“地中海式饮食”是指有利于健康的,简单、清淡以及富含营养的膳食模式,是居住在地中海地区的居民(主要以意大利南部和希腊为代表)所特有的饮食模式。大量研究表明,地中海饮食模式可预防心脏病、糖尿病,降低癌症发生率,逆转心血管疾病,减少炎症性肠病的发生[1]。研究者发现,橄榄油和红葡萄酒作为地中海饮食中的特征成分,在疾病的预防中发挥着非常重要的作用,而这又跟它们的重要组分——羟基酪醇(HT)密切相关[2]。文献报道,HT在抗氧化、预防癌症、抑菌抗炎、降脂等方面具体良好的生物学活性[2]。目前,国内外对HT的研究主要集中在医药学领域和保健行业,而在畜禽生产领域研究较少,这主要是因为人们对HT的来源、合成方法不了解,对其在动物体内的消化吸收和代谢途径以及在动物体内能发挥的生物学功能及作用机制不清楚。因此,本文旨在综述HT的研究进展,总结其来源、合成方法及其在动物体内的消化吸收和代谢途径,阐述其主要生理功能与作用机制以及在畜禽动物生产中的应用前景,以期为“禁抗限抗”下的畜牧行业开发一种新型天然绿色添加剂。
1 HT的来源、性质及合成方法HT是一种主要存在于橄榄果实、橄榄叶及橄榄油中的单组分酚类物质,其化学名称为3,4-二羟基苯乙醇,分子式为C8H10O3,相对分子质量为154.16。HT是一种两亲性酚(亲水亲脂),因此其生物利用度相对较高[2]。研究表明,以游离形式存在的HT占初榨橄榄油中总酚类物质的6.0%[3]。其主要产生于橄榄苦苷的水解作用,而这种水解作用发生在橄榄的成熟、贮藏和食用加工过程中[4]。故其中的HT浓度受橄榄的品种、成熟度及加工工艺等因素影响[5]。其次,在红葡萄酒和白葡萄酒中也有少量HT存在[6]。不同来源中HT的浓度范围如表 1所示。目前已研究出3种HT主要的合成方法,即天然提取法、化学合成法和生物合成法[1]。在诸多化学合成法中,以多巴胺为原料合成HT是最成功的一种方法,但工艺复杂且产量不足,成本昂贵。而生物合成方法不需要使用任何重金属催化剂,也没有苛刻的条件,其中利用结构与HT相似的化合物进行单步生物转化的方法具有较高的容积产率和产品效价,然而,昂贵的底物是这种方法实现工业规模生产的主要瓶颈。故目前在工业生产中大多还是采用天然提取法,即在橄榄加工的副产品和工业废水中提取HT,既保护了生态环境又得到了高价值的产物。
|
|
表 1 HT在不同来源组分中的浓度范围 Table 1 Concentration range of HT in different components from different sources[7] |
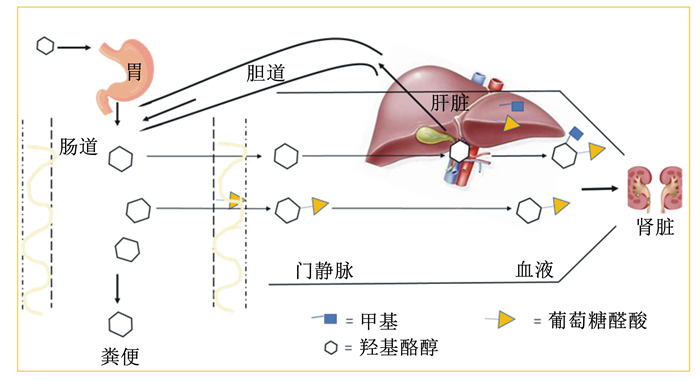
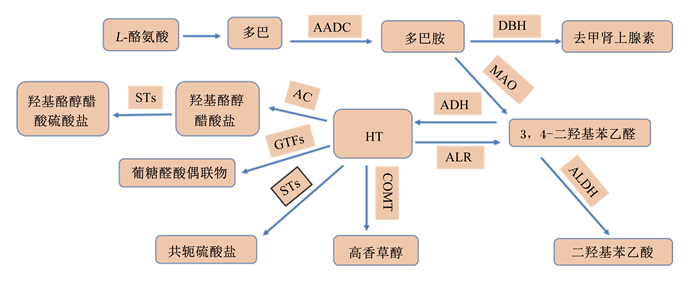
HT及其衍生物在动物胃酸性环境下是可以稳定存在的[8]。研究表明,橄榄油中的酚类物质很容易被机体吸收,其中HT是通过被动扩散的方式被动物机体吸收的[9],主要吸收部位在小肠和结肠,并呈现一定的剂量依赖性,其吸收速率因动物种类的不同而不同,平均吸收总量可达66%[10]。吸收后在小肠和肝脏内进行重要的代谢[11],其吸收途径如图 1所示。Tuck等[12]在小鼠上的试验表明,HT作为橄榄油溶液口服时的生物利用度为99%,当以水溶液形式口服时为75%,且静脉注射和口服没有显著差异。也有研究报道,HT的生物利用度在较大范围区间波动[13],这可能是由于动物种类、生长阶段、激素水平以及性别等差异导致的[14]。如Domínguez-Perles等[15]研究报道,雌性鼠对HT的吸收利用率明显优于雄性鼠。HT一旦被机体吸收,就会发生共轭反应,主要包括甲基化和葡萄糖醛酸化[16],并很快成为血浆高密度脂蛋白(HDL)的一部分,从而降低机体组织胆固醇的沉积,以抵抗动脉粥样硬化,保护动物心血管[17]。HT作为目前唯一能够穿过血脑屏障的酚类物质,其在肌肉、睾丸、肝脏、大脑等组织中具有良好的分布能力,并最终在动物肾脏和肝脏中积累[18]。吸收入血后,HT在机体中的下游代谢产物主要包括高香草醇[19]、羟基酪醇醋酸硫酸盐[20]等。而近年研究发现,机体摄入HT后,通过与肠道微生物之间的作用,产生的重要代谢物是酪氨酸(Tyr),而不是高香草醇[15]。部分HT及其代谢产物会被重新导向胆道排泄途径,进行肠肝循环,这延长了HT和代谢物在机体中的存留时间,提高了其生物利用度[21]。另外,HT还是动物机体中多巴胺的一种代谢物[22],研究发现,HT在脑组织中可能与多巴胺能通路相互作用,并保护多巴胺能神经元[23]。其代谢途径如图 2所示。因此,如何在动物机体内存在内源性HT的情况下,探究外源性HT在不同动物模型中的消化吸收代谢率及生物利用度需要进一步深入研究。
|
图 1 HT在体内的吸收途径 Fig. 1 Absorption pathway of HT in vivo |
|
DBH:多巴胺β-羟化酶dopamine β-hydroxylase;AADC:芳香酸脱羧酶aromatic acid decarboxylase;MAO:单胺氧化酶monoamine oxidase;ADH:乙醇脱氢酶ethanol dehydrogenase;ALDH:乙醛脱氢酶acetaldehyde dehydrogenase;ALR:乙醛还原酶acetaldebyde reductase;AC:脂肪酰转移酶aliphatic acyl transferase;GTFs:葡糖转移酶glucosyltransferase;STs:磺基转移酶sulfotransferase;COMT:儿茶酚氧位甲基转移酶atechol-O-methyl transferase。 图 2 HT在体内的代谢途径 Fig. 2 Metabolic pathway of HT in vivo |
HT是一种具有多种生物活性的强抗氧化剂[24]。其抗氧化能力是绿茶的15倍,是辅酶Q的3倍[25]。这一方面,与其结构中具有多个酚羟基关系密切,它的酚羟基可以作为氢的供体,对多种活性氧具有清除作用,能够将单线态的氧还原成活性较低的三线态氧,降低氧自由基产生的可能性,同时通过反应生成的活性较低的多酚自由基可以清除各种自由基,从而打断自由基氧化的链式反应[26]。并且其2个邻位酚羟基还能通过与金属离子的螯合作用来阻止活性氧的形成[27]。研究表明,HT及其主要代谢物硫酸盐和葡糖苷酸,可以通过清除自由基来保护Caco-2细胞、肾细胞、红细胞免受氧化损伤[28-30]。另一方面,研究表明,在外周血单核细胞中,HT通过降低过氧化脂质、活性氧的含量和增加抗氧化酶的活性,减轻二噁英诱导的氧化应激,防止细胞内DNA被破坏,并减少了细胞形态学的变化[31]。HT还能诱导激活核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)在细胞核内的表达,从而促进了下游诸多抗氧化酶体系包括γ-谷氨酰-半胱氨酸连接酶(γ-GCL)、醌氧化还原酶1(NQO1)和血红素氧合酶-1(HO-1)的活化[32]。Martín等[33]在人肝癌细胞HepG2培养试验中发现,HT增强了谷胱甘肽过氧化物酶(GPx)、谷胱甘肽还原酶(GR)和谷胱甘肽S-转移酶(GST)的表达和活性。该试验还表明,HT对细胞抗氧化酶的作用与其增强了Nrf2向细胞核转运的能力有关,从而促进与抗氧化防御系统相关基因的表达,有助于细胞抵抗氧化应激。
