2. 动物抗病营养教育部重点实验室, 成都 611130
2. Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Sichuan Agricultural University, Chengdu 611130, China
动物脂质代谢紊乱会引起脂质沉积异常,导致代谢性疾病,如脂肪肝和高血脂等的发生,最终影响动物生长性能[1]。因此,保证动物脂质代谢正常对于促进动物生长至关重要。α-硫辛酸(α-lipoic acid,ALA)是丙酮酸脱氢酶中的辅助因子,通过参与三羧酸循环调节能量代谢[2-3]。研究发现,饲粮中添加ALA可以提高肉鸡[4]和草鱼(Ctenopharyngodon idella)[5]的生长性能,降低小鼠肝脏[6]和大鼠血清中三酰甘油的含量[7]以及HepG2细胞中脂质的积累[8]。本文就ALA在动物体内的消化吸收和代谢、促进动物生长及调节脂质代谢的机制进行综述。
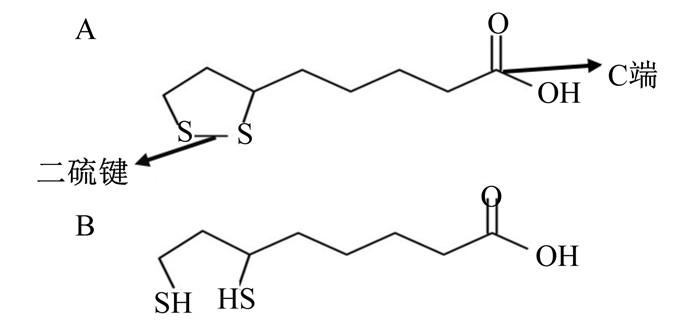
1 ALA的结构、吸收与代谢 1.1 ALA的结构及其在体内存在的形式ALA是一种含硫八碳羧酸,是对映异构体R-ALA和S-ALA的混合物,在生物体内广泛存在[3]。ALA含有一个二硫键(图 1-A),一旦进入细胞二硫键就被还原为二氢硫辛酸(dihydrolipoic acid,DHLA)(图 1-B),并与蛋白质氨基酸残基的氨基结合形成酰胺键,可经脂酰胺酶作用裂解释放具有生理功能的ALA[9-10]。
|
图 1 α-硫辛酸(A)和二氢硫辛酸(B)的化学结构 Fig. 1 Chemical structure of α-lipoic acid (A) and dihydrothioctic acid (B)[10] |
ALA是一种类维生素类物质,既具有脂溶性,也具有水溶性,其经消化道吸收后可经血液循环运送到动物机体各处发挥相应的生理作用[5, 11]。ALA的转运需要载体蛋白[12]。动物体内的ALA可以来自食物,也可以经由线粒体内一系列的酶促合成[13]。2种来源的ALA都可被迅速吸收或转运,并在体内迅速代谢,绝大部分的ALA在24 h内经肾脏代谢后以尿液的形式排出[14]。在体内,ALA主要经过β-氧化进行代谢,产物为二氧化碳(CO2)及一系列的含硫化合物[14]。研究发现,在大鼠腹膜内注射用14C标记的ALA 3~6 h后尿液放射性达到最高,CO2的产量在3 h时达到峰值,此后逐渐下降,约75%的标记物在24 h内排泄[15]。进一步研究发现,用14C标记的ALA饲喂大鼠、小鼠、犬后,超过80%的放射性是在肾脏中检测出来的,这说明ALA在肠道经过有效的吸收并代谢[14]。因此,口服ALA可能不被用作代谢辅助因子,而是触发了一系列的生化活性[13]。
2 ALA对动物生长的影响及可能作用机制 2.1 ALA促进动物生长研究发现,ALA提高了肉鸡[16-18]、幼龄皱纹盘鲍(Haliotis discus hannai Ino)[19]、草鱼[5]以及中华绒鳌蟹(Eriocheir sinensis)[20]的体增重,提高了肉鸡[17-18]和草鱼[5]的采食量以及草鱼[5]、肉鸡[4]的饲料效率,促进了动物生长。但是,ALA抑制了大鼠[21]摄食及体重增加,并对断奶仔猪[22]的采食量无显著影响,这可能与动物种类、ALA添加水平的不同有关。
2.2 ALA促进动物生长的可能作用机制动物生长与其消化功能密切相关。动物的消化功能受到肠道内消化酶活性的影响[23]。研究发现,饲粮中添加适宜水平的ALA提高了小鼠肠道淀粉酶、胰蛋白酶的活性[24]和HepG2细胞中脂肪酶的表达[6]。在鱼类上的研究表明,肠道健康是保证其快速生长的前提条件[25]。肠道健康与肠炎抵抗能力[26]、肠道细胞间紧密连接蛋白的表达[27]、肠道抗凋亡能力[28]以及肠道抗氧化能力密切相关。研究表明,ALA的补充可以降低大鼠肠道损伤[26],增加草鱼肠道紧密连接相关蛋白闭合蛋白(claudin)、紧密连接蛋白-1(ZO-1)等的表达[29],抑制肠道细胞凋亡并显著提高草鱼的生长性能[29]。肠道谷胱甘肽过氧化物酶(GSH-Px)和超氧化物歧化酶(SOD)的活性以及谷胱甘肽(GSH)和维生素C的含量增加可以反映动物肠道抗氧化能力的增强[30]。研究发现,ALA提高了大鼠肠道GSH-Px和SOD活性[27]以及非酶抗氧化物GSH[31]和维生素C[27]的含量。研究表明,ALA的补充可以保障动物肠道健康。以上结果表明,ALA可能通过提高肠道消化吸收能力,维持肠道健康来促进动物生长。
