膳食氨基酸调节肠内动态平衡对维持肠道健康和预防肠道疾病具有重要作用[1]。蛋氨酸(methionine,Met)又名甲硫氨酸或甲硫基丁氨酸,其一碳代谢物S-腺苷甲硫氨酸(S-adenosylmethionine,SAM)是控制肠道代谢稳态的关键介质,同时也是维持肠道干细胞(intestinal stem cells,ISCs)活性的重要调节因子[2-4]。Met通过调控Wnt/β-连环蛋白(β-catenin)和哺乳动物雷帕霉素靶蛋白敏感型复合体1(mTORC1)等信号通路的转导,保护肠上皮结构和功能的完整性[5]。本文综述了肠道中Met的吸收代谢、Met对ISCs和肠上皮发育的影响及其可能的机制,以期为肠道精准营养调控提供思路。
1 Met在小肠中的吸收和代谢Met是构成机体的必需氨基酸之一,分为L型和D型。与Met相关的转运系统有A型(ATA1、ATA2和ATA3)和B0型(B0AT1和B0AT2)等。肠道中的Met主要依赖肠上皮细胞刷状缘上的B0型转运载体进入细胞内[6-8]。被吸收的L-Met可直接参与蛋白质的合成,而D-Met则需要在氨基酸氧化酶的作用下去掉1个氨基生成α-酮-蛋氨酸,接着在转氨酶的作用下连上1个氨基形成L-Met才能参与合成蛋白质[9]。研究表明,人工合成的DL-Met由50%的D-Met和50%的L-Met构成,DL-Met转化为L-Met需要消耗1.75个ATP,而D-Met转化为L-Met需要消耗3.5个ATP[10]。
肠细胞吸收的Met一部分通过门静脉入肝脏,被肝细胞加工后排入血液供机体利用,另一部分Met则留在肠细胞中被代谢利用[11]。首先,Met在ATP和蛋氨酸腺苷转移酶1型(methionine adenosine transferase 1A,MAT1A)的作用下活化为SAM,SAM作为辅酶参与转甲基作用,失去1个甲基转变为S-腺苷高半胱氨酸(S-adenosylhomocysteine,SAH)[12];SAH再经水解生成高半胱氨酸(homocysteine,Hcy),完成转甲基途径[13-14]。Hcy依次在β-胱硫醚合成酶和γ-胱硫醚酶的作用下生成半胱氨酸(cysteine,Cys),参与谷胱甘肽、胱氨酸和牛磺酸等生物活性物质的合成,完成转硫途径[15-16]。此外,SAM还可通过转胺丙基作用参与生物胺(包括精胺、亚精胺和腐胺)的形成。
2 Met增强肠道屏障功能及抗氧化能力 2.1 Met增强肠道屏障功能肠细胞通过紧密连接形成一道完整的防线以隔绝营养物质、毒素和抗原在细胞旁空间的自由交换[17-18]。而这些紧密连接蛋白质的表达和分布又受营养素的动态调控。Met作为功能性氨基酸促进蛋白质合成,维持肠道屏障功能完整性[19-20]。Chen等[21]研究表明,饲粮添加L-Met增加断奶仔猪空肠上皮细胞跨膜电阻值,提示Met增强仔猪肠道屏障功能。Tsukita等[22]进一步证实,添加Met组的仔猪肠道闭合蛋白-3(Claudin-3)表达水平显著增加。本课题组给小鼠灌胃L-Met和蛋氨酸羟基类似物(2-hydroxy-4-methylthio butanoic acid,HMB)发现,二者并未提高空肠中紧密连接蛋白-1(ZO-1)和闭合蛋白-1(Claudin-1)的表达量;然而,在呕吐毒素损伤条件下,L-Met和液体HMB均能显著降低血清中脂多糖(lipopolysaccharide,LPS)含量,提高肠道中二胺氧化酶(diamine oxidase,DAO)活性和紧密连接蛋白表达量。此外,绒毛上Mucin2+细胞和隐窝中Lysozyme+细胞数量增加,提示Met及其羟基类似物增强了因呕吐毒素中毒的小鼠肠道化学和免疫屏障功能[23]。
2.2 Met增强肠道抗氧化能力饲粮中适量添加Met及其羟基类似物,可以在一定程度上改善早期断奶仔猪肠道的抗氧化能力[24]。一方面,Met经氧化转化为甲硫氨酸亚砜(methionine sulfoxide,MetO),而普遍存在的甲硫氨酸亚砜还原酶可以将亚砜还原为Met。这个氧化还原循环使Met残基几乎能被所有种类的活性氧(reactive oxygen species,ROS)修饰反应,提供有效的抗氧化防御[25-26];另一方面,Met通过转硫作用促进Cys参与谷胱甘肽的合成,而谷胱甘肽作为一种重要的抗氧化剂,具有清除体内的自由基、保护蛋白质和酶等分子中的巯基不被ROS氧化的作用[27-28]。此外,Met代谢产生多胺、牛磺酸等生物活性物质,同样能参与清除自由基、抵抗脂质过氧化的功能。
3 Met及其一碳代谢产物促进肠上皮更新和再生肠道是机体更新最快的组织之一。肠上皮易受到外源因子的攻击,为了维持肠道稳态平衡,ISCs保持增殖以替换受损的肠细胞[29]。Met及其代谢产物可以促进细胞增殖,增强细胞免疫功能,加速肠黏膜的快速更新和再生[30]。而Met的缺乏则会降低仔猪肠上皮细胞活力,抑制小肠黏膜生长,导致肠绒毛萎缩[31]。此外,Met循环的产物多胺能诱导仔猪肠上皮细胞及隐窝细胞增殖,增加绒毛高度和宽度,在黏膜发育的早期也能刺激其分化[32-33];Met的另一种代谢产物——精胺同样能增加仔猪小肠黏膜重量,提高黏膜中蛋白质、DNA和RNA的含量,促进仔猪肠道发育,缺失会抑制哺乳动物细胞增殖中的翻译过程[34-35]。