综上所述,HT一方面因其自身结构具有多个酚羟基,从而可以有效清除机体内自由基、阻碍活性氧生成;另一方面,可以激活抗氧化酶相关通路,促进机体内抗氧化酶体系中的酶的合成及相关基因的表达,从而提高机体抗氧化能力。
3.2 预防癌症大量文献报道,HT通过不同作用途径和机制在预防肝癌、结肠癌、乳腺癌、前列腺癌等癌症中发挥着重要作用。机体内,磷酸化蛋白激酶B(AKt)参与调节细胞周期,其可抑制磷脂酰肌醇3-激酶(PI3K-AKt)通路,从而抑制肿瘤细胞增殖、促进肿瘤细胞凋亡,核转录因子-κB(NF-κB)是PI3K-AKt途径的下游组件之一,参与调节肝癌发展过程的各个阶段[34]。Zhao等[35]通过制备原位肝癌模型进行研究,发现HT能激活AKt并活化NF-κB基因,阻碍PI3K-AKt的磷酸化,抑制NF-κB基因产物[原癌基因(c-myc)、细胞周期素D(cyclinD1)、Bcl-2蛋白、抗凋亡蛋白(Bcl-XL)、环氧化酶-2(COX-2)和血管内皮生长因子]的合成,从而发挥其抑制肝癌细胞增殖、诱导肝癌细胞凋亡的作用。在体外结肠癌模型试验中,HT可阻滞结肠腺癌细胞(HT-29)的细胞周期停留在S期,抑制肿瘤细胞的生长、增殖,诱导肿瘤细胞凋亡,抑制肿瘤细胞扩散,发挥其广谱抗癌作用[36]。Fabiani等[37]对人幼粒白血病细胞(HL-60)进行研究,发现HT阻滞细胞周期的G1期,在细胞G0/G1期呈比例增加,在S和G2/M期呈比例下降。其作用机制包括:直接抑制细胞周期蛋白依赖性激酶、诱导细胞周期蛋白依赖性激酶抑制剂生成、阻滞细胞增殖信号、引起细胞凋亡、促进HL-60细胞分化。该研究进一步提出HT(100 μmol/L)可引起细胞周期蛋白依赖性激酶抑制剂1A(P21WAF/Cip1)和细胞周期蛋白依赖性激酶抑制剂1B(P27Kip1)表达水平增加,抑制细胞周期蛋白依赖性激酶-6表达,使细胞周期停滞在G2/M期和G0/G1期,在S期促使细胞凋亡,且对正常细胞没有毒性。Sirianni等[38]研究表明,HT能阻断雌二醇诱导的乳腺癌细胞表达,并对人表皮生长因子受体2(HER2)诱导的乳腺癌细胞(MCF-7和MCF-7/HER2)产生细胞毒活性,从而发挥其对乳腺癌的预防作用。另外,Luo等[39]研究结果显示,HT可降低人前列腺癌细胞(PC-3、DU-145)的存活率。其主要是通过产生超氧化物,使线粒体功能发生障碍,从而诱导癌细胞凋亡。
3.3 抑菌抗炎大量研究表明,HT具有良好的抗菌活性[40-42]。Ramírez-Tortose等[7]在体外试验中发现,低浓度HT具有对呼吸道和胃肠道致病原如肠炎弧菌、霍乱弧菌、伤寒杆菌、流感嗜血杆菌、金黄色葡萄球菌、莫拉菌的抗菌活性,抑菌浓度甚至低于某些抗生素如氨苄青霉素。而Medina-Martínez等[43]却发现低浓度HT抑制细菌生长的能力较差,需达400 μg/mL才能抑制大肠杆菌菌株生长,这种现象可能是由于HT在营养丰富的培养基中发生了氧化,减弱了其抗菌作用。还有部分研究表明,HT的类似物能高效破坏真菌细胞膜从而表现出抗真菌和抗原生动物的作用[44]。在抗炎方面,Yao等[45]研究表明,HT对血管内皮细胞炎症的保护作用部分是通过沉默调节相关蛋白6抗体(sirt6)介导的PKM2信号通路实现的。另外,研究表明,HT对COX-2介导的炎症具有延迟但持续的抗炎作用,并且可与传统的短效抗炎药配伍使用[46]。Scoditti等[47]在体外试验中发现,HT可以抑制小鼠3T3-L1脂肪细胞中氧化物酶体增殖物激活受体γ(PPARγ)信号通路,从而显示抗炎活性。体内研究也有相关报道,Liu等[48]在C58BL/6J小鼠饲粮中添加50 mg/(kg·d) HT,持续8周,显著降低了肝脏炎症标记物白细胞介素(IL)-1β、IL-6的含量,减少了肝脏中肿瘤坏死因子-α(TNF-α)、IL-1β、IL-6、Toll样4受体(TLR4)和磷酸化磷酸激酶(p-JNK)的表达,而且抑制了血液中脂多糖(LPS)的释放。其中的分子机制至少部分与下调COX-2和iNOS的基因表达、促进膜联蛋白A1的合成以及抑制丝裂原活化蛋白激酶p38 MAPK磷酸化和NF-κB的核易位有关[49]。而最新研究发现,HT及其与肠道微生物作用产生的代谢物Tyr可以通过调节p38和ERK1/2 MAPK,在肠道水平上阻止病理意义上的一氧化氮(NO)过量产生,并集中在肠道内,从而显著提高了其对炎症的保护活性[50]。
3.4 降脂作用研究报道,HT可降低脂肪细胞的分化和增殖,并减少脂肪细胞脂滴的数量和大小[51-53]。Scoditti等[54]在人脂肪细胞(SGBS)的培养试验中发现,HT显著减少炎症相关miRNAs(mir-155-5-p、miR-34a-5p、let-7c-5p)在细胞和胞液中的表达,抑制NF-κB激活和活性氧的产生,从而抑制脂肪细胞基因表达。而Garcia-Contreras等[55]在怀孕母猪饲粮中添加1.5 mg/(kg·d)HT结果发现,HT没有影响胎儿脂肪的沉积,但显著提高了胎儿体内必需脂肪酸的合成,并显著影响了ω-6和ω-3脂肪酸的比例,从而改善了动物宫内生长迟缓症状。这说明尽管HT在体外和体内对脂肪代谢的影响存在一定差异,但都是有益影响。此外,Echeverría等[56]在大鼠上的试验表明,HT与二十碳五烯酸(EPA)联合使用可以显著减少非酒精性脂肪肝(NAFLD)的发生,这一作用显示出HT的附加性,而其中主要作用是依赖于HT增强了抗氧化能力,抑制了肝脏脂肪变性。HT在体外和体内均具有抗氧化活性,可清除活性氧自由基,减少肝细胞内脂滴数量,使肝细胞内脂滴体积缩小。从而减少了细胞内三酰甘油堆积,并且显著降低了脂质的合成[57]。HT还可以通过调节内质网应激改善胰岛素抵抗,预防饮食诱导肥胖小鼠的肝脂肪变性[58]。综上可知,HT一方面可以直接降低脂肪细胞的分化和增殖,以减少脂肪细胞脂滴的数量和大小;另一方面,又可以通过调节炎症相关因子的表达,以及依赖其强抗氧化能力来间接减少脂质合成和沉积。
