微量元素锌(zinc,Zn)被称为“生命元素”,含量在人体内仅次于“铁”。Zn参与哺乳动物基因组中10%蛋白质的合成,它的缺乏会导致机体生长发育受阻、皮肤角质化以及软骨细胞增生等[1-2];相反,Zn的过量摄入会造成“Zn中毒”,抑制机体免疫功能[3-4]。Zn存在有机Zn和无机Zn 2种形式[5],其中无机Zn的摄入是通过在肠腔中转换成Zn2+,并与胰腺分泌的Zn配体形成络合物进入小肠上皮细胞,再通过基底膜进入门静脉与血清白蛋白形成血清白蛋白Zn复合体,随血液循环到达全身各个器官组织;而有机Zn多以氨基酸螯合物的形态存在,其吸收存在2种可能:其一,氨基酸螯合物解离后进入肠道参与物质代谢,通过肠系膜入血,进入器官组织;其二,Zn直接以氨基酸螯合物的形式,借助氨基酸转运载体穿过肠细胞膜进入血液循环[6]。
1 Zn的转运载体及其转运机制机体内Zn平衡主要通过小肠摄取、肾脏重吸收和粪便排出维持。肠细胞主要通过Zn转运载体摄入Zn,且摄入量与吸收效率成反比[7-8]。哺乳动物的Zn转运载体分为2种:Zn调控转运蛋白家族(zinc-regulated transporter-like proteins,ZIP)和阳离子扩散辅助蛋白家族(cation diffusion facilitator family,CDF),二者均具有跨膜结构域[9-10]。ZIP也称SLC39A,编码14种蛋白,即ZIP1~ZIP14,促进Zn进入胞浆;CDF又称SLC30A,编码10种转运蛋白,即ZnT1~ZnT10,促进Zn从胞浆流向胞外或核内(图 1)[11-13]。
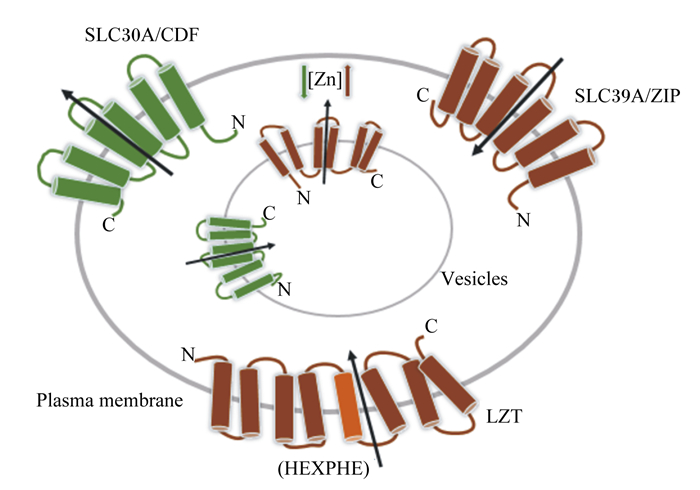
|
Zn:锌zinc;SLC30A/CDF:阳离子扩散辅助蛋白家族cation diffusion facilitator family;SLC39A/ZIP:Zn调控转运蛋白家族znic-regulated transporter-like proteins;LZT:锌转运蛋白的LIV-1亚家族LIV-1 subfamily of zinc transporters;HEXPHE:LZT中脯氨酸和谷氨酸残基proline and glutamate residues in LZT;plasma membrane:浆膜;vesicles:囊泡。 图 1 Zn的跨膜转运 Fig. 1 Transmembrane transport of Zn[13] |
研究表明,ZIP3位于细胞顶膜上,通过介导胞浆Zn的跨膜转运过程,构造囊泡“Zn池”[14-15]。当Zn浓度降低时,肠细胞转运载体蛋白ZIP4活性上升,并被募集到细胞的表面,促进Zn的吸收;ZIP4基因敲除会导致肠道结构完整性被破坏、隐窝凋亡和肠道干细胞微环境稳态紊乱,而外源补充Zn可逆转这一现象[16]。ZIP5定位在肠上皮细胞、胰腺腺泡细胞和肾细胞的基底外侧膜上,通过促进细胞吸收膳食Zn和“囊泡池”释放Zn 2种方式来维持细胞Zn水平[17]。ZIP6又称LIV-1(SLC39A6),其主要功能是将Zn2+转运到细胞质基质中[18]。ZIP7定位于内质网和高尔基体,作为细胞内Zn进出的“看门人”,具有与ZIP5相似的作用,此外还参与预防Zn在高尔基体中的过度积累造成的“Zn中毒”[19]。而ZnT1位于细胞的基底外侧膜上,通过调节自身活性降低过高的Zn2+浓度[20-21]。ZnT5~ZnT7可将胞浆内Zn转运至高尔基体内,参与蛋白质的加工、分拣和运输。除上述2类转运载体外,二价金属离子转运蛋白1(divalent metal transporter 1,DMT1)、金属硫蛋白1(metallothionein,MT1)和锌转运蛋白LIV-1亚家族(LIV-1 subfamily of zinc transporters,LZT)等也参与了Zn的吸收。DMT1主要参与二价金属离子的吸收过程,通过影响锌转运蛋白的功能和表达,在Zn吸收过程中发挥着重要作用[22];MT1可通过增加ZnT1诱导内源性Zn的组织分布,决定了各组织Zn含量的不同[23];LZT则有一个独特的基序(HEXPHEXGD),具有将Zn跨膜运输的功能[24]。总之,Zn浓度变化需要Zn转运载体ZIP、CDF、MT1和DMT1等协同调节以维持Zn在体内的稳态[25-26]。
