。
PEPCK催化糖异生作用的第一步,将草酰乙酸盐转变成磷酸烯醇式丙酮酸盐[46]。研究发现,Cr3+能够通过在动物体内形成核酸衍生物从而直接抑制PEPCK活性[47]。Gardner等[48]证实了INS自身也可抑制PEPCK活性,这种抑制是通过抑制其PEPCK基因的表达造成的,而铬具有协调INS作用的生物学作用,因此,铬的补充能够通过加强INS作用进一步间接抑制PEPCK活性。本试验结果与上述研究相类似,添加CrPic和CrMet显著抑制了鲤肝胰脏的PEPCK活性,PEPCK活性的减少可能导致糖异生作用减缓,从而减少内源性葡萄糖的生成。
G6PDH是糖代谢的磷酸戊糖途径的关键酶,它促使磷酸戊糖核酸合成、烟酰胺腺嘌呤二核苷酸磷酸(NADPH)的合成反应并维持细胞的氧化还原状态[49]。本试验中,添加3种铬源未均对G6PDH活性产生显著影响,这与Pan等[29]对罗非鱼的研究结果相一致。
4 结论在葡萄糖含量为35%的饲料中分别添加2.92 mg/kg Cr2O3、16.46 mg/kg CrPic、2.52 mg/kg CrMet对于鲤的生长、饲料利用以及糖利用能力均有促进效果,其中以CrMet效果最好,CrPic次之,而Cr2O3效果最差。
| [1] |
HATLEN B, GRISDALE-HELLAND B, HELLAND S J. Growth, feed utilization and body composition in two size groups of Atlantic halibut (Hippoglossus hippoglossus) fed diets differing in protein and carbohydrate content[J]. Aquaculture, 2005, 249(1/2/3/4): 401-408. |
| [2] |
HEMRE G I, MOMMSEN T P, KROGDAHL A. Carbohydrates in fish nutrition:effects on growth, glucose metabolism and hepatic enzymes[J]. Aquaculture Nutrition, 2002, 8(3): 175-194. DOI:10.1046/j.1365-2095.2002.00200.x |
| [3] |
STONE D A J. Dietary carbohydrate utilization by fish[J]. Reviews in Fisheries Science, 2003, 11(4): 337-369. DOI:10.1080/10641260390260884 |
| [4] |
LI X F, LIU W B, LU K L, et al. Dietary carbohydrate/lipid ratios affect stress, oxidative status and non-specific immune responses of fingerling blunt snout bream, Megalobrama amblycephala[J]. Fish & Shellfish Immunology, 2012, 33(2): 316-323. |
| [5] |
戈贤平, 刘波, 谢骏, 等. 饲料中不同碳水化合物水平对翘嘴红鲌生长及血液指标和糖代谢酶的影响[J]. 南京农业大学学报, 2007, 30(3): 88-93. |
| [6] |
JEEJEEBHOY K N, CHU R C, MARLISS E B, et al. Chromium deficiency, glucose intolerance and neuropathy reversed by chromium supplementation in a patient receiving long-term total parenteral nutrition[J]. The American Journal of Clinical Nutrition, 1977, 30(4): 531-538. DOI:10.1093/ajcn/30.4.531 |
| [7] |
SHWARTZ K, MERTZ W. Chromium (Ⅲ) and glucose tolerance factor[J]. Archives of Biochemistry and Biophysics, 1959, 85(1): 292-295. DOI:10.1016/0003-9861(59)90479-5 |
| [8] |
ANDERSON R A, MERTZ W. Glucose tolerance factor:an essential dietary agent[J]. Trends in Biochemical Sciences, 1977, 2(12): 277-279. DOI:10.1016/0968-0004(77)90280-8 |
| [9] |
HOFFMAN N J, PENQUE B A, HABEGGER K M, et al. Chromium enhances insulin responsiveness via AMPK[J]. The Journal of Nutritional Biochemistry, 2014, 25(5): 565-572. DOI:10.1016/j.jnutbio.2014.01.007 |
| [10] |
LIU T L, WEN H, JIANG M, et al. Effect of dietary chromium picolinate on growth performance and blood parameters in grass carp fingerling, Ctenopharyngodon idellus[J]. Fish Physiology and Bioehemistry, 2010, 36(3): 565-572. DOI:10.1007/s10695-009-9327-5 |
| [11] |
SELCUK Z, TIRIL S U, ALAGIL F, et al. Effects of dietary L-carnitine and chromium picolinate supplementations on performance and some serum parameters in rainbow trout (Oncorhynchus mykiss)[J]. Aquaculture International, 2010, 18(2): 213-221. DOI:10.1007/s10499-008-9237-z |
| [12] |
WANG J, AI Q H, MAI K S, et al. Dietary chromium polynicotinate enhanced growth performance, feed utilization, and resistance to Cryptocaryon irritans in juvenile large yellow croaker (Larmichthys crocea)[J]. Aquaculture, 2014, 432: 321-326. DOI:10.1016/j.aquaculture.2014.05.027 |
| [13] |
PAN Q, LIU S, ZHENG C, et al. The effect of chromium-nicotinic acid on growth, feed utilization and tissue composition in hybrid tilapia, Oreochromis niloticus×O. aureus[J]. Acta Hydrobiologica Sinca, 2002, 26(2): 197-200. |
| [14] |
ARUNKUMAR R I, RAJASEKARAN P, MICHAEL R D. Differential effect of chromium compounds on the immune response of the African mouth breeder Oreochromis mossambicus (Peters)[J]. Fish & Shellfish Immunology, 2000, 10(8): 667-676. |
| [15] |