4 HT在畜禽动物生产中的应用潜力 4.1 改善动物肠道健康研究报道,橄榄油及其组分能够调节老鼠肠道菌群,改善动物肠道健康[59]。Echeverría等[56]在PM2.5诱导的氧化应激小鼠试验中发现,HT可增加小鼠肠道菌群丰富度,减少致病菌,调节小鼠的微生物结构,有效纠正PM2.5引起的氧化应激失衡,进而改善NF-κB通路和胰岛素信号通路。此外,HT还可以通过改变肠道菌群的组成和改善肠壁的完整性来改善动物肥胖和胰岛素抵抗[48]。研究表明,饲粮添加50 mg/kg的HT显著提升小鼠肠道乳酸菌的数量,特别是强效乳酸菌,而对厚壁菌门/拟杆菌门的比例基本没有影响[59]。虽然目前还没有关于HT对肠道菌群影响的专门研究,但已有动物模型和细胞培养的试验表明,HT能够通过调节相关基因的表达来提高动物机体内源性肠道免疫能力[60]。因此,HT在改善畜禽动物肠道健康方面具有巨大潜力。
4.2 保护动物肝脏Pan等[61]报道,单独添加HT对小鼠肝脏缺血/再灌注损伤具有保护作用,其作用机制是通过降低血清转氨酶水平,抑制细胞凋亡,进而延缓肝细胞外基质沉积并清除肝细胞内活性氧自由基以减少肝细胞内脂滴数量。其次,炎性细胞因子TNF-α、IL-6和趋化因子巨噬细胞炎性蛋白-2在肝脏缺血/再灌注损伤中发挥着重要作用。人肝细胞体外试验表明,经HT处理后,血清中TNF-α、IL-6和趋化因子巨噬细胞炎性蛋白-2含量明显降低[62]。另外,HT与EPA配伍使用,能显著减弱高脂饲粮(HFD)诱导的小鼠肝脂肪变性,对非酒精性脂肪肝起到了重要的保护作用[63]。HT与DHA联用则完全预防了HFD引起的肝脏脂肪变性及伴随的促炎症状态,抑制了脂肪生成和氧化应激信号,并恢复了脂肪酸氧化能力[64]。有文献报道,HT也能通过减少细胞凋亡、增加抗氧化活性和增加肝细胞活力等途径发挥其保护动物肝脏的作用[65-67]。综上可知,HT在保护畜禽动物肝脏方面,可能发挥着重要作用。
4.3 促进动物骨骼发育研究报道,HT可以通过降低线粒体内源发动蛋白1(OPA1)的裂解,增加AKT和糖原合成酶激酶3(GSK3)的β磷酸化来抑制氧化应激诱导的线粒体功能障碍,从而显著减少成骨细胞凋亡,这也表明HT可能是一种预防动物骨质疏松症的有效营养素[68]。Mahmoudi等[69]在双酚A诱导的甲状腺功能衰退的雌鼠饲粮中添加HT,显著提高了甲状腺激素水平,改善了泌乳雌鼠的甲状腺细胞功能,并促进了小鼠的生长,其特征是改善了股骨骨组织以及微观结构。但也有少数研究表明,高浓度的HT可能抑制成骨细胞以及脂肪生成,导致骨质流失[70]。这表明使用外源添加剂辅助动物生长时,剂量不能超过动物所能承受的范围,而适宜浓度的HT对生产中畜禽动物的跛行、站立不稳等病症可能具有一定的预防作用。
4.4 作为动物饲料添加剂的独特优势首先,从来源上看,HT的主要来源就是橄榄果实、橄榄叶及相关加工品,而我国橄榄品种资源极为丰富,目前栽培较广的品种有:檀香、惠圆、公本、猎腰榄、茶窖榄、青心等,主要分布在福建、台湾、广东、广西、云南等地区。且橄榄对土壤适应性较广,江河沿岸,丘陵山地,红黄壤、石砾土均可栽培,只要土层深厚,排水良好即可生长良好。从其性质上看,HT是一种两亲性酚类物质,是目前发现的生物利用度最高的一种酚类,其在动物体内能够迅速被吸收到血液和组织中,凭借其强抗氧化能力,清除体内自由基。另外,HT是目前发现的唯一能够穿过血脑屏障的酚类,也是神经递质多巴胺的一种代谢物,故在保护畜禽动物神经方面也具有巨大潜力。
5 小结HT具有抗氧化、预防癌症、抑菌抗炎、降脂等多种生理功能,且在改善畜禽动物肠道健康、保护动物肝脏、促进动物骨骼发育等方面具有巨大潜力。凭借其丰富的来源及优良的性质,HT作为一种天然的饲料添加剂颇具开发价值。然而,目前HT的研究主要是在体外模型和小鼠模型中,在畜禽动物生产上的应用鲜见报道。并且在实际生产中,HT的提纯成本昂贵,故市面上HT的商品形式多为油状黏稠液体,少见纯度高的粉末形式,因此在饲粮中添加时,混合可能不均匀,难以达到预期饲养效果,可通过逐级混匀或制粒的方法来提高HT在畜禽饲粮中的利用度。随着提取工艺的进步,HT必将克服成本昂贵、混合不均的问题。未来对HT的研究方向还应包括探讨HT与线粒体功能之间的关系以及HT与动物肠道微生物的相互作用机制研究,在不同畜禽动物饲粮中的最佳添加浓度、生物利用度和作用机制也还需要进一步探究。
| [1] |
BRITTON J, DAVIS R, O'CONNOR K E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol[J]. Applied Microbiology and Biotechnology, 2019, 103(15): 5957-5974. DOI:10.1007/s00253-019-09914-9 |
| [2] |
ROBLES-ALMAZAN M, PULIDO-MORAN M, MORENO-FERNANDEZ J, et al. Hydroxytyrosol:bioavailability, toxicity, and clinical applications[J]. Food Research International, 2018, 105: 654-667. DOI:10.1016/j.foodres.2017.11.053 |
| [3] |
OWEN R W, MIER W, GIACOSA A, et al. Phenolic compounds and squalene in olive oils:the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene[J]. Food and Chemical Toxicology, 2000, 38(8): 647-659. DOI:10.1016/S0278-6915(00)00061-2 |
| [4] |
CHAROENPRASERT S, MITCHELL A. Factors influencing phenolic compounds in table olives (Olea europaea)[J]. Journal of Agricultural and Food Chemistry, 2012, 60(29): 7081-7095. DOI:10.1021/jf3017699 |
