2. 湖南畜禽安全生产协同创新中心, 长沙 410128;
3. 湖南家禽安全生产工程技术研究中心, 长沙 410128
2. Hunan Co-Innovation Center of Animal Production Safety, Changsha 410128, China;
3. Hunan Engineering Research Center of Poultry Production Safety, Changsha 410128, China
虾青素(astaxanthin)又名变胞藻黄素或虾红素,存在于各种微生物和海洋动物中如红法夫酵母、微藻、三文鱼、磷虾以及复杂植物和一些鸟类中[1-2],天然虾青素主要来源于海鲜的提取[3-4]。虾青素在1938年被德国化学家理查德·库恩首次从龙虾体内提取并被鉴定,其抗氧化活性远远优于其他类胡萝卜素,是维生素C的6 000倍,是辅酶Q10的800倍,是维生素E的550~1 000倍,是花青素的200倍,因此,被称为“抗氧化之王”[5]。随着抗生素禁用,虾青素凭借其天然、无残留、具有抗氧化及提高机体免疫功能的特点,成为一种具有潜力的绿色添加剂,但现阶段关于虾青素在动物生产中应用的研究较少。因此,本文阐述了虾青素的生理功能,总结了其在动物生产中的作用效果与可能的作用机制,旨在为虾青素开发和利用提供理论参考。
1 虾青素的化学结构和存在形式 1.1 虾青素的化学结构虾青素(分子式C40H52O4),即3,3′-二羟基-4,4,-二酮基-β,β′-胡萝卜素,熔点为224 ℃,不溶于水,易溶于有机溶剂[6],化学结构如图 1所示。由于共轭双键的存在,虾青素容易发生顺反异构,生成大量的几何异构体,顺式异构体中氢原子有空间位阻,因此,虾青素主要以全反式异构体形式存在。图 1-A中为虾青素立体异构体形式3S、3′S;图 1-B和图 1-C分别为单酯和双酯形式;图 1-D和图 1-E分别为立体异构体3R、3′S和3R、3′R。天然虾青素主要以3S、3′S和3R、3′R为主,而合成虾青素以3S、3′S,3R、3′S,3R、3′R的比值为1 : 2 : 1固定存在[1, 7-8]。
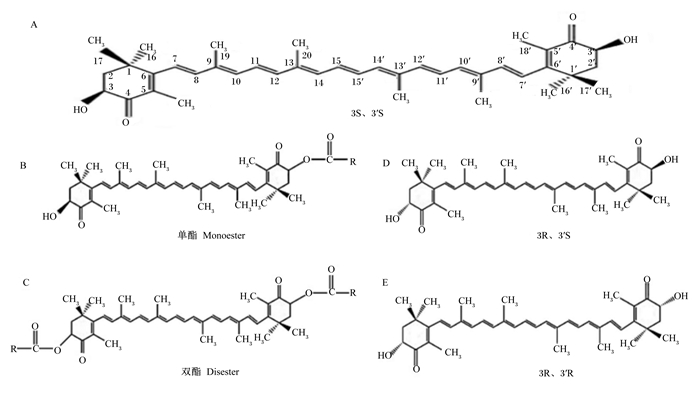
|
图 1 虾青素的化学结构 Fig. 1 Chemical structure of astaxanthin |
由于共轭双键具有疏水性,虾青素在细胞膜中两端的紫罗兰酮环分别嵌入磷脂双分子层,连接细胞膜的内外[6]。自然界中的虾青素主要以游离态和酯化态2种形式存在,化学合成的虾青素均为3S、3′R的游离态。在生物体内天然虾青素主要以3S、3′S或3R、3′R的酯化形式存在,其中红法夫酵母中的虾青素主要以酯化的3R、3′R为主[9],雨生红球藻中的虾青素含5%游离酯、25%双酯和70%单酯[5],主要以3S、3′S为主。游离态的虾青素容易氧化,可与蛋白质和脂质结合,形成蓝灰色的复合物,在受到光和热等环境因素胁迫时发生游离现象,呈现出红色。
2 虾青素的吸收代谢机制虾青素的生物利用率较低,主要是由于其水溶性和分散性差,因此,消化液中有限的溶解度会影响肠上皮细胞对虾青素的吸收,使其与乳糜微粒结合进入到淋巴中[3, 10-11]。动物摄入的虾青素在小肠的混合胶束中发生溶解,这些胶束是胆汁酸、磷脂、胆固醇、脂肪酸和单酰基甘油的混合物。虾青素通过胞质膜磷脂双层膜的自由扩散从胶束转移到上皮细胞,被肠黏膜细胞部分吸收后与乳糜微粒结合,含有虾青素的乳糜微粒进入血液后被脂蛋白脂肪酶消化,储存在肝脏中,然后与极低密度脂蛋白(very low density lipoprotein,VLDL)、低密度脂蛋白(low density lipoprotein,LDL)和高密度脂蛋白(high density lipoprotein,HDL)结合,最终通过体循环运输到皮肤、肌肉和性腺等组织中。肝脏是类胡萝卜素的主要代谢器官,可将虾青素分解成其他色素或者不含色素的代谢物,并随胆汁分泌到肠道,进行重吸收,未被代谢的虾青素则被重新包装成VLDL重新进入肝脏,最后经肾脏排出。到达肌肉组织中的虾青素通过疏水键与肌动球蛋白结合形成复合物,沉积在肌节和结缔组织当中,在VLDL和LDL流经血液时,部分复合物被胆固醇酯转移蛋白(cholesterol ester transfer protein,CETP)转移到HDL中,运送至到皮肤和性腺,在性腺和卵细胞中,虾青素与卵黄脂蛋白可形成复合物并沉积[12]。虾青素酯在被吸收和转运前,需要被胆固醇酯酶水解[13],Coombes等[14]发现人血液中游离的虾青素含量可达0.19 μmol/L,说明游离态虾青素可能在人体循环中被优先吸收或选择性转运[15]。此外,动物试验也显示,虾青素的生物利用度受其酯化状态的影响[16],表明将其添加到食品、饮料和医药产品中时需使用表面活性剂和其他载体[17]。
由于人工合成的虾青素均为游离态,所以其稳定性低于天然虾青素,而且不能以酯化形式存在,产生多变的颜色,在体内的吸收率和沉积率均低于天然虾青素。人工合成虾青素可能因混入各种杂质或非天然副产物等引起生物利用安全性问题。
3 虾青素的主要生物学功能 3.1 着色功能虾青素进入生物体后可以不经修饰或生物转化而直接贮存沉积在组织中,与肌红蛋白非特异性结合,呈现出红色[18]。虾青素的着色机制主要包括2种:第1种即蛋白质复合物中虾青素的物理排列,与游离的虾青素(双键角度约50°)相比,虾青素的C6-C7键的扭曲角度大大降低,形成6-S-反式构象,呈现出蓝灰色。此外,在甲壳素中配对的虾青素之间通过激子偶联,端环与蛋白质结合形成的多烯链共面,并沿复合形式的共轭阵列增强重叠,从而使甲壳类动物的壳呈现出红色[19-20]。第2种涉及虾青素在络合过程中发生的可逆电离,被认为是α-羟基环己酮残基去质子化和质子转移的结果。虾青素在酸和碱诱导下,与去质子化的蛋白质结合形成烯醇化物,一端与3号氧原子和组氨酸之间的氢键结合,另一端与4号氧原子和水-酪氨酸对之间的氢键结合,高温条件会导致氢键断裂,呈现出由蓝灰色至红色的变化[21]。
3.2 抗氧化功能活性氧簇(reactive oxygen species,ROS)过量是导致机体氧化损伤的主要因素。机体内过量的ROS通过链式反应与蛋白质、脂质、碳水化合物及核酸生成不同氧化产物,造成氧化损伤。虾青素具有诱捕活性氧、增强细胞阻断氧化应激的能力[22],通过清除过量的ROS以终止链式反应,发挥其抗氧化功能[23]。虾青素有比胡萝卜素更长的共轭体系,较长的共轭体系使得虾青素分子可以更强、更活跃地吸收单线态氧的能量,并将吸收的能量以热能的形式耗散。其抗氧化功能主要源于其分子结构,通过两端极性紫罗兰酮环和磷脂双分子层融合,以自由扩散的方式穿过细胞膜[24],虾青素可以存在于细胞膜和脂蛋白中,不改变双层膜的结构完整性或电子密度,其多烯链能捕获细胞膜中的自由基,末端环可以清除细胞膜外部和内部的自由基[18]。最新研究发现,幽门螺旋杆菌会导致线粒体中ROS水平上升,使胃肠上皮细胞中超氧化物歧化酶的活性降低,而用5 μmol/L虾青素预处理胃肠上皮细胞后,其超氧化物歧化酶的活性不会受到幽门螺旋杆菌的影响,说明虾青素可能对线粒体具有保护作用[25]。