GATTA P P, THOMPSON K D, SMULLEN R, et al. Dietary organic chromium supplementation and its effect on the immune response of rainbow trout (Oncorhynchus mykiss)[J]. Fish & Shellfish Immunology, 2001, 11(5): 371-382. |
| [16] |
RAWLES S D, GATLIN D M. Carbohydrate utilization in striped bass (Morone saxatilis) and sunshine bass (Morone chrysops×M. saxatilis)[J]. Aquaculture, 1998, 161(1/2/3/4): 201-212. |
| [17] |
WEDEMEYER G A. Effects of rearing conditions on the health and physiological quality of fish in intensive culture[M]//IWAMA G K, PICKERING A D, SUMPTER J P, et al. Fish stress and health in aquaculture. Cambridge: Cambridge University Press, 1997: 35-71.
|
| [18] |
MARCELLO P C, GUSTAVO S C, THALITA R P, et al. Acute aerocystitis in Nile tilapia bred in net cages and supplemented with chromium carbochelate and Saccharomyces cerevisiae[J]. Fish & Shellfish Immunology, 2014, 36(1): 284-290. |
| [19] |
AHMED A R, MOODY A J, FISHER S A, et al. Growth performance and starch utilization in common carp (Cyprinus carpio L.) in response to dietary chromium chloride supplementation[J]. Journal of Trace Elements in Medicine Biology, 2013, 27(1): 45-51. DOI:10.1016/j.jtemb.2012.05.006 |
| [20] |
HERTZ Y, MADER Z, HEPHER B, et al. Glucose metabolism in the common carp (Cyprinus carpio L.):the effects of cobalt and chromium[J]. Aquaculture, 1989, 76(3/4): 255-267. |
| [21] |
EVANS G W, BOWMAN T D. Chromium picolinate increases membrane fluidity and rate of insulin internalization[J]. Journal of Inorganic Biochemistry, 1992, 46(4): 243-250. DOI:10.1016/0162-0134(92)80034-S |
| [22] |
AOAC. Official methods of analysis of AOAC international[S]. 16th ed. Arlington: AOAC International, 1995.
|
| [23] |
HASSID W Z. Chemical procedures for analysis of polysaccharides[J]. Methods in Enzymology, 1957, 3: 34-50. DOI:10.1016/S0076-6879(57)03345-5 |
| [24] |
潘庆, 毕英佐, 颜惜玲, 等. 有机铬对奥尼罗非鱼生长和糖利用的影响[J]. 水生生物学报, 2002, 26(4): 393-399. |
| [25] |
SHIAU S Y, LIN S F. Effect of supplemental dietary chromium and vanadium on the utilization of different carbohydrates in tilapia, Oreochromis niloticus×O. aureus[J]. Aquaculture, 1993, 110(3/4): 321-330. |
| [26] |
SHIAU S Y, LIANG H S. Carbohydrate utilization and digestibility by tilapia, Oreochromis niloticus×O. aureus, are affected by chromic oxide inclusion in the diet[J]. The Journal of Nutrition, 1995, 125(4): 976-982. |
| [27] |
SHIAU S Y, SHY S M. Dietary chromic oxide inclusion level required to maximize glucose utilization in hybrid tilapia, Oreochromis niloticus×O. aureus[J]. Aquaculture, 1998, 161(1/2/3/4): 357-364. |
| [28] |
SHIAU S Y, CHEN M J. Carbohydrate utilization by tilapia (Oreochromis niloticus×O. aureus) as influenced by different chromium sources[J]. The Journal of Nutrition, 1993, 123(10): 1747-1753. DOI:10.1093/jn/123.10.1747 |
| [29] |
PAN Q, LIU S, TAN Y G, et al. The effect of chromium picolinate on growth and carbohydrate utilization in tilapia, Oreochromis niloticus×Oreochromis aureus[J]. Aquaculture, 2003, 225(1/2/3/4): 421-429. |
| [30] |
GATTA P P, PIVA A, PAOLINI M, et al. Effects of dietary organic chromium on gilthead seabream (Sparus aurata L.) performances and liver microsomal metabolism[J]. Aquaculture Research, 2001, 32(1): 60-69. |
| [31] |
BUREAU D P, KIRKLAND J B, CHO C Y. The effects of dietary chromium supplementation on performance, carcass yield and blood glucose of rainbow trout (Oncorhynchus mykiss) fed two practical diets[J]. Journal of Animal Science, 1995, 73(1S): 194-194. |
| [32] |
MERTZ W. Chromium occurrence and function in biological systems[J]. Physiological Reviews, 1969, 49(2): 163-239. DOI:10.1152/physrev.1969.49.2.163 |
| [33] |
SEERLEY R W. Organic chromium and manganese in human nutrition[C]//Proceeding of Alltech's ninth annual symposium. Lyons: [s. n. ], 1993: 41-51.