4 Met刺激ISCs分裂位于肠道黏膜隐窝底部的ISCs是驱动肠上皮稳态平衡和再生的关键[36-37]。ISCs包括活跃型的隐窝基部柱状细胞(crypt base columnar cell,CBC)和沉默型的+4位干细胞[38]。CBC由亮氨酸重复单位的G蛋白质偶联受体5(leucinerich-repeat-containing G-protein-coupled receptor 5,Lgr5)标记,镶嵌在潘氏细胞中,负责维持肠黏膜的更新;+4位干细胞由B细胞特异性单株鼠白血病病毒插入位点1(B cell specific moloney murine leukemia virus insertion site 1,Bmi1)标记,在损伤条件下被激活[39-40]。本课题组研究表明,添加Met能刺激Lgr5和Bmi1表达,促进ISCs扩增,从而逆转呕吐毒素诱导的肠上皮损伤[23]。Saito等[41]同样发现,Met缺乏会下调类肠团中Lgr5 mRNA丰度,抑制干细胞增殖活性。Obata等[42]通过对果蝇饥饿和复饲的研究证明,饲粮Met通过SAM调节蛋白质合成并影响ISCs分裂。
4.1 Met与ISCs巢ISCs处于一个动态且复杂的由潘氏细胞、肠间质细胞和肌成纤维细胞组成的微环境(即干细胞巢)中[43]。大量研究证实,功能性氨基酸不仅仅是合成蛋白质的原料,也可能通过改变干细胞巢中的成分,直接或间接影响到ISCs发育。Met残基可通过与氧自由基作用影响细胞微环境中K+浓度参与细胞离子通道功能的调节。此外,SAM促进体内硫化氢(H2S)形成,促进环磷腺苷(cAMP)的生成、激活细胞K+通道[44]。上述结果提示干细胞巢中Met及代谢产物有调控离子通道、控制物质进出的作用。然而目前关于Met对干细胞巢影响的研究仍不够深入,其对ISCs的精准调控机制仍待进一步解析。
4.2 Met通过其一碳代谢产物调节Wnt/β-catenin信号通路Wnt/β-catenin是ISCs增殖的首要驱动力[45]。Wnts能够与细胞膜表面的卷曲蛋白质受体(frizzleds,FZDs)及其辅助受体低密度脂蛋白质受体相关蛋白质(low-density lipoprotein receptor-related proteins,LRPs)的胞外活性区域结合,将信号传递给蓬乱蛋白质(dishevelled,Dvl),抑制由大肠腺瘤息肉蛋白质(adenomatosis polyposis coli,APC)、酪氨酸激酶1(casein kinase 1,CK1)、轴抑制蛋白质2(axis inhibition protein 2,Axin2)和糖合成酶激酶3β(glycogen synthase kinase 3β,GSK3β)等组成的降解复合体的形成,阻断β-catenin降解,使得细胞质中稳定的β-catenin转移到细胞核中与T细胞特异性转录因子(T cell-specific transcription factor,TCF)结合,启动c-Myc癌基因(cancer-Myc,c-Myc)、细胞周期蛋白质D1(cell-cycle protein cyclin D1,Cyclin D1)和Lgr5等靶基因的转录[46-47]。本课题组研究表明,激活Wnt/β-catenin可促进干细胞扩增为类肠团[48-49]。
Albrecht等[50]研究发现,在Met缺乏的培养基中补充Met或SAM均会激活Wnt/β-catenin信号,而同时加入蛋氨酸腺苷转移酶2型(methionine adenosine transferase 2A,MAT2A)阻断Met转化为SAM,Met对Wnt/β-catenin的调节作用消失,说明Met能够通过一碳代谢控制细胞内SAM水平从而调控Wnt/β-catenin活性。细胞内SAM含量降低会抑制Wnt/β-catenin信号传导,同时也下调了Wnt诱导的溶酶体活性。虽然目前尚不清楚Wnt/β-catenin信号是否通过溶酶体重新连接代谢途径,但是毫无疑问,SAM是溶酶体保持活性所必需的。此外,SAM是精氨酸甲基转移酶1(arginine methyl transferase 1,PRMT1)的甲基供体[51]。作为Wnt信号成分之一,PRMT1在隐窝中表达水平较高,其活性也随着SAM水平的降低而降低。Zhou等[51]进一步发现,SAM可促进胞质中的PRMT1和GSK3锚定在内溶酶体,从而加速GSK3的降解。由此可知,SAM调控Wnt/β-catenin主要通过PRMT1诱导GSK3的降解。
4.3 Met及其一碳代谢产物调节mTORC1信号通路mTORC1能整合营养素在内等多种细胞外的信号,通过与真核起始因子3(eukaryotic initiation factor,eIF3)结合,磷酸化下游效应蛋白质核糖体蛋白S6激酶1(ribosomal protein S6 kinase polypeptide 1,S6K1),使S6K1从eIF3上释放而被活化,活化的S6K1可磷酸化S6核糖体蛋白质,从而促进人类延伸因子1α启动子(elonggation factor 1α,EF1α)和多聚腺苷酸(polyadenylic acid,polyA)等蛋白质的翻译及表达[52-53]。此外,mTORC1能磷酸化4E结合蛋白质1(4E binding protein 1,4EBP1),使其解除对真核翻译起始因子4E(eukaryotic translation initiation factor 4E,eIF4E)的抑制作用,进而启动和促进转录过程,合成新的蛋白质,提高细胞增殖活性[54-56]。哺乳动物雷帕霉素靶蛋白(mTOR)在小肠上皮的稳态和再生过程中发挥了不同的调节作用,即在正常条件下,mTOR可调控吸收性和分泌性肠细胞的末端成熟,而处于损伤后的修复状态时,mTORC1是ISCs活性维持的关键信号,它的缺失将导致肠上皮萎缩[57]。研究表明,抑制结节性硬化2(tuberous sclerosis 2,TSC2)可上调mTORC1信号,从而促进Notch胞内结构域(notch intracellular domain,NICD)和Hes1蛋白质的表达,激活Notch信号通路,降低细胞分化[58-60]。相反,雷帕霉素(rapamycin,Rapa)处理后,可降低mTORC1介导的Notch信号上调,细胞的分化能力得到改善[61]。