| [5] |
BRENES M, GARCÍA A, GARCÍA P, et al. Phenolic compounds in Spanish olive oils[J]. Journal of Agricultural and Food Chemistry, 1999, 47(9): 3535-3540. DOI:10.1021/jf990009o |
| [6] |
MOTILVA M J, MACIÀ A, ROMERO M P, et al. Human bioavailability and metabolism of phenolic compounds from red wine enriched with free or nano-encapsulated phenolic extract[J]. Journal of Functional Foods, 2016, 25: 80-93. DOI:10.1016/j.jff.2016.05.013 |
| [7] |
RODRÍGUEZ-MORATÓ J, XICOTA L, FITÓ M, et al. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases[J]. Molecules, 2015, 20(3): 4655-4680. DOI:10.3390/molecules20034655 |
| [8] |
PEREIRA-CARO G, SARRIÁ B, MADRONA A, et al. Digestive stability of hydroxytyrosol, hydroxytyrosyl acetate and alkyl hydroxytyrosyl ethers[J]. International Journal of Food Sciences and Nutrition, 2012, 63(6): 703-707. DOI:10.3109/09637486.2011.652943 |
| [9] |
MANNA C, GALLETTI P, MAISTO G, et al. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells[J]. FEBS Letters, 2000, 470(3): 341-344. DOI:10.1016/S0014-5793(00)01350-8 |
| [10] |
VISSERS M N, ZOCK P L, ROODENBURG A J C, et al. Olive oil phenols are absorbed in humans[J]. The Journal of Nutrition, 2002, 132(3): 409-417. DOI:10.1093/jn/132.3.409 |
| [11] |
GRANADOS-PRINCIPAL S, QUILES J L, RAMIREZ-TORTOSA C L, et al. Hydroxytyrosol:from laboratory investigations to future clinical trials[J]. Nutrition Reviews, 2010, 68(4): 191-206. DOI:10.1111/j.1753-4887.2010.00278.x |
| [12] |
TUCK K L, FREEMAN M P, HAYBALL P J, et al. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats[J]. The Journal of Nutrition, 2001, 131(7): 1993-1996. DOI:10.1093/jn/131.7.1993 |
| [13] |
GONZALEZ-SANTIAGO M, FONOLLÁ J, LOPEZ-HUERTAS E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins[J]. Pharmacological Research, 2010, 61(4): 364-370. |
| [14] |
DE BOCK M, THORSTENSEN E B, DERRAIK J G B, et al. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract[J]. Molecular Nutrition & Food Research, 2013, 57(11): 2079-2085. |
| [15] |
DOMÍNGUEZ-PERLES R, AUÑÓN D, FERRERES F, et al. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC[J]. European Journal of Nutrition, 2017, 56(1): 215-224. DOI:10.1007/s00394-015-1071-2 |
| [16] |
SUÁREZ M, VALLS R M, ROMERO M P, et al. Bioavailability of phenols from a phenol-enriched olive oil[J]. British Journal of Nutrition, 2011, 106(11): 1691-1701. DOI:10.1017/S0007114511002200 |
| [17] |
FERNÁNDEZ-ÁVILA C, MONTES R, CASTELLOTE A I, et al. Fast determination of virgin olive oil phenolic metabolites in human high-density lipoproteins[J]. Biomedical Chromatography, 2015, 29(7): 1035-1041. DOI:10.1002/bmc.3389 |
| [18] |
D'ANGELO S, MANNA C, MIGLIARDI V, et al. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil[J]. Drug Metabolism and Disposition, 2001, 29(11): 1492-1498. |