3.3 免疫功能炎症的特点是血浆和细胞产生促炎因子的能力增强,其中,巨噬细胞产生ROS是引发机体产生炎症反应的关键潜在因素[26-27]。有研究表明,虾青素能减少巨噬细胞中ROS的积累,抑制核因子-κB(nuclear factor-kappaB,NF-κB)诱导的炎性介质的产生[28-30]。核因子E2相关因子2(nuclear factor erythroid-2-related factor2,Nrf2)通过增加抗氧化酶的产生和抑制NF-κB信号通路使机体抗炎功能增强[31-32]。虾青素除在抑制核因子-κB p65(p65 nuclear factor-kappaB,NF-κB p65)易位方面发挥作用外,还通过激活Nrf2通路,限制炎性介质产生[33]。此外,虾青素能阻断Toll样受体4的表达,抑制NF-κB p65的磷酸化和NF-κB的降解[34],达到抗炎效果。Han等[35]发现虾青素抑制了信号转导和转录激活因子3的DNA结合活性,从而抑制脂多糖诱导的氧化反应、神经炎症反应和淀粉样蛋白形成。Park等[36]发现虾青素通过抑制NF-κB抑制蛋白的降解,进而抑制NF-κB的活化,抑制了炎性细胞因子如前列腺素E2(prostaglandin E2,PGE2)、肿瘤坏死因子(tumor necrosis factor,TNF)、白细胞介素-1β(interleukin-1β,IL-1β)、诱导性一氧化氮合酶(inducible nitric oxide synthase,iNOS)、环氧合酶2(cyclooxygenase 2,COX2)的表达,以及减少一氧化氮(nitric oxide,NO)的产生。因此,虾青素主要通过抑制巨噬细胞中ROS的积累和下调促炎因子途径提高机体免疫的功能。
3.4 预防心血管疾病氧化应激和炎症是动脉粥样硬化性心血管疾病的病理生理特征。虾青素是一种潜在的治疗动脉粥样硬化性心血管疾病的药物[37-38],对心肌[39-40]、脑[41]、肝脏[42]、肾脏[43]等不同缺血再灌注损伤体内模型具有保护作用。此外,虾青素能降低高脂饮食引起的高脂血症大鼠的凝血、血小板聚集和纤溶活性,这些作用与虾青素降低血脂和脂蛋白含量、产生抗氧化剂和保护内皮细胞有关[44]。临床研究也发现,虾青素具有轻微的降血糖作用[45]。然而,关于虾青素的代谢和药代动力学等方面的认识还有待进一步研究。
3.5 抗癌作用虾青素对不同类型的癌症都具有抑制作用,包括口腔癌[46]、膀胱癌[47]、结肠癌[48-49]、白血病[50]和肝细胞癌[51]、肺癌[52]和乳腺癌[53]等。其抗癌作用归因于其具有选择性抑制细胞增殖和调节细胞凋亡的功能[54-55]。此外,虾青素的抗癌机制与细胞膜的稳定性和膜蛋白基因表达有关,它通过改变膜稳定性和基因表达量来调节细胞间物质交换,维持细胞的正常功能[56-57]。
3.6 保护神经系统虾青素被认为是一种潜在的神经保护剂,因为它能够跨越脑血屏障,保护大脑免受急性损伤和慢性神经退行性病变的伤害[58]。有研究表明,虾青素具有促进或维持神经可塑性的潜力,可以通过促进神经发生和促进神经功能的增强而增强认知功能[59],Lobos等[60]发现,淀粉样蛋白β肽寡聚体会促使神经元线粒体产生过量的ROS,导致神经元损伤,而虾青素能够通过钙神经素/活化T细胞核因子(nuclear factors of activated T cells, NFAT)阻止淀粉样蛋白β肽寡聚体对突触的毒性作用。此外,虾青素对帕金森病诱导的神经衰退过程具有抑制作用[61],Lee等[62]研究表明,虾青素对1-甲基-4-苯基-1, 2, 3, 6-四氢吡啶(MPTP)诱导的帕金森病(idiopathic Parkinson’s disease,PD)小鼠模型中黑质神经元凋亡具有缓解作用,这种作用可归因于B淋巴细胞瘤-2(B-cell lymphoma-2,Bcl-2)蛋白表达的上调和促凋亡基因Bax(Bcl-associated x protein,Bax)的表达降低,从而抑制半胱氨酸天冬氨酸蛋白酶3的活化。Altunrende等[63]研究表明,虾青素对钙离子稳态具有保护作用,而钙离子能调节神经元中的许多细胞过程,通过调节突触处谷氨酸的释放量保护神经元功能的正常发挥。
4 虾青素在动物生产中的应用 4.1 虾青素在猪生产中的应用Do等[64]研究表明,饲粮中添加0.5 mg/kg虾青素促进了热应激下母猪卵母细胞的成熟,进而可提高其受精率及胚胎存活率,使热应激下母猪繁殖性能提高,这可能是虾青素缓解了高温对猪卵母细胞减数分裂的负面影响。Basioura等[65]和Lee等[66]发现,虾青素对公猪精液质量具有保护作用,可能是虾青素降低了精液中ROS的含量,从而提高了精子的寿命。林建坤等[67]研究表明,饲粮中添加4 400 mg/kg虾青素和双乙酸钠的混合制剂能够显著提高28日龄三元杂交断奶仔猪血清和空肠黏膜超氧化物歧化酶、谷胱甘肽过氧化物酶的活性,提高了机体抗氧化能力,促进空肠细胞结构和功能的完整性,提高肠道对养分的消化能力,从而提高了生长性能。此外,由于肌肉中的不饱和脂肪酸和蛋白质分子与空气中的氧气结合形成过氧化物,会导致脂质分解和多肽链断裂,使肌肉的持水性和风味下降[68],虾青素具有强大的抗氧化功能和着色功能,可通过其在肌肉中的沉积,降低肌肉的脂质和蛋白质氧化速度,有望提高肉品质。
4.2 虾青素在禽类生产中的应用虾青素具有提高肉鸡生长性能、改善肉品质的作用。付兴周等[69]研究发现,肉鸡饲粮中添加1%虾青素复合添加剂,其平均日增重和饲料转化率显著提高,宰后肌肉的pH下降速度显著降低。Perenlei等[70]研究表明,饲粮中添加20 mg/kg含虾青素的酵母粉可显著增加肉鸡屠宰后肌肉的红度(a*)和黄度(b*)值,降低烹饪损失,且120 h时虾青素组的总游离氨基酸含量显著高于对照组。Inoue等[71]也发现,饲粮中添加0.15%富含虾青素的干细胞粉可显著增加肉鸡肌肉的红度和黄度。究其原因,虾青素可以在血浆、肝脏、性腺和大腿肌肉中富集,一方面由于其着色功能增加了胸肌和腿部肌肉的红度和黄度值,另一方面降低了热应激状态下肌肉中丙二醛(MDA)的含量,提高了肌肉的抗氧化能力。