Met调控ISCs增殖活性,促进蛋白质合成。然而Met调节ISCs中蛋白质合成的机制还不是十分清楚。Gu等[62]发现Met活化成SAM可通过直接与SAMTOR结合,从而破坏SAMTOR-GATOR1复合物。而GATOR1(包括DEPDC5、NPRL2和NPRL3)通过与其子复合体Rag鸟苷三磷酸酶(Rag GTPase)相互作用抑制mTORC1信号通路。同时,Inoki等[63]表示GSK3能通过依赖于腺苷酸活化蛋白质激酶(adenylate activated protein kinase,AMPK)启动的磷酸化方式激活TSC2来抑制mTORC1通路,而SAM能加速GSK3的降解。因此,Met可能通过SAM抑制SAMTOR-GATOR1和GSK3/TSC2,从而激活mTORC1信号通路,促进干细胞中蛋白质的合成。Met介导Wnt/β-catenin和mTORC1信号转导机制如图 1所示。
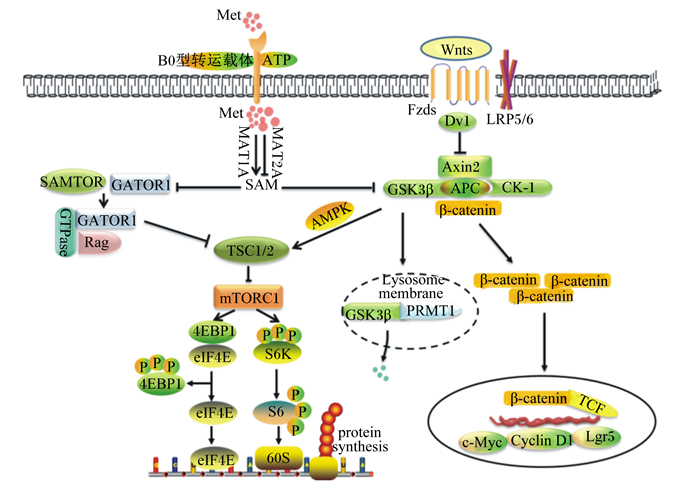
|
Met:蛋氨酸methionine;MAT1A:蛋氨酸腺苷转移酶1型methionine adenosine transferase 1A;MAT2A:蛋氨酸腺苷转移酶2型methionine adenosine transferase 2A;SAMTOR:S-腺苷甲硫氨酸传感器S-adenosylmethionine sensor upstream of mTORC1;GATOR1:Rag1的GTP酶激活蛋白质Gap activity toward Rags 1;SAM:S-腺苷甲硫氨酸S-adenosylmethionine;GSK3β:糖合成酶激酶3β glycogen synthase kinase 3β;APC:腺瘤息肉蛋白质adenomatosis polyposis coli;CK-1:肌酸激酶-1 creatine kinase-1;Dvl:蓬乱蛋白质dishevelled;LRP5/6:低密度脂蛋白质受体相关蛋白质5/6 low-density lipoprotein receptor-related proteins 5/6;Axin2:轴抑制蛋白质2 axis inhibition protein 2;GTPase:GTP酶guanosine triphosphatase;Rag:重组酶激活基因recombinase activating gene;TSC1/2:结节性硬化1/2 tuberous sclerosis 1/2;AMPK:腺苷酸活化蛋白质激酶adenylate activated protein kinase;β-catenin:β-连环蛋白;mTORC1:哺乳动物雷帕霉素靶蛋白敏感型复合体1 mammalian target-sensitive complex of rapamycin 1;4EBP1:4E结合蛋白质1 4E binding protein 1;eIF4E:真核翻译起始因子4E eukaryotic translation initiation factor 4;S6K:效应蛋白S6K1 ribosomalprotein S6 kinase;S6:S6核糖体蛋白质S6 ribosomal protein;60S:60S亚基60S subunit; GSK3β:糖合成酶激酶3β glycogen synthase kinase 3β;PRMT1:精氨酸甲基转移酶1 arginine methyl transferase 1;TCF:T细胞特异性转录因子T cell-specific transcription factor;c-Myc:c-Myc癌基因cancer-Myc;Cyclin D1:细胞周期蛋白质D1 cell-cycle protein cyclin D1;Lgr5:亮氨酸重复单位的G蛋白质偶联受体5 leucinerich-repeat-containing G-protein-coupled receptor 5;TCF:T细胞特异性转录因子T cell-specific transcription factor;protein synthesis:蛋白质合成;Lysosome membrane:溶酶体膜。 图 1 Met介导Wnt/β-catenin和mTORC1信号转导机制 Fig. 1 Regulatory mechanisms of Wnt/β-catenin and mTORC1 signaling pathways by Met |
ISCs与肠道内容物紧密接触,Met及其一碳代谢产物通过调控ISCs增殖和分化活性维持肠道上皮结构和功能完整性,这一过程依赖于Wnt/β-catenin和mTORC1等信号通路的介导。此外,TSC2整合的Wnt和mTORC1信号可能是Met促进ISCs中蛋白质合成的途径之一。然而,除GSK3和GATOR外,胞内SAM与Wnt/β-catenin和mTORC1之间是否还存在其他关联的关键靶标以及胞外Met能否通过膜受体直接调控Wnt/β-catenin和mTORC1信号通路仍需进一步挖掘。目前,关于Met及其一碳代谢产物调节ISCs功能的研究多建立在非常态下,而且缺少与其他氨基酸之间的互作效应。因此,考虑将现有的成果集合成营养调控技术体系应用于新型饲料添加剂和药物研发需关注:1)Met及其一碳代谢产物对不同物种的适宜添加水平;2)常态和非常态下Met及其一碳代谢产物功能的差异性比较;3)Met及其一碳代谢产物精准靶向,需要鉴定肠细胞中的蛋氨酸转运感应体以及其在细胞内的关键作用靶标;4)Met及其一碳代谢产物与其他营养素的协同或拮抗作用。