此外,在虾上的研究表明,肠道微生物菌群对维持机体营养代谢有重要作用[32]。研究发现,高脂饲粮中补充ALA降低肠道中有害菌大肠杆菌的数量,提高了有益菌乳酸杆菌的数量[33]。以上研究表明,ALA可能通过提高动物肠道抗氧化能力、促进肠道菌群平衡来提高动物的消化功能。
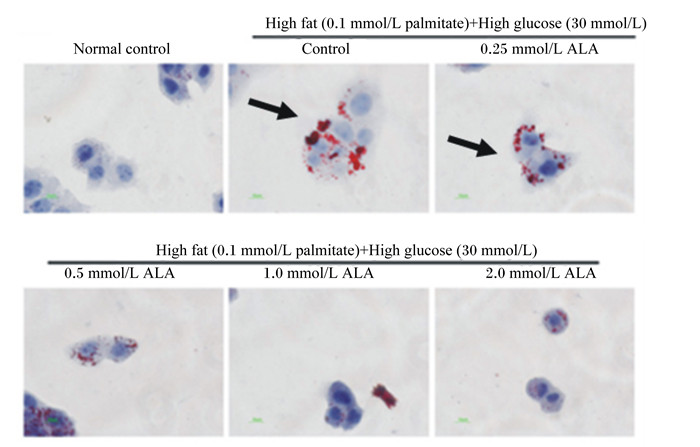
3 ALA对动物脂质代谢的影响及可能作用途径 3.1 ALA对动物脂质沉积的影响脂质沉积取决于三酰甘油合成和储存以及脂质动员和脂质氧化的相对强度[34]。研究发现,ALA降低小鼠[6]肝脏和大鼠[7]血清中三酰甘油的含量以及HepG2细胞中脂质的积累[8](如图 2所示:用油红O对HepG2细胞进行染色,脂滴呈红色,细胞核呈蓝色)。甘油三酯酶(ATGL)[35]、激素敏感性脂酶(HSL)[36]是脂肪分解过程中的关键酶,乙酰辅酶A羧化酶(ACC)[8]和脂肪酸合酶(FAS)[37]和二酯酰甘油酰基转移酶2(DGAT2)[38]是脂质合成中的关键调节酶,这几种酶表达量的改变可以反映脂质代谢情况。研究发现,ALA提高了HepG2细胞中ATGL和3T3L1脂肪细胞中HSL的表达[36],可以降低HepG2细胞中ACC[8]、肉仔鸡肝脏FAS[37]以及罗非鱼肝脏和脂肪组织中DGAT2 mRNA的表达[38]。以上研究表明,ALA可能通过提高脂质分解酶表达、降低脂质合成酶表达抑制脂质的沉积。
|
Normal control:正常组;High fat (0.1 mmol/L palmitate)+High glucose (30 mmol/L):高脂高糖组(1 mmol/L棕榈酸酯,30 mmol/L)。 图 2 高脂高糖环境中添加ALA后HepG2细胞中脂质积累情况 Fig. 2 Lipid accumulation in HepG2 cells after adding ALA in high fat and high glucose environment[6] |
胰岛素是调节脂质合成的关键激素,具有抑制脂质分解、加速脂肪酸利用的功能;瘦素则可以加速机体储备脂肪[39],胰岛素和瘦素的含量可以反映脂质代谢情况。研究发现,ALA降低大鼠胰岛B细胞[40]以及血浆[21]胰岛素的含量,减少3T3L1脂肪细胞[41]和人肝脏[42]中瘦素的含量。因此,ALA可能通过调节脂质代谢相关激素的分泌调节脂质代谢。
综上所述,ALA可能通过提高动物脂质分解酶、抑制脂质合成酶以及脂肪合成相关激素的含量,抑制脂质沉积,最终调节动物体内脂质代谢平衡。
3.2 ALA影响脂质代谢的作用机制 3.2.1 ALA抑制脂质沉积的作用机制脂肪酸结合蛋白参与细胞内脂肪酸的运输,可将脂肪酸从细胞膜运送到脂质合成的部位[43]。研究发现,ALA可以降低罗非鱼肝脏中脂肪酸结合蛋白的表达[44]。研究表明,ALA可能通过抑制脂质的从头合成来抑制脂质的积累。脂肪组织的生长包括脂肪细胞体积的增加以及脂肪细胞的分化,脂肪细胞分化的同时伴随着细胞分化相关基因表达的急剧增加[45]。过氧化物酶体增值物激活受体γ(peroxisome proliferators-activated receptors,PPARγ)是脂肪细胞分化的重要转录因子[46],其含量的改变可以反映脂质氧化程度。c-Fos、c-Jun、myc在细胞分化早期阶段表达,可以驱动3T3L1前脂肪细胞从G0期到G1期[47],从而促进脂肪细胞的分化,其表达量的增加可以反映脂肪细胞分化增强。研究发现,ALA可以降低小鼠3T3L1细胞[48]和大鼠肝脏[49]PPARγ的表达量以及3T3L1前脂肪细胞中c-Fos、c-Jun的表达量[48]。以上结果表明,ALA可能通过抑制脂肪细胞的分化抑制脂质积累。