在蛋鸡方面,吴斯诺等[72]发现,饲粮中添加80 mg/kg虾青素显著提高了太行鸡的生产性能,降低了料蛋比,同时改善了蛋黄颜色和哈氏单位。王钧艺等[73]研究表明,蛋黄颜色在一定范围内随着虾青素复合添加剂含量的增加而加深。这是由于蛋黄颜色是蛋形成过程中,虾青素与脂蛋白结合,通过体循环进入到蛋黄中,转化成棕油酸二酯在蛋黄内沉积,使蛋黄的黄色加深或呈现出红色[74]。
4.3 虾青素在水产养殖中的应用饲料中添加一定量的虾青素可以提高大黄鱼幼鱼[75]和锦鲤[76]的生长性能、凡纳滨对虾[77]等水产品的存活率,其原因是虾青素可以增强鱼虾的免疫力,提高对高氮、低氧环境的耐受力[78-79]。虾青素还显著提高了水产品的色素沉积,对于甲壳类动物,如蟹、虾等,色素沉积主要在壳、性腺和肝胰腺上,而对肉色无显著影响;就鱼类而言,鱼体颜色鲜艳,而鱼肉却是白色。李小兵等[80]通过向金曼龙饲料中添加200 mg/kg的虾青素发现,在第15天时鱼体、鱼鳞、鱼鳍、鱼皮中色素沉积量达最高值,且不同组织部位的含量从高至低依次为鱼皮、鱼鳍、鱼鳞、鱼体、鱼头、鱼肉,体现了虾青素在不同部位沉积能力的差异。虾青素的作用效果与添加量有关,但其含量沉积过高会对机体产生额外的代谢,因此,多余的虾青素会通过代谢排出体外。
5 小结综上所述,虾青素具有多种生理功能,作为饲料添加剂在动物生产中具有提高动物的免疫力和产品品质的功能,但目前在动物生产应用方面的研究较少,且作用机制尚不清楚。因此,需要进一步深入研究虾青素在动物生产中的适宜添加量、作用效果和作用机制,为虾青素在动物生产中的利用提供数据参考。此外,最新研究表明,利用聚合物[81-82]、脂质[83-84]和环糊精体系[85]对虾青素的溶解度和稳定性均有改善,但对于包被后虾青素的抗氧化能力尚缺乏评估,因此,需深入研究来确定虾青素载体系统的生物学效价,以期为虾青素在动物生产中更好的应用提供理论依据。
| [1] |
FASSETT R G, COOMBES J S. Astaxanthin:a potential therapeutic agent in cardiovascular disease[J]. Marine Drugs, 2011, 9(3): 447-465. DOI:10.3390/md9030447 |
| [2] |
CABALLO C, COSTI E M, SICILIA M D, et al. Determination of supplemental feeding needs for astaxanthin and canthaxanthin in salmonids by supramolecular solvent-based microextraction and liquid chromatography-UV/VIS spectroscopy[J]. Food Chemistry, 2012, 134(2): 1244-1249. |
| [3] |
NAGAO A. Absorption and metabolism of dietary carotenoids[J]. BioFactors, 2011, 37(2): 83-87. DOI:10.1002/biof.151 |
| [4] |
SANDMANN G. Carotenoid biosynthesis in microorganisms and plants[J]. European Journal of Biochemistry, 1994, 223(1): 7-24. DOI:10.1111/j.1432-1033.1994.tb18961.x |
| [5] |
LORENZ R T, CYSEWSKI G R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin[J]. Trends in Biotechnology, 2000, 18(4): 160-167. DOI:10.1016/S0167-7799(00)01433-5 |
| [6] |
AMBATI R R, PHANG S M, RAVI S, et al. Astaxanthin:sources, extraction, stability, biological activities and its commercial applications—a review[J]. Marine Drugs, 2014, 12(1): 128-152. DOI:10.3390/md12010128 |
| [7] |
European Food Safety Authority (EFSA). Opinion of the scientific panel on additives and products or substances used in animal feed (FEEDAP) on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance[J]. EFSA Journal, 2005, 3(6): 223. DOI:10.2903/j.efsa.2005.223 |
| [8] |
牟志春, 张明, 张艺兵, 等. 高效液相色谱法快速鉴别人工合成虾青素养殖的三文鱼[J]. 食品科学, 2009, 30(22): 318-320. DOI:10.3321/j.issn:1002-6630.2009.22.075 |
| [9] |
孙伟红, 林洪, 翟毓秀, 等. 红发夫酵母中3R, 3'R-虾青素的分离纯化和结构鉴定[J]. 食品科学, 2014, 35(11): 79-82. DOI:10.7506/spkx1002-6630-201411016 |
| [10] |
BILIA A R, ISACCHI B, RIGHESCHI C, et al. Flavonoids loaded in nanocarriers:an opportunity to increase oral bioavailability and bioefficacy[J]. Food and Nutrition Sciences, 2014, 5(13): 1212-1227. DOI:10.4236/fns.2014.513132 |
| [11] |
SMALL D M, PENKETT S A, CHAPMAN D. Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy[J]. Biochimica et Biophysica Acta:Lipids and Lipid Metabolism, 1969, 176(1): 178-189. DOI:10.1016/0005-2760(69)90086-1 |
| [12] |