| [19] |
WU Y T, LIN L C, TSAI T H. Measurement of free hydroxytyrosol in microdialysates from blood and brain of anesthetized rats by liquid chromatography with fluorescence detection[J]. Journal of Chromatography A, 2009, 1216(16): 3501-3507. DOI:10.1016/j.chroma.2008.10.116 |
| [20] |
RUBIO L, MACIÀ A, VALLS R M, et al. A new hydroxytyrosol metabolite identified in human plasma:hydroxytyrosol acetate sulphate[J]. Food Chemistry, 2012, 134(2): 1132-1136. DOI:10.1016/j.foodchem.2012.02.192 |
| [21] |
SERRA A, RUBIO L, BORRÀS X, et al. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake[J]. Molecular Nutrition & Food Research, 2012, 56(3): 486-496. |
| [22] |
DE LA TORRE R, COVAS M I, PUJADAS M A, et al. Is dopamine behind the health benefits of red wine?[J]. European Journal of Nutrition, 2006, 45(5): 307-310. DOI:10.1007/s00394-006-0596-9 |
| [23] |
SCHAFFER S, MVELLER W E, ECKERT G P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells[J]. Pharmacological Research, 2010, 62(4): 322-327. |
| [24] |
COVAS M I, DE LA TORRE R, FITÓ M. Virgin olive oil:a key food for cardiovascular risk protection[J]. British Journal of Nutrition, 2015, 113(Suppl.2): S19-S28. |
| [25] |
MARTINEZ L, ROS G, NIETO G. Hydroxytyrosol:health benefits and use as functional ingredient in meat[J]. Medicines, 2018, 5(1): 13. DOI:10.3390/medicines5010013 |
| [26] |
DE LA CRUZ J P, RUIZ-MORENO M I, GUERRERO A, et al. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain[J]. The Journal of Nutritional Biochemistry, 2015, 26(5): 549-555. DOI:10.1016/j.jnutbio.2014.12.013 |
| [27] |
VISIOLI F, POLI A, GALL C. Antioxidant and other biological activities of phenols from olives and olive oil[J]. Medicinal Research Reviews, 2002, 22(1): 65-75. |
| [28] |
DEIANA M, INCANI A, ROSA A, et al. Hydroxytyrosol glucuronides protect renal tubular epithelial cells against H2O2 induced oxidative damage[J]. Chemico-Biological Interactions, 2011, 193(3): 232-239. DOI:10.1016/j.cbi.2011.07.002 |
| [29] |
PAIVA-MARTINS F, SILVA A, ALMEIDA V, et al. Protective activity of hydroxytyrosol metabolites on erythrocyte oxidative-induced hemolysis[J]. Journal of Agricultural and Food Chemistry, 2013, 61(27): 6636-6642. DOI:10.1021/jf4016202 |
| [30] |
RODRIGUEZ-RAMIRO I, MARTÍN M Á, RAMOS S, et al. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress[J]. Toxicology, 2011, 288(1/2/3): 43-48. |
| [31] |
ILAVARASI K, KIRUTHIGA P V, PANDIAN S K, et al. Hydroxytyrosol, the phenolic compound of olive oil protects human PBMC against oxidative stress and DNA damage mediated by 2, 3, 7, 8-TCDD[J]. Chemosphere, 2011, 84(7): 888-893. DOI:10.1016/j.chemosphere.2011.06.017 |
| [32] |