增强子结合蛋白(C/EBP)是成熟脂肪细胞发育中重要的特征因子,C/EBPβ可以诱导PPARγ表达[50]。核因子-κB(NF-κB)活性增强会抑制脂肪细胞的分化[51]。丝裂原活化蛋白激酶(MAPK)可以调控脂肪形成相关转录因子的表达[48]。研究发现,ALA可以通过激活MAPK抑制3T3L1脂肪细胞中C/EBP的表达[48],以及增强黑色素瘤细胞[52]中NF-κB的活性。以上研究表明,ALA可能通过MAPK途径抑制脂肪细胞分化来抑制脂质的积累,但还需要更加深入系统的研究。
3.2.2 ALA促进脂质分解的作用机制脂肪酸的β-氧化是ATP产生的重要途径,主要由线粒体的功能蛋白促进[53],线粒体的含量可以反映脂肪酸氧化程度[54]。哺乳动物的脂肪有2种类型,包括白色脂肪细胞和棕色脂肪细胞,棕色脂肪细胞比白色脂肪细胞存储的脂质更少、线粒体含量更高[46]。参与细胞呼吸和ATP合成的线粒体蛋白COXIV和线粒体DNA的相对丰度可以反映线粒体的含量。研究发现,ALA增加了人体脂肪细胞中线粒体的数量[54]以及大鼠骨骼肌中COXIV的含量[35]和线粒体DNA的相对丰度[55]。此外,解偶联蛋白1(UCP1)是棕色脂肪细胞中特有的线粒体内膜蛋白[46],能增加能量的消耗促进脂质氧化[21]。研究发现,ALA提高了大鼠脂肪细胞中UCP1蛋白[54]以及mRNA[21]的表达。因此,ALA可能通过增加棕色脂肪细胞以及线粒体的含量促进动物体内脂质的氧化。脂质氧化相关酶肉毒碱棕榈酰转移酶1(CPT1)、过氧化物酶体增殖活化受体γ共激活因子-1α(PGC-1α)以及脂质氧化相关激素脂联素表达量的升高标志着脂质氧化代谢的增强[37, 56-57]。腺苷酸激活蛋白激酶(AMPK)是一种细胞能量感受器,激活AMPK可以促进脂质的氧化分解[40]。研究发现,ALA通过激活AMPK提高了大鼠[35]、小鼠[58]骨骼肌中PGC-1α、大鼠骨骼肌中CPT1[35]以及3T3L1脂肪细胞中脂联素[41]的表达。钙调蛋白依赖性蛋白激酶(CAMKK)可以通过Ca2+/钙调蛋白结合而被激活[59]。沉默信息调节因子2相关酶1(SIRT1)是一类NAD(+)-依赖性脱乙酰酶[6]。CAMKK和沉默信息调节因子2相关酶1(SIRT1)活性的增加都可以激活AMPK[59]。有研究发现,ALA可以增加C2C12细胞中Ca2+的浓度[59]以及HepG2细胞中NAD+/NADH的比值[8], 从而激活AMPK。以上研究表明,ALA可能通过增加CAMKK和SIRT1的活性激活AMPK,进而促进脂质的分解及氧化调节脂质代谢。另外有研究发现,在下丘脑中ALA可以抑制摄食[21],这可能与饱食后胰岛素分泌增加抑制脂解作用有关,但需要进一步的研究。
4 小结综上所述,ALA能够通过提高动物肠道消化酶的活性、维持肠道结构完整性及肠道菌群平衡来促进动物生长。动物体内脂质代谢的平衡与减少机体脂质过度沉积及促进氧化供能密切相关。ALA通过促进脂质分解,抑制脂质的合成,最终减少脂质的积累。在动物体内ALA可能通过激活MAPK抑制脂肪细胞的分化,从而抑制脂质的积累,并可能通过激活AMPK途径增加脂质分解相关酶及激素的表达,进而促进脂质的分解。
| [1] |
JUNG T S, KIM T S, SHIN H J, et al. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats[J]. Liver Internatonal, 2012, 32(10): 1565-1573. DOI:10.1111/j.1478-3231.2012.02857.x |
| [2] |
WANG M, WANG Q, GAO X, et al. Conditional knock-out of lipoic acid protein ligase 1 reveals redundancy pathway for lipoic acid metabolism in Plasmodium berghei malaria parasite[J]. Parasites & Vectors, 2017, 10: 315. |