|
| [34] |
刘太亮. 草鱼对饲料中铬需要量的研究[D]. 硕士学位论文. 武汉: 华中农业大学, 2009.
|
| [35] |
孙敏敏. 有机铬对尼罗罗非鱼生长、IR和GLUT基因表达的影响[D]. 硕士学位论文. 泰安: 山东农业大学, 2013.
|
| [36] |
PAGE T G, SOUTHERN L L, WARD T L, et al. Effect of chromium picolinate on growth and serum and carcass traits of growing-finishing pigs[J]. Journal of Animal Science, 1993, 71(3): 656-662. DOI:10.2527/1993.713656x |
| [37] |
BOLEMAN S J, BIDNER T D, MCMILLIN K W, et al. Effects of postmortem time of calcium chloride injuction on beef tenderness and drip, cooking and total loss[J]. Meat Scuence, 1995, 39(1): 5-41. |
| [38] |
MOONEY K W, CROMWELL G L. Efficacy of chromium picolinate and chromium chloride as potential carcass modifiers in swine[J]. Journal of Animal Science, 1997, 75(10): 2661-2671. DOI:10.2527/1997.75102661x |
| [39] |
GANG X, XU Z R, WU S H, et al. Effects of chromium picolinate on growth performance, carcass charcteristies, serum metabolites and metabolism of lipid in pig[J]. Asian-Australlan Journal of Animal Science, 2001, 14(2): 258-262. DOI:10.5713/ajas.2001.258 |
| [40] |
NRC. The role of chromium in animal nutrition[S]. Washington, D. C. : National Academy Press, 2012.
|
| [41] |
STEELE N C, ROSEBROUGHT R W. Effect of trivalent chromium on hepatic lipogenesis by the turkey poult[J]. Poultry Science, 1981, 60(3): 617-622. DOI:10.3382/ps.0600617 |
| [42] |
CAMPBELL W W, POLANSKY M M, BRYDEN N A, et al. Exercise training and dietary chromium effects on glycogen synthase, phosphorylase and total protein in rats[J]. The Journal of Nutriton, 1989(4): 653-660. |
| [43] |
李洪霞. 吡啶甲醇铬对罗非鱼生长, 肌肉品质及抗应激能力的影响[D]. 硕士学位论文. 泰安: 山东农业大学, 2014.
|
| [44] |
AMOIKON E K, FERNANDEZ J M, SOUTHERN L L, et al. Effect of chromium tripicolinate on growth, glucose tolerance, insulin sensitivity, plasma metabolites, and growth hormone in pigs[J]. Journal of Animal Science, 1995, 73(4): 1123-1130. DOI:10.2527/1995.7341123x |
| [45] |
SAHIN N, ONDERCI M, SAHIN K. Effects of dietary chromium and zinc on egg production, egg quality, and some blood metabolites of laying hens reared under low ambient temperature[J]. Biological Trace Element Research, 2002, 85(1): 47-58. DOI:10.1385/BTER:85:1 |
| [46] |
NRC. Nutrient requirements of fish and shrimp[S]. Washington, D. C. : National Academies Press, 2011.
|
| [47] |
KRAMER P, NOWAK T. The preparation and characterization of Cr (Ⅲ) and Co (Ⅲ) complexes of GDP and GTP and their interactions with avian phosphoenolpyruvate carboxykinase[J]. Journal of Inorganic Biochemistry, 1988, 32(2): 135-151. DOI:10.1016/0162-0134(88)80022-9 |
| [48] |
GARDNER G E, PETHICK D W, SMITH G. Effect of chromium chelative supplementation of the metabolism of glycogen and lipid in adult Merino sheep[J]. Australia Journal of Agriculture Research, 1988, 49: 137. |
| [49] |
CAPPAI G, SONGINI M, DORIA A, et al. Increased prevalence of proliferative retinopathy in patients with typeⅠdiabetes who are deficient in glucose-6-phosphate dehydrogenase[J]. Diabetologia, 2011, 54(6): 1539-1542. DOI:10.1007/s00125-011-2099-3 |