RAJASINGH H, ØYEHAUG L, VÅGE D I, et al. Carotenoid dynamics in Atlantic salmon[J]. BMC Biology, 2006, 4: 10. DOI:10.1186/1741-7007-4-10 |
| [13] |
JACOBSSON L S, YUAN X M, ZIEDÉN B, et al. Effects of α-tocopherol and astaxanthin on LDL oxidation and atherosclerosis in WHHL rabbits[J]. Atherosclerosis, 2004, 173(2): 231-237. |
| [14] |
COOMBES J S, SHARMAN J E, FASSETT R G. Astaxanthin has no effect on arterial stiffness, oxidative stress, or inflammation in renal transplant recipients:a randomized controlled trial (the XANTHIN trial)[J]. The American Journal of Clinical Nutrition, 2016, 103(1): 283-289. DOI:10.3945/ajcn.115.115477 |
| [15] |
CORAL-HINOSTROZA G N, YTRESTØYL T, RUYTER B, et al. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3'R/S isomers of astaxanthin fatty acyl diesters[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2004, 139(1/2/3): 99-110. |
| [16] |
YANG Y, PHAM T X, WEGNER C J, et al. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice[J]. British Journal of Nutrition, 2014, 112(11): 1797-1804. DOI:10.1017/S0007114514002554 |
| [17] |
ANARJAN N, TAN C P. Developing a three component stabilizer system for producing astaxanthin nanodispersions[J]. Food Hydrocolloids, 2013, 30(1): 437-447. |
| [18] |
MCNULTY H P, BYUN J, LOCKWOOD S F, et al. Differential effects of carotenoids on lipid peroxidation due to membrane interactions:X-ray diffraction analysis[J]. Biochimica et Biophysica Acta:Biomembranes, 2007, 1768(1): 167-174. DOI:10.1016/j.bbamem.2006.09.010 |
| [19] |
STRAMBI A, DURBEEJ B. Excited-state modeling of the astaxanthin dimer predicts a minor contribution from exciton coupling to the bathochromic shift in crustacyanin[J]. The Journal of Physical Chemistry B, 2009, 113(15): 5311-5317. DOI:10.1021/jp810754s |
| [20] |
CHRISTENSSON N, žÍDEK K, MAGDAONG N C M, et al. Origin of the bathochromic shift of astaxanthin in lobster protein:2D electronic spectroscopy investigation of β-crustacyanin[J]. The Journal of Physical Chemistry B, 2013, 117(38): 11209-11219. DOI:10.1021/jp401873k |
| [21] |
BEGUM S, CIANCI M, DURBEEJ B, et al. On the origin and variation of colors in lobster carapace[J]. Physical Chemistry Chemical Physics, 2015, 17(26): 16723-16732. DOI:10.1039/C4CP06124A |
| [22] |
RAO A R, BASKARAN V, SARADA R, et al. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass—a repeated dose study[J]. Food Research International, 2013, 54(1): 711-717. DOI:10.1016/j.foodres.2013.07.067 |
| [23] |
XUE X L, HAN X D, LI Y, et al. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis[J]. Stem Cell Research & Therapy, 2017, 8: 7. |
| [24] |
ZULUAGA M, GUEGUEN V, LETOURNEUR D, et al. Astaxanthin-antioxidant impact on excessive reactive oxygen species generation induced by ischemia and reperfusion injury[J]. Chemico-Biological Interactions, 2018, 279: 145-158. DOI:10.1016/j.cbi.2017.11.012 |
| [25] |