| [1] |
GOTTARDO E T, PROKOSKI K, HORN D, et al. Regeneration of the intestinal mucosa in Eimeria and E. coli challenged broilers supplemented with amino acids[J]. Poultry Science, 2016, 95(5): 1056-1065. DOI:10.3382/ps/pev356 |
| [2] |
SHOVELLER A K, STOLL B, BALL R O, et al. Nutritional and functional importance of intestinal sulfur amino acid metabolism[J]. The Journal of Nutrition, 2005, 135(7): 1609-1612. DOI:10.1093/jn/135.7.1609 |
| [3] |
TANG S, FANG Y, HUANG G, et al. Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development[J]. The EMBO Journal, 2017, 36(21): 3175-3193. DOI:10.15252/embj.201796708 |
| [4] |
BURGESS R J, AGATHOCLEOUS M, MORRISON S J. Metabolic regulation of stem cell function[J]. Journal of Internal Medicine, 2014, 276(1): 12-24. |
| [5] |
YAN S L, LONG L N, ZONG E Y, et al. Dietary sulfur amino acids affect jejunal cell proliferation and functions by affecting antioxidant capacity, Wnt/β-catenin, and the mechanistic target of rapamycin signaling pathways in weaning piglets[J]. Journal of Animal Science, 2018, 96(12): 5124-5133. DOI:10.1093/jas/sky349 |
| [6] |
韩春晓, 苗翠. 蛋氨酸的功能及代谢吸收过程[J]. 现代畜牧兽医, 2013(9): 75-80. DOI:10.3969/j.issn.1672-9692.2013.09.021 |
| [7] |
BAKER D H, BOEBEL K P. Utilization of the D-and L-isomers of methionine and methionine hydroxy analogue as determined by chick bioassay[J]. The Journal of Nutrition, 1980, 110(5): 959-964. DOI:10.1093/jn/110.5.959 |
| [8] |
GAO X, SANDERSON S M, DAI Z W, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism[J]. Nature, 2019, 572(7769): 397-401. |
| [9] |
霍迪. 对于断奶仔猪L-蛋氨酸与DL-蛋氨酸的生物利用率是相同的[J]. 饲料与畜牧, 2016(1): 56-58. |
| [10] |
WANG W W, WANG J, WU S G, et al. Bioavailability of L-methionine relative to DL-methionine in broiler chickens[J]. Italian Journal of Animal Science, 2019, 18(1): 1231-1238. DOI:10.1080/1828051X.2019.1641433 |
| [11] |
TSUKAMOTO H, LU S C. Current concepts in the pathogenesis of alcoholic liver injury[J]. The FASEB Journal, 2001, 15(8): 1335-1349. DOI:10.1096/fj.00-0650rev |
| [12] |
RAMANI K, LU S C. Methionine adenosyltransferases in liver health and diseases[J]. Liver Research, 2017, 1(2): 103-111. |
| [13] |
BROSNAN J T, BROSNAN M E. The sulfur-containing amino acids:an overview[J]. The Journal of Nutrition, 2006, 136(6): 1636S-1640S. DOI:10.1093/jn/136.6.1636S |
| [14] |