ZHU L, LIU Z B, FENG Z H, et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase Ⅱ detoxifying enzyme systems in retinal pigment epithelial cells[J]. Journal of Nutritional Biochemistry, 2010, 21(11): 1089-1098. DOI:10.1016/j.jnutbio.2009.09.006 |
| [33] |
MARTÍN M A, RAMOS S, GRANADO-SERRANO A B, et al. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells[J]. Molecular Nutrition & Food Research, 2010, 54(7): 956-966. |
| [34] |
SAXENA N K, SHARMA D, DING X K, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells[J]. Cancer Research, 2007, 67(6): 2497-2507. DOI:10.1158/0008-5472.CAN-06-3075 |
| [35] |
ZHAO B L, MA Y, XU Z L, et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways[J]. Cancer Letters, 2014, 347(1): 79-87. DOI:10.1016/j.canlet.2014.01.028 |
| [36] |
NOTARNICOLA M, PISANTI S, TUTINO V, et al. Effects of olive oil polyphenols on fatty acid synthase gene expression and activity in human colorectal cancer cells[J]. Genes and Nutrition, 2011, 6(1): 63-69. |
| [37] |
FABIANI R, ROSIGNOLI P, DE BARTOLOMEO A, et al. Inhibition of cell cycle progression by hydroxytyrosol is associated with upregulation of cyclin-dependent protein kinase inhibitors p21WAF1/Cip1 and p27Kip1 and with induction of differentiation in HL60 cells[J]. The Journal of Nutrition, 2008, 138(1): 42-48. DOI:10.1093/jn/138.1.42 |
| [38] |
SIRIANNI R, CHIMENTO A, DE LUCA A, et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation[J]. Molecular Nutrition & Food Research, 2010, 54(6): 833-840. |
| [39] |
LUO C, LI Y, WANG H, et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells[J]. Current Cancer Drug Targets, 2013, 13(6): 625-639. DOI:10.2174/15680096113139990035 |
| [40] |
BISIGNANO G, TOMAINO A, LO CASCIO R, et al. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol[J]. The Journal of Pharmacy and Pharmacology, 1999, 51(8): 971-974. DOI:10.1211/0022357991773258 |
| [41] |
DE LEONARDIS A, ARETINI A, ALFANO G, et al. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity[J]. European Food Research and Technology, 2008, 226(4): 653-659. DOI:10.1007/s00217-007-0574-3 |
| [42] |
AISSA I, KHARRAT N, ALOUI F, et al. Valorization of antioxidants extracted from olive mill wastewater[J]. Biotechnology and Applied Biochemistry, 2017, 64(4): 579-589. DOI:10.1002/bab.1509 |
| [43] |
MEDINA-MARTÍNEZ M S, TRUCHADO P, CASTRO-IBÁÑEZ I, et al. Antimicrobial activity of hydroxytyrosol:a current controversy[J]. Bioscience, Biotechnology, and Biochemistry, 2016, 80(4): 801-810. DOI:10.1080/09168451.2015.1116924 |
| [44] |
DIALLINAS G, RAFAILIDOU N, KALPAKTSI I, et al. Hydroxytyrosol (HT) analogs act as potent antifungals by direct disruption of the fungal cell membrane[J]. Frontiers in Microbiology, 2018, 9: 2624. DOI:10.3389/fmicb.2018.02624 |
| [45] |
YAO F, YANG G D, XIAN Y S, et al. The protective effect of hydroxytyrosol acetate against inflammation of vascular endothelial cells partly through the SIRT6-mediated PKM2 signaling pathway[J]. Food & Function, 2019, 10(9): 5789-5803. |