KIM S H, LIM J W, KIM H. Astaxanthin prevents decreases in superoxide dismutase 2 level and superoxide dismutase activity in Helicobacter pylori-infected gastric epithelial cells[J]. Journal of Cancer Prevention, 2019, 24(1): 54-58. DOI:10.15430/JCP.2019.24.1.54 |
| [26] |
BALCE D R, LI B Q, ALLAN E R O, et al. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms[J]. Blood, 2011, 118(15): 4199-4208. DOI:10.1182/blood-2011-01-328906 |
| [27] |
KAULMANN A, BOHN T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention[J]. Nutrition Research, 2014, 34(11): 907-929. DOI:10.1016/j.nutres.2014.07.010 |
| [28] |
LEE S J, BAI S K, LEE K S, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing IκB kinase-dependent NF-κB activation[J]. Molecules and Cells, 2003, 16(1): 97-105. |
| [29] |
JOUNG E J, GWON W G, SHIN T, et al. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components[J]. Journal of Applied Phycology, 2017, 29(1): 563-573. DOI:10.1007/s10811-016-0954-9 |
| [30] |
HU B Y, ZHANG H, MENG X L, et al. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages[J]. Journal of Ethnopharmacology, 2014, 153(3): 846-853. DOI:10.1016/j.jep.2014.03.059 |
| [31] |
SUN C C, LI S J, YANG C L, et al. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-κB signaling pathway[J]. Journal of Biological Chemistry, 2015, 290(29): 17784-17795. DOI:10.1074/jbc.M115.655019 |
| [32] |
KOBAYASHI E H, SUZUKI T, FUNAYAMA R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription[J]. Nature Communications, 2016, 7: 11624. DOI:10.1038/ncomms11624 |
| [33] |
KOBAYASHI E H, SUZUKI T, FUNAYAMA R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription[J]. Nature Communications, 2016, 7: 11624. DOI:10.1038/ncomms11624 |
| [34] |
LI M Y, SUN L, NIU X T, et al. Astaxanthin protects lipopolysaccharide-induced inflammatory response in Channa argus through inhibiting NF-κB and MAPKs signaling pathways[J]. Fish & Shellfish Immunology, 2019, 86: 280-286. |
| [35] |
HAN J H, LEE Y S, IM J H, et al. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway[J]. Marine Drugs, 2019, 17(2): 123. DOI:10.3390/md17020123 |
| [36] |
PARK J H, YEO I J, HAN J H, et al. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model[J]. Experimental Dermatology, 2018, 27(4): 378-385. DOI:10.1111/exd.13437 |
| [37] |
KISHIMOTO Y, YOSHIDA H, KONDO K. Potential anti-atherosclerotic properties of astaxanthin[J]. Marine Drugs, 2016, 14(2): 35. DOI:10.3390/md14020035 |
| [38] |
VISIOLI F, ARTARIA C. Astaxanthin in cardiovascular health and disease:mechanisms of action, therapeutic merits, and knowledge gaps[J]. Food & Function, 2017, 8(1): 39-63. |
| [39] |
ADLURI R S, THIRUNAVUKKARASU M, ZHAN L J, et al. Cardioprotective efficacy of a novel antioxidant mix VitaePro against ex vivo myocardial ischemia-reperfusion injury[J]. Cell Biochemistry and Biophysics, 2013, 67(2): 281-286. DOI:10.1007/s12013-011-9300-7 |
| [40] |