| [3] |
PARK S, KARUNAKARAN U, JEOUNG N H, et al. Physiological effect and therapeutic application of alpha lipoic acid[J]. Current Medicinal Chemistry, 2014, 21(32): 3636-3645. DOI:10.2174/0929867321666140706141806 |
| [4] |
DÍAZ-CRUZ A, SERRET M, RAMÍREZ G, et al. Prophylactic action of lipoic acid on oxidative stress and growth performance in broilers at risk of developing ascites syndrome[J]. Avian Pathology, 2003, 32(6): 645-653. |
| [5] |
LIU H X, ZHOU X Q, JIANG W D, et al. Optimal α-lipoic acid strengthen immunity of young grass carp (Ctenopharyngodon idella) by enhancing immune function of head kidney, spleen and skin[J]. Fish & Shellfish Immunology, 2018, 80: 600-617. |
| [6] |
KUO Y T, LIN T H, CHEN W L, et al. Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells[J]. European Journal of Pharmacology, 2012, 692(1/2/3): 10-18. |
| [7] |
HUONG D T T, IDE T. Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats[J]. British Journal of Nutrition, 2008, 100(1): 79-87. |
| [8] |
YANG Y, LI W, LIU Y, et al. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway[J]. The Journal of Nutritional Biochemistry, 2014, 25(11): 1207-1217. DOI:10.1016/j.jnutbio.2014.06.001 |
| [9] |
MAYR J A, FEICHTINGER R G, TORT F, et al. Lipoic acid biosynthesis defects[J]. Journal of Inherited Metabolic Disease, 2014, 37(4): 553-563. DOI:10.1007/s10545-014-9705-8 |
| [10] |
KÜTTER M T, ROMANO L A, VENTURA-LIMA J, et al. Antioxidant and toxicological effects elicited by alpha-lipoic acid in aquatic organisms[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2014, 162: 70-76. |
| [11] |
N RC. Nutrient requirements of fish and shrimp[M]. Washington, D.C.: The National Academies Press, 2011.