WEICKHMANN A K, KELLER H, WURM J P, et al. The structure of the SAM/SAH-binding riboswitch[J]. Nucleic Acids Research, 2019, 47(5): 2654-2665. DOI:10.1093/nar/gky1283 |
| [15] |
JEON J S, OH J J, KWAK H C, et al. Age-related changes in sulfur amino acid metabolism in male C57BL/6 mice[J]. Biomolecules & Therapeutics, 2018, 26(2): 167-174. |
| [16] |
FOUAD A M, RUAN D, LIN Y C, et al. Effects of dietary methionine on performance, egg quality and glutathione redox system in egg-laying ducks[J]. British Poultry Science, 2016, 57(6): 818-823. DOI:10.1080/00071668.2016.1222603 |
| [17] |
LI Q R, ZHANG Q, WANG C Y, et al. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats[J]. Journal of Cellular and Molecular Medicine, 2009, 13(9B): 4061-4076. DOI:10.1111/j.1582-4934.2009.00975.x |
| [18] |
STEED E, BALDA M S, MATTER K. Dynamics and functions of tight junctions[J]. Trends in Cell Biology, 2010, 20(3): 142-149. DOI:10.1016/j.tcb.2009.12.002 |
| [19] |
RAMALINGAM A, WANG X X, GABELLO M, et al. Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern[J]. American Journal of Physiology-Cell Physiology, 2010, 299(5): C1028-C1035. DOI:10.1152/ajpcell.00482.2009 |
| [20] |
SKROVANEK S, VALENZANO M C, MULLIN J M. Restriction of sulfur-containing amino acids alters claudin composition and improves tight junction barrier function[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2007, 293(3): R1046-R1055. DOI:10.1152/ajpregu.00072.2007 |
| [21] |
CHEN Y, LI D F, DAI Z L, et al. L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets[J]. Amino Acids, 2014, 46(4): 1131-1142. DOI:10.1007/s00726-014-1675-5 |
| [22] |
TSUKITA S, FURUSE M. The structure and function of claudins, cell adhesion molecules at tight junctions[J]. Annals of the New York Academy of Sciences, 2000, 915(1): 129-135. |
| [23] |
ZHOU J Y, WANG Z, ZHANG S W, et al. Methionine and its hydroxyl analogues improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating Wnt/β-catenin signaling[J]. Journal of Agricultural and Food Chemistry, 2019, 67(41): 11464-11473. DOI:10.1021/acs.jafc.9b04442 |
| [24] |
LI H, WAN H F, MERCIER Y, et al. Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue[J]. British Journal of Nutrition, 2014, 112(6): 855-867. DOI:10.1017/S000711451400172X |
| [25] |
KAYA A, KOC A, LEE B C, et al. Compartmentalization and regulation of mitochondrial function by methionine sulfoxide reductases in yeast[J]. Biochemistry, 2018, 49(39): 8618-8625. |