HE W K, SU Q, LIANG J B, et al. The protective effect of nicorandil on cardiomyocyte apoptosis after coronary microembolization by activating Nrf2/HO-1 signaling pathway in rats[J]. Biochemical and Biophysical Research Communications, 2018, 496(4): 1296-1301. DOI:10.1016/j.bbrc.2018.02.003 |
| [41] |
LU Y P, LIU S Y, SUN H, et al. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo[J]. Brain Research, 2010, 1360: 40-48. DOI:10.1016/j.brainres.2010.09.016 |
| [42] |
LI J J, WANG F, XIA Y J, et al. Astaxanthin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy via the ROS/MAPK pathway in mice[J]. Marine Drugs, 2015, 13(6): 3368-3387. DOI:10.3390/md13063368 |
| [43] |
QIU X F, FU K, ZHAO X Z, et al. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice[J]. Journal of Translational Medicine, 2015, 13: 28. DOI:10.1186/s12967-015-0388-1 |
| [44] |
DENG Z Y, SHAN W G, WANG S F, et al. Effects of astaxanthin on blood coagulation, fibrinolysis and platelet aggregation in hyperlipidemic rats[J]. Pharmaceutical Biology, 2017, 55(1): 663-672. DOI:10.1080/13880209.2016.1261905 |
| [45] |
URSONIU S, SAHEBKAR A, SERBAN M C, et al. Lipid profile and glucose changes after supplementation with astaxanthin:a systematic review and meta-analysis of randomized controlled trials[J]. Archives of Medical Science, 2015, 11(2): 253-266. |
| [46] |
KAVITHA K, KOWSHIK J, KISHORE T K K, et al. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer[J]. Biochimica et Biophysica Acta:General Subjects, 2013, 1830(10): 4433-4444. DOI:10.1016/j.bbagen.2013.05.032 |
| [47] |
TANAKA T, MORISHITA Y, SUZUI M, et al. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin[J]. Carcinogenesis, 1994, 15(1): 15-19. DOI:10.1093/carcin/15.1.15 |
| [48] |
NAGENDRAPRABHU P, SUDHANDIRAN G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFκB and COX-2[J]. Investigational New Drugs, 2011, 29(2): 207-224. DOI:10.1007/s10637-009-9342-5 |
| [49] |
YASUI Y, HOSOKAWA M, MIKAMI N, et al. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines[J]. Chemico-Biological Interactions, 2011, 193(1): 79-87. DOI:10.1016/j.cbi.2011.05.006 |
| [50] |
ZHANG X, ZHAO W E, HU L Q, et al. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells[J]. Archives of Biochemistry and Biophysics, 2011, 512(1): 96-106. DOI:10.1016/j.abb.2011.05.004 |
| [51] |
SONG X D, WANG M R, ZHANG L X, et al. Changes in cell ultrastructure and inhibition of JAK1/STAT3 signaling pathway in CBRH-7919 cells with astaxanthin[J]. Toxicology Mechanisms and Methods, 2012, 22(9): 679-686. |
| [52] |
LIAO K S, WEI C L, CHEN J C, et al. Astaxanthin enhances pemetrexed-induced cytotoxicity by downregulation of thymidylate synthase expression in human lung cancer cells[J]. Regulatory Toxicology and Pharmacology, 2016, 81: 353-361. DOI:10.1016/j.yrtph.2016.09.031 |
| [53] |