|
| [12] |
TAKAISHI N, YOSHIDA K, SATSU H, et al. Transepithelial transport of α-lipoic acid across human intestinal Caco-2 cell monolayers[J]. Journal of Agricultural and Food Chemistry, 2007, 55(13): 5253-5259. DOI:10.1021/jf063624i |
| [13] |
SHAY K P, MOREAU R F, SMITH E J, et al. Alpha-lipoic acid as a dietary supplement:molecular mechanisms and therapeutic potential[J]. Biochimica et Biophysica Acta:General Subjects, 2009, 1790(10): 1149-1160. DOI:10.1016/j.bbagen.2009.07.026 |
| [14] |
SCHUPKE H, HEMPEL R, PETER G, et al. New metabolic pathways of alpha-lipoic acid[J]. Drug Metabolism and Disposition, 2001, 29(6): 855-862. |
| [15] |
HARRISON E H, MCCORMICK D B. The metabolism of dl-[1, 6-14C] lipoic acid in the rat[J]. Archives of Biochemistry and Biophysics, 1974, 160(2): 514-522. DOI:10.1016/0003-9861(74)90428-7 |
| [16] |
YASIN M, ASGHAR A, ANJUM F M, et al. Oxidative stability enhancement of broiler bird meats with α-lipoic acid and α-tocopherol acetate supplemented feed[J]. Food Chemistry, 2012, 131(3): 768-773. DOI:10.1016/j.foodchem.2011.09.031 |
| [17] |
IMIK H, OZLU H, GUMUS R, et al. Effects of ascorbic acid and α-lipoic acid on performance and meat quality of broilers subjected to heat stress[J]. British Poultry Science, 2012, 53(6): 800-808. DOI:10.1080/00071668.2012.740615 |
| [18] |
SRILATHA T, REDDY V R, QUDRATULLAH S, et al. Effect of alpha-lipoic acid and vitamin E in diet on the performance, antioxidation and immune response in broiler chicken[J]. International Journal of Poultry Science, 2010, 9(7): 678-683. DOI:10.3923/ijps.2010.678.683 |
| [19] |
ZHANG W B, CHEN Q Y, MAI K S, et al. Effects of dietary α-lipoic acid on the growth and antioxidative responses of juvenile abalone Haliotis discus hannai Ino[J]. Aquaculture Research, 2010, 41(11): e781-e787. DOI:10.1111/j.1365-2109.2010.02592.x |
| [20] |
XU C, WANG X D, HAN F L, et al. α-lipoic acid regulate growth, antioxidant status and lipid metabolism of Chinese mitten crab Eriocheir sinensis:optimum supplement level and metabonomics response[J]. Aquaculture, 2019, 506: 94-103. DOI:10.1016/j.aquaculture.2019.03.029 |
| [21] |
KIM M S, PARK J Y, NAMKOONG C, et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase[J]. Nature Medicine, 2004, 10(7): 727-733. DOI:10.1038/nm1061 |
| [22] |
MADDOCK K R, CARROLL J A, BERG E P, et al. Evaluation of the potential role of alpha-lipoic acid with regard to health and performance of wealing pigs[J]. Journal of Animal and Neterinary Advaces, 2003, 10: 554-563. |
| [23] |
周洋, 彭艳, 周小秋. 植物精油对动物生长和免疫力的影响及其作用机制[J]. 动物营养学报, 2018, 30(1): 57-63. |
| [24] |
鲍伟光, 郝月, 崔艳军, 等. 敌草快和硫辛酸对育肥猪肠道结构及消化功能的影响[J]. 动物营养学报, 2016, 28(10): 3264-3274. DOI:10.3969/j.issn.1006-267x.2016.10.030 |
| [25] |
ESLAMLOO K, AKHAVAN S R, FALLAH F J, et al. Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress[J]. Fish & Shellfish Immunology, 2014, 37(1): 147-153. |
| [26] |
GUVEN A, TUNC T, TOPAL T, et al. α-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine[J]. Surgery Today, 2008, 38(11): 1029-1035. DOI:10.1007/s00595-007-3752-9 |
| [27] |
EPSTEIN M D, TCHERVENKOV J I, ALEXANDER J W, et al. Increased gut permeability following burn trauma[J]. Archives of Surgery, 1991, 126(2): 198-200. DOI:10.1001/archsurg.1991.01410260086012 |
| [28] |
DUAN X D, FENG L, JIANG W D, et al. Dietary soybean β-conglycinin suppresses growth performance and inconsistently triggers apoptosis in the intestine of juvenile grass carp (Ctenopharyngodon idella) in association with ROS-mediated MAPK signalling[J]. Aquaculture Nutrition, 2019, 25(4): 770-782. |
| [29] |
刘华西.α-硫辛酸对生长中期草鱼生产性能、功能器官健康以及肌肉品质的作用及其机制[D].硕士学位论文.雅安: 四川农业大学, 2018.