| [26] |
LIM J M, KIM G, LEVINE R L. Methionine in proteins:it's not just for protein initiation anymore[J]. Neurochemical Research, 2019, 44(1): 247-257. DOI:10.1007/s11064-017-2460-0 |
| [27] |
SHAH N A, KHAN M R. Increase of glutathione, testosterone and antioxidant effects of Jurenia dolomiaea on CCl4 induced testicular toxicity in rat[J]. BMC Complementary and Alternative Medicine, 2017, 17: 206. DOI:10.1186/s12906-017-1718-z |
| [28] |
KENGEN J, DEGLASSE J P, NEVEU M A, et al. Biomarkers of tumour redox status in response to modulations of glutathione and thioredoxin antioxidant pathways[J]. Free Radical Research, 2018, 52(2): 256-266. |
| [29] |
YOUSEFI M, NAKAUKA-DDAMBA A, BERRY C T, et al. Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mtorc1 in reserve stem cells[J]. Stem Cell Reports, 2018, 10(3): 703-711. |
| [30] |
WITHERS H R. Regeneration of intestinal mucosa after irradiation[J]. Cancer, 1971, 28(1): 75-81. |
| [31] |
BAUCHART-THEVRET C, STOLL B, CHACKO S, et al. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs[J]. American Journal of Physiology-Endocrinology and Metabolism, 2009, 296(6): E1239-E1239. DOI:10.1152/ajpendo.91021.2008 |
| [32] |
WU G Y, FLYNN N E, KNABE D A. Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets[J]. American Journal of Physiology-Endocrinology and Metabolism, 2000, 279(2): E395-E402. DOI:10.1152/ajpendo.2000.279.2.E395 |
| [33] |
VAN WETTERE W H E J, WILLSON N L, PAIN S J, et al. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics[J]. Animal, 2016, 10(10): 1655-1659. DOI:10.1017/S1751731116000446 |
| [34] |
MANDAL S, MANDAL A, JOHANSSON H E, et al. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(6): 2169-2174. DOI:10.1073/pnas.1219002110 |
| [35] |
FANG T T, LIU G M, CAO W, et al. Spermine:new insights into the intestinal development and serum antioxidant status of suckling piglets[J]. RSC Advances, 2016, 6(37): 31323-31335. DOI:10.1039/C6RA05361K |
| [36] |
BARKER N. Adult intestinal stem cells:critical drivers of epithelial homeostasis and regeneration[J]. Nature Reviews Molecular Cell Biology, 2014, 15(1): 19-33. |
| [37] |
POTTEN C S, LOEFFLER M. Stem cells:attributes, cycles, spirals, pitfalls and uncertainties:lessons for and from the crypt[J]. Development, 1990, 110(4): 1001-1020. |
| [38] |
CLEVERS H. The intestinal crypt, a prototype stem cell compartment[J]. Cell, 2013, 154(2): 274-284. |