BHARATHIRAJA S, MANIVASAGAN P, BUI N Q, et al. Cytotoxic induction and photoacoustic imaging of breast cancer cells using astaxanthin-reduced gold nanoparticles[J]. Nanomaterials, 2016, 6(4): 78. DOI:10.3390/nano6040078 |
| [54] |
KOWSHIK J, BABA A B, GIRI H, et al. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer[J]. PLoS One, 2014, 9(10): e109114. DOI:10.1371/journal.pone.0109114 |
| [55] |
ZHANG J J, XU P, WANG Y L, et al. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission[J]. Journal of Cellular and Molecular Medicine, 2015, 19(9): 2215-2231. DOI:10.1111/jcmm.12609 |
| [56] |
TORTELOTE G G, REIS R R, DE ALMEIDA MENDES F, et al. Complexity of the Wnt/β-catenin pathway:searching for an activation model[J]. Cellular Signalling, 2017, 40: 30-43. DOI:10.1016/j.cellsig.2017.08.008 |
| [57] |
KOWALCZYK T, SITAREK P, SKAŁA E, et al. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells[J]. Cytotechnology, 2019, 71(1): 165-180. DOI:10.1007/s10616-018-0274-9 |
| [58] |
SHEN H, KUO C C, CHOU J, et al. Astaxanthin reduces ischemic brain injury in adult rats[J]. The FASEB Journal, 2009, 23(6): 1958-1968. DOI:10.1096/fj.08-123281 |
| [59] |
GRIMMIG B, KIM S H, NASH K, et al. Neuroprotective mechanisms of astaxanthin:a potential therapeutic role in preserving cognitive function in age and neurodegeneration[J]. GeroScience, 2017, 39(1): 19-32. DOI:10.1007/s11357-017-9958-x |
| [60] |
LOBOS P, BRUNA B, CORDOVA A, et al. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers[J]. Neural Plasticity, 2016, 2016: 3456783. |
| [61] |
GRIMMIG B, MORGANTI J, NASH K, et al. Immunomodulators as therapeutic agents in mitigating the progression of Parkinson's disease[J]. Brain Sciences, 2016, 6(4): 41. DOI:10.3390/brainsci6040041 |
| [62] |
LEE D H, KIM C S, LEE Y J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro[J]. Food and Chemical Toxicology, 2011, 49(1): 271-280. DOI:10.1016/j.fct.2010.10.029 |
| [63] |
ALTUNRENDE M E, GEZEN-AK D, ATASOY I L, et al. The role of astaxanthin on transcriptional regulation of NMDA receptors voltage sensitive calcium channels and calcium binding proteins in primary cortical neurons[J]. Archives of Neuropsychiatry, 2018, 55(4): 295. |
| [64] |
DO L T K, LUU V V, MORITA Y, et al. Astaxanthin present in the maturation medium reduces negative effects of heat shock on the developmental competence of porcine oocytes[J]. Reproductive Biology, 2015, 15(2): 86-93. DOI:10.1016/j.repbio.2015.01.002 |
| [65] |
BASIOURA A, BOSCOS C M, PARRILLA I, et al. Effect of astaxanthin on the quality of boar sperm stored at 17 ℃, incubated at 37 ℃ or under in vitro conditions[J]. Reproduction in Domestic Animals, 2018, 53(2): 463-471. DOI:10.1111/rda.13133 |
| [66] |
LEE E, KIM D. Effects of astaxanthin on miniature pig sperm cryopreservation[J]. BioMed Research International, 2018, 2018: 6784591. |
| [67] |
林建坤, 郭瑞萍. 虾青素和双乙酸钠联用对断奶仔猪生产性能和抗氧化能力的影响[J]. 饲料研究, 2014(13): 28-32. |
| [68] |