| [46] |
YONEZAWA Y, KIHARA T, IBI K, et al. Olive-derived hydroxytyrosol shows anti-inflammatory effect without gastric damage in rats[J]. Biological and Pharmaceutical Bulletin, 2019, 42(7): 1120-1127. DOI:10.1248/bpb.b18-00979 |
| [47] |
SCODITTI E, MASSARO M, CARLUCCIO M A, et al. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes[J]. PLoS One, 2015, 10(6): e0128218. DOI:10.1371/journal.pone.0128218 |
| [48] |
LIU Z Q, WANG N N, MA Y N, et al. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice[J]. Frontiers in Microbiology, 2019, 10: 390. DOI:10.3389/fmicb.2019.00390 |
| [49] |
GINER E, ANDU'JAR I, RECIO M C, et al. Oleuropein ameliorates acute colitis in mice[J]. Journal of Agricultural and Food Chemistry, 2011, 59(24): 12882-12892. DOI:10.1021/jf203715m |
| [50] |
SERRELI G, MELIS M P, CORONA G, et al. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites:insight into the mechanism of action[J]. Food and Chemical Toxicology, 2019, 125: 520-527. DOI:10.1016/j.fct.2019.01.039 |
| [51] |
DRIRA R, SAKAMOTO K. Modulation of adipogenesis, lipolysis and glucose consumption in 3T3-L1 adipocytes and C2C12 myotubes by hydroxytyrosol acetate:a comparative study[J]. Biochemical and Biophysical Research Communications, 2013, 440(4): 576-581. DOI:10.1016/j.bbrc.2013.09.106 |
| [52] |
DRIRA R, SAKAMOTO K. Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes[J]. European Journal of Nutrition, 2014, 53(3): 743-750. DOI:10.1007/s00394-013-0578-7 |
| [53] |
STEFANON B, COLITTI M. Original research:hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation[J]. Experimental Biology and Medicine, 2016, 241(16): 1796-1802. DOI:10.1177/1535370216654226 |
| [54] |
SCODITTI E, CARPI S, MASSARO M, et al. Hydroxytyrosol modulates adipocyte gene and miRNA expression under inflammatory condition[J]. Nutrients, 2019, 11(10): 2493. DOI:10.3390/nu11102493 |
| [55] |
GARCIA-CONTRERAS C, VAZQUEZ-GOMEZ M, PARDO Z, et al. Polyphenols and IUGR pregnancies:effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues[J]. Nutrients, 2019, 11(7): 1534. DOI:10.3390/nu11071534 |
| [56] |
ECHEVERRÍA F, VALENZUELA R, BUSTAMANTE A, et al. Attenuation of high-fat diet-induced rat liver oxidative stress and steatosis by combined hydroxytyrosol- (HT-) eicosapentaenoic acid supplementation mainly relies on HT[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 5109503. |
| [57] |
LUCAS R, COMELLES F, ALCÁNTARA D, et al. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters:a potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions[J]. Journal of Agricultural and Food Chemistry, 2010, 58(13): 8021-8026. DOI:10.1021/jf1009928 |
| [58] |