| [39] |
GEHART H, CLEVERS H. Tales from the crypt:new insights into intestinal stem cells[J]. Nature Reviews Gastroenterology & Hepatology, 2019, 16(1): 19-34. |
| [40] |
YAN K S, CHIA L A, LI X N, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(2): 466-471. DOI:10.1073/pnas.1118857109 |
| [41] |
SAITO Y, IWATSUKI K, HANYU H, et al. Effect of essential amino acids on enteroids:methionine deprivation suppresses proliferation and affects differentiation in enteroid stem cells[J]. Biochemical and Biophysical Research Communications, 2017, 488(1): 171-176. DOI:10.1016/j.bbrc.2017.05.029 |
| [42] |
OBATA F, TSUDA-SAKURAI K, YAMAZAKI T, et al. Nutritional control of stem cell division through S-adenosylmethionine in Drosophila intestine[J]. Developmental Cell, 2018, 44(6): 741-751. DOI:10.1016/j.devcel.2018.02.017 |
| [43] |
MERAN L, BAULIES A, LI V S W. Intestinal stem cell niche:the extracellular matrix and cellular components[J]. Stem Cells International, 2017, 2007: 7970385. |
| [44] |
YAN X H, SAKAMOTO K, KIMURA J. P15 effects of NaHS on E-4031 sensitive K+ current and apamin-sensitive K+ current in L6 myotubes[J]. Nitric Oxide, 2014, 39(Suppl.1): S20. |
| [45] |
LI X G, ZHU M, CHEN M X, et al. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway[J]. Toxicology Letters, 2019, 305: 19-31. DOI:10.1016/j.toxlet.2019.01.008 |
| [46] |
ZHOU J Y, HUANG D G, ZHU M, et al. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress[J]. Journal of Cellular Physiology, 2020. DOI:10.1002/jcp.29492 |
| [47] |
FLANAGAN D J, PHESSE T J, BARKER N, et al. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5+ stem cells[J]. Stem Cell Reports, 2015, 4(5): 759-767. DOI:10.1016/j.stemcr.2015.03.003 |
| [48] |
ZHOU J Y, ZHANG S W, LIN H L, et al. Hydrolyzed wheat gluten alleviates deoxynivalenol-induced intestinal injury by promoting intestinal stem cell proliferation and differentiation via upregulation of Wnt/β-catenin signaling in mice[J]. Food and Chemical Toxicology, 2019, 131: 110579. DOI:10.1016/j.fct.2019.110579 |
| [49] |
朱秋杰, 周加义, 梁少杰, 等. Wnt/β-连环蛋白信号驱动小肠上皮更新和再生机制的研究进展[J]. 动物营养学报, 2019, 31(11): 4995-5002. |
| [50] |
ALBRECHT L V, BUI M H, DE ROBERTIS E M. Canonical Wnt is inhibited by targeting one-carbon metabolism through methotrexate or methionine deprivation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(8): 2987-2995. DOI:10.1073/pnas.1820161116 |
| [51] |