林映才, 马现永, 蒋宗勇. 猪肉氧化与营养调控[J]. 饲料工业, 2008, 29(16): 1-6. DOI:10.3969/j.issn.1001-991X.2008.16.001 |
| [69] |
付兴周, 路志芳, 李东. 虾青素复合添加剂对肉鸡生长性能及肉质的影响[J]. 畜牧与兽医, 2017, 49(1): 27-30. |
| [70] |
PERENLEI G, TOJO H, OKADA T, et al. Effect of dietary astaxanthin rich yeast, Phaffia rhodozyma, on meat quality of broiler chickens[J]. Animal Science Journal, 2014, 85(10): 895-903. DOI:10.1111/asj.12221 |
| [71] |
INOUE H, SHIMAMOTO S, TAKAHASHI H, et al. Effects of astaxanthin-rich dried cell powder from Paracoccus carotinifaciens on carotenoid composition and lipid peroxidation in skeletal muscle of broiler chickens under thermo-neutral or realistic high temperature conditions[J]. Animal Science Journal, 2019, 90(2): 229-236. DOI:10.1111/asj.13141 |
| [72] |
吴斯诺, 臧素敏, 郭欣, 等. 日粮添加虾青素对太行鸡生产性能和蛋品质的影响[J]. 中国家禽, 2018, 40(14): 32-35. |
| [73] |
王钧艺, 闫研, 金辉东, 等. 虾青素复合添加剂对蛋鸡生产性能、蛋品质、蛋黄抗氧化指标和蛋黄中虾青素含量的影响[J]. 动物营养学报, 2018, 30(7): 2700-2706. DOI:10.3969/j.issn.1006-267x.2018.07.030 |
| [74] |
范志勇, 沈静玲, 贺建华, 等. 家禽产品着色的原理及应用[J]. 中国家禽, 2004, 26(18): 26-27. DOI:10.3969/j.issn.1004-6364.2004.18.009 |
| [75] |
LI M, WU W J, ZHOU P P, et al. Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea[J]. Aquaculture, 2014, 434: 227-232. DOI:10.1016/j.aquaculture.2014.08.022 |
| [76] |
崔培, 周文礼, 刘芳, 等. 虾青素对红白锦鲤体色、生长的影响[J]. 水产科技情报, 2013, 40(1): 37-40. DOI:10.3969/j.issn.1001-1994.2013.01.009 |
| [77] |
裴素蕊, 管越强, 马云婷. 饲料中添加虾青素对凡纳滨对虾生长、存活和抗氧化能力的影响[J]. 水产科学, 2009, 28(3): 126-129. DOI:10.3969/j.issn.1003-1111.2009.03.003 |
| [78] |
CHIEN Y H, SHIAU W C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate[J]. Journal of Experimental Marine Biology and Ecology, 2005, 318(2): 201-211. DOI:10.1016/j.jembe.2004.12.016 |
| [79] |
PAN C H, CHIEN Y H, HUNTER B. The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin[J]. Journal of Experimental Marine Biology and Ecology, 2003, 297(1): 107-118. DOI:10.1016/j.jembe.2003.07.002 |
| [80] |
李小兵, 黎雪梅, 郑曙明, 等. 饲料中添加虾青素对金曼龙鱼体色的影响[J]. 饲料研究, 2013(11): 74-79. |
| [81] |
BILIA A R, ISACCHI B, RIGHESCHI C, et al. Flavonoids loaded in nanocarriers:an opportunity to increase oral bioavailability and bioefficacy[J]. Food and Nutrition Sciences, 2014, 5(13): 1212-1327. DOI:10.4236/fns.2014.513132 |
| [82] |
LIN S F, CHEN Y C, CHEN R N, et al. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads[J]. PLoS One, 2016, 11(4): e0153685. DOI:10.1371/journal.pone.0153685 |
| [83] |
MCCLEMENTS D J. Nanoemulsions versus microemulsions:terminology, differences, and similarities[J]. Soft Matter, 2012, 8(6): 1719-1729. DOI:10.1039/C2SM06903B |
| [84] |
MEHNERT W, MÄDER K. Solid lipid nanoparticles:production, characterization and applications[J]. Advanced Drug Delivery Reviews, 2001, 47(2/3): 165-196. |
| [85] |
ASTRAY G, GONZALEZ-BARREIRO C, MEJUTO J C, et al. A review on the use of cyclodextrins in foods[J]. Food Hydrocolloids, 2009, 23(7): 1631-1640. DOI:10.1016/j.foodhyd.2009.01.001 |