WANG N N, LIU Y, MA Y N, et al. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice[J]. The Journal of Nutritional Biochemistry, 2018, 57: 180-188. DOI:10.1016/j.jnutbio.2018.03.018 |
| [59] |
GAVAHIAN M, KHANEGHAH A M, LORENZO J M, et al. Health benefits of olive oil and its components:impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases[J]. Trends in Food Science & Technology, 2019, 88: 220-227. |
| [60] |
DEIANA M, SERRA G, CORONA G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds[J]. Food & Function, 2018, 9(8): 4085-4099. |
| [61] |
PAN S H, LIU L X, PAN H Y, et al. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice[J]. Molecular Nutrition & Food Research, 2013, 57(7): 1218-1227. |
| [62] |
BHOGAL R H, CURBISHLEY S M, WESTON C J, et al. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation[J]. Liver Transplantation, 2010, 16(11): 1303-1313. DOI:10.1002/lt.22157 |
| [63] |
ECHEVERRIA F, VALENZUELA R, BUSTAMANTE A, et al. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism:attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration[J]. Food & Function, 2019, 10(9): 6170-6183. |
| [64] |
SOTO-ALARCÓN S A, ORTIZ M, ORELLANA P, et al. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevents liver steatosis and related parameters in mice subjected to high-fat diet:a molecular approach[J]. Biofactors, 2019, 45(6): 930-943. DOI:10.1002/biof.1556 |
| [65] |
PRIORE P, SICULELLA L, GNONI G V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes[J]. Journal of Nutritional Biochemistry, 2014, 25(7): 683-691. DOI:10.1016/j.jnutbio.2014.01.009 |
| [66] |
RUBIO-SENENT F, DE ROOS B, DUTHIE G, et al. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats[J]. European Journal of Nutrition, 2015, 54(8): 1287-1295. DOI:10.1007/s00394-014-0807-8 |
| [67] |
RODRÍGUEZ-GUTIÉRREZ G, RUBIO-SENENT F, GÓMEZ-CARRETERO A, et al. Selenium and sulphur derivatives of hydroxytyrosol:inhibition of lipid peroxidation in liver microsomes of vitamin E-deficient rats[J]. European Journal of Nutrition, 2019, 58(5): 1847-1851. DOI:10.1007/s00394-018-1733-y |
| [68] |
CAI W J, CHEN Y, SHI L X, et al. AKT-GSK3β signaling pathway regulates mitochondrial dysfunction-associated OPA1 cleavage contributing to osteoblast apoptosis:preventative effects of hydroxytyrosol[J]. Oxidative Medicine and Cellular Longevity, 2019, 2019: 4101738. |
| [69] |
MAHMOUDI A, GHORBEL H, FEKI I, et al. Oleuropein and hydroxytyrosol protect rats' pups against bisphenol A induced hypothyroidism[J]. Biomedicine & Pharmacotherapy, 2018, 103: 1115-1126. |
| [70] |
ANTER J, QUESADA-GÓMEZ J M, DORADO G, et al. Effect of hydroxytyrosol on human mesenchymal stromal/stem cell differentiation into adipocytes and osteoblasts[J]. Archives of Medical Research, 2016, 47(3): 162-171. DOI:10.1016/j.arcmed.2016.06.006 |