|
| [30] |
WEI S P, JIANG W D, WU P, et al. Dietary magnesium deficiency impaired intestinal structural integrity in grass carp (Ctenopharyngodon idella)[J]. Scientific Reports, 2018, 8: 12705. DOI:10.1038/s41598-018-30485-8 |
| [31] |
DADHANIA V P, TRIPATHI D N, VIKRAM A, et al. Intervention of α-lipoic acid ameliorates methotrexate-induced oxidative stress and genotoxicity:a study in rat intestine[J]. Chemico-Biological Interactions, 2010, 183(1): 85-97. DOI:10.1016/j.cbi.2009.10.020 |
| [32] |
倪江, 杨维仁. 肠道微生物与宿主代谢关系的研究进展[J]. 饲料博览, 2012(7): 9-12. DOI:10.3969/j.issn.1001-0084.2012.07.003 |
| [33] |
张蓉, 孙进, 李亚欣, 等. 硫辛酸对高脂日粮小鼠肠道氧化还原状态与微生物菌群的影响[J]. 食品工业科技, 2010, 31(9): 321-324, 369. |
| [34] |
ZHENG J L, LUO Z, HU W, et al. Different effects of dietary Zn deficiency and excess on lipid metabolism in yellow catfish Pelteobagrus fulvidraco[J]. Aquaculture, 2015, 435: 10-17. DOI:10.1016/j.aquaculture.2014.09.011 |
| [35] |
LI Z Y, DUNGAN C M, CARRIER B, et al. Alpha-lipoic acid supplementation reduces mTORC1 signaling in skeletal muscle from high fat fed, obese zucker rats[J]. Lipids, 2014, 49(12): 1193-1201. DOI:10.1007/s11745-014-3964-x |
| [36] |
FERNÁNDEZ-GALILEA M, PÉREZ-MATUTE P, PRIETO-HONTORIA P L, et al. Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes[J]. Journal of Lipid Research, 2012, 53(11): 2296-2306. DOI:10.1194/jlr.M027086 |
| [37] |
WANG Y F, EVERAERT N, SONG Z G, et al. Alpha-lipoic acid impairs body weight gain of young broiler chicks via modulating peripheral AMPK[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2017, 211: 34-40. |
| [38] |
HUNG Y H, CARREIRO A L, BUHMAN K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat[J]. Biochimica et Biophysica Acta:Molecular and Cell Biology of Lipids, 2017, 1862(6): 600-614. DOI:10.1016/j.bbalip.2017.02.014 |
| [39] |
郭英杰. 丙酮酸补充对运动机体身体成分和脂肪代谢的影响及机理的研究[M]. 北京: 北京体育大学出版社, 2009.