ZHOU R, XIE Y Q, HU H, et al. Molecular mechanism underlying PRMT1 dimerization for SAM binding and methylase activity[J]. Journal of Chemical Information and Modeling, 2015, 55(12): 2623-2632. DOI:10.1021/acs.jcim.5b00454 |
| [52] |
HOLZ M K, BALLIF B A, GYGI S P, et al. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events[J]. Cell, 2005, 123(4): 569-580. DOI:10.1016/j.cell.2005.10.024 |
| [53] |
ZHU M, WANG X Q. Regulation of mTORC1 by small GTPases in response to nutrients[J]. The Journal of Nutrition, 2020. DOI:10.1093/jn/nxz301 |
| [54] |
BURNETT P E, BARROW R K, COHEN N A, et al. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(4): 1432-1437. DOI:10.1073/pnas.95.4.1432 |
| [55] |
LE BACQUER O, PETROULAKIS E, PAGLIALUNGA S, et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2[J]. The Journal of Clinical Investigation, 2007, 117(2): 387-396. DOI:10.1172/JCI29528 |
| [56] |
ZHOU J Y, HUANG D G, QIN Y C, et al. mTORC1 signaling activation increases intestinal stem cell activity and promotes epithelial cell proliferation[J]. Journal of Cellular Physiology, 2019, 234(10): 19028-19038. DOI:10.1002/jcp.28542 |
| [57] |
SAMPSON L L, DAVIS A K, GROGG M W, et al. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice[J]. The FASEB Journal, 2015, 30(3): 1263-1275. |
| [58] |
ZHOU Y, RYCHAHOU P, WANG Q, et al. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium[J]. Cell Death & Disease, 2015, 6(2): e1631. |
| [59] |
KARBOWNICZEK M, ZITSERMAN D, KHABIBULLIN D, et al. The evolutionarily conserved TSC/Rheb pathway activates Notch in tuberous sclerosis complex and Drosophila external sensory organ development[J]. The Journal of Clinical Investigation, 2010, 120(1): 93-102. DOI:10.1172/JCI40221 |
| [60] |
LIANG S J, LI X G, WANG X Q. Notch signaling in mammalian intestinal stem cells:determining cell fate and maintaining homeostasis[J]. Current Stem Cell Research & Therapy, 2019, 14(7): 583-590. |
| [61] |
MA J H, MENG Y, KWIATKOWSKI D J, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade[J]. The Journal of Clinical Investigation, 2010, 120(1): 103-114. DOI:10.1172/JCI37964 |
| [62] |
GU X, OROZCO J M, SAXTON R A, et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway[J]. Science, 2017, 358(6364): 813-818. DOI:10.1126/science.aao3265 |
| [63] |
INOKI K, OUYANG H J, ZHU T Q, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth[J]. Cell, 2006, 126(5): 955-968. DOI:10.1016/j.cell.2006.06.055 |