|
| [40] |
TARGONSKY E D, DAI F, KOSHKIN V, et al. α-Lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells[J]. Diabetologia, 2006, 49(7): 1587-1598. DOI:10.1007/s00125-006-0265-9 |
| [41] |
PRIETO-HONTORIA P, PÉREZ-MATUTE P, FERNÁNDEZ-GALILEA M, et al. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats[J]. European Journal of Nutrition, 2013, 52(2): 779-787. |
| [42] |
RAHMANABADI A, MAHBOOB S, AMIRKHIZI F, et al. Oral α-lipoic acid supplementation in patients with non-alcoholic fatty liver disease:effects on adipokines and liver histology features[J]. Food & Function, 2019, 10(8): 4941-4952. |
| [43] |
STORCH J, THUMSER A E A. The fatty acid transport function of fatty acid-binding proteins[J]. Biochimica et Biophysica Acta:Molecular and Cell Biology of Lipids, 2000, 1486(1): 28-44. DOI:10.1016/S1388-1981(00)00046-9 |
| [44] |
XU F N, XU C, XIAO S S, et al. Effects of α-lipoic acid on growth performance, body composition, antioxidant profile and lipid metabolism of the GIFT tilapia (Oreochromis niloticus) fed high-fat diets[J]. Aquaculture Nutrition, 2019, 25(3): 585-596. |
| [45] |
GREGOIRE F M, SMAS C M, SUL H S. Understanding adipocyte differentiation[J]. Physiological Reviews, 1998, 78(3): 783-809. DOI:10.1152/physrev.1998.78.3.783 |
| [46] |
ROSEN E D, MACDOUGALD O A. Adipocyte differentiation from the inside out[J]. Nature Reviews Molecular Cell Biology, 2006, 7(12): 885-896. DOI:10.1038/nrm2066 |
| [47] |
SMAS C M, CHEN L, ZHAO L, et al. Transcriptional repression of pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation[J]. Journal of Biological Chemistry, 1999, 274(18): 12632-12641. DOI:10.1074/jbc.274.18.12632 |
| [48] |
CHO K J, MOON H E, MOINI H, et al. α-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways[J]. Journal of Biological Chemistry, 2003, 278(37): 34823-34833. DOI:10.1074/jbc.M210747200 |
| [49] |
CASTRO M C, MASSA M L, SCHINELLA G, et al. Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet[J]. Biochimica et Biophysica Acta:General Subjects, 2013, 1830(1): 2226-2232. |
| [50] |
HAMM J K, PARK B H, FARMER S R. A Role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes[J]. Journal of Biological Chemistry, 2001, 276(21): 18464-18471. DOI:10.1074/jbc.M100797200 |
| [51] |
RUAN H, HACOHEN N, GOLUB T R, et al. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes:nuclear factor-κB activation by TNF-α is obligatory[J]. Diabetes, 2002, 51(5): 1319-1336. |
| [52] |
DHAWAN P, RICHMOND A. A novel NF-κ B-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells[J]. Journal of Biological Chemistry, 2002, 277(10): 7920-7928. DOI:10.1074/jbc.M112210200 |
| [53] |
PAN H, LI L Y, LI J M, et al. Inhibited fatty acid β-oxidation impairs stress resistance ability in Nile tilapia (Oreochromis niloticus)[J]. Fish & Shellfish Immunology, 2017, 68: 500-508. |
| [54] |
FERNÁDEZ-GALILEA M, PÉREZ-MATUTE P, PRIETO-HONTORIA P L, et al. α-lipoic acid treatment increases mitochondrial biogenesis and promotes beige adipose features in subcutaneous adipocytes from overweight/obese subjects[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1851(3): 273-281. |
| [55] |
WANG Y, LI X J, GUO Y M, et al. α-lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-γ coactivator-1α signaling in the skeletal muscle of aged mice[J]. Metabolism, 2010, 59(7): 967-976. DOI:10.1016/j.metabol.2009.10.018 |
| [56] |
MOTOSHIMA H, WU X D, SINHA M K, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes:effects of insulin and rosiglitazone[J]. The Journal of Clinical Endocrinology & Metabolism, 2002, 87(12): 5662-5667. |
| [57] |
DRAKE J C, ALWAY S E, HOLLANDER J M, et al. AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle[J]. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology, 2010, 299(6): R1546-R1554. DOI:10.1152/ajpregu.00337.2010 |
| [58] |
JÄGER S, HANDSCHIN C, St-PIERRE J, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α[J]. Proceedings of the National Academy of Sciences, 2007, 104(29): 12017-12022. DOI:10.1073/pnas.0705070104 |
| [59] |
SHEN Q W, ZHU M J, TONG J, et al. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by α-lipoic acid in C2C12 myotubes[J]. American Journal of Physiology:Cell Physiology, 2007, 293(4): C1395-C1403. DOI:10.1152/ajpcell.00115.2007 |