2 Zn促进肠道发育 2.1 Zn制剂目前,动物生产中运用到的Zn饲料添加剂主要有2种类型:无机Zn和Zn氨基酸螯合物。因为蛋氨酸锌(Zn-Met)、赖氨酸锌(Zn-Lys)、天冬氨酸锌(Zn-Asp)和甘氨酸锌(Zn-Gly)等Zn氨基酸螯合物在动物体内具有比无机Zn更好的生物利用度,所以逐渐被人们认识和接受[27-30]。Huang等[31]证实,相较于硫酸锌(ZnSO4),添加Zn-Met和Zn-Gly处理肠上皮细胞更能提高细胞活力,改善细胞状态;并且Zn转运体MT1和ZnT1 mRNA丰度显著增高,这可能是促进有机Zn有效吸收的机制之一。
2.2 Zn促进肠道发育肠上皮每3~5 d会更新1次,其动力是位于隐窝底部的肠道干细胞(intestinal stem cell,ISC)的扩增(包括增殖、迁移和分化)。隐窝中存在的多种生长因子、细胞因子和细胞外基质分子构成了“干细胞微环境”,其对ISC增殖和分化过程提供必要的支持,以维持肠道上皮稳态[32]。Amcheslavsky等[33]报道,锌指蛋白(zinc finger protein,ZFP)能促进果蝇肠道干细胞分化,其被敲除后干细胞分化功能丧失,干细胞微环境平衡被破坏。Ohashi等[34]研究表示,肠道隐窝中高度表达的ZIP7被敲除后,潘氏细胞功能严重衰弱,肠道干细胞发生凋亡。补充Zn能够增强肠道干细胞增殖能力,增加肠吸收细胞、杯状细胞和肠内分泌细胞数量,改善肠上皮形态结构[35-36]。上述研究提示Zn是肠道干细胞及分化细胞维持正常功能的关键因子。
小肠黏膜刷状缘膜(brush-border membrane,BBM)可增加小肠表面积和肠内膜阻力,促进营养物质的消化吸收。研究表明,Zn-Met能通过增加转运蛋白质和BBM酶的活性,改善肠道形态结构,促进鸡肠绒毛的发育[27]。同时,Zn-Met或Zn-Gly还可显著增加肉仔鸡小肠金属硫蛋白mRNA丰度,促进自身被肠细胞吸收[37]。此外,Zn制剂能调节肠道屏障功能。Zhang等[38]发现,饲粮添加氧化锌(ZnO)可以上调断奶仔猪的闭合蛋白(occludin)和紧密连接蛋白-1(zonula occludens protein-1,ZO-1)的表达,降低肠道通透性,提高肠道机械屏障功能;这与Shao等[39]在Caco-2细胞上开展的Zn对肠上皮细胞屏障影响的试验结果一致。ZnSO4则被报道可通过促进乳酸菌在肠道定植并分泌酸性物质抑制有害菌生长,从而增加肠道微生物屏障功能[40]。
肠道黏膜抗氧化及免疫功能是维持肠上皮稳态的2项重要指标。早在2000年,Cario等[41]就发现,补充Zn能提高肠黏膜的抗氧化能力,加快肠上皮细胞更新代谢,改善缺Zn诱导的肠炎。同时,血清中的抗氧化指标,包括超氧化物歧化酶(superoxide dismutase,SOD)和谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)的活性在添加Zn-Gly后显著增加[30]。此外,Han等[42]研究表明,壳聚糖Zn可显著提高仔猪黏膜免疫力,减少肠上皮凋亡细胞数,促进肠道黏膜发育。
3 Zn驱动肠上皮损伤后修复肠上皮易受到毒素和病原体等外源有毒有害物的攻击,肠道损伤后的快速修复是维持肠道结构和功能完整性的关键。研究表明,Zn口服液或饲粮中添加Zn可降低肠黏膜的通透性,改善腹泻引起的肠道损伤[43-45]。Tran等[46]研究发现,乳清来源的生长因子提取物(whey growth factor extract,WGFE)可改善甲氨蝶呤(methotrexate,MTX)引起的肠道损伤,而Zn和WGFE结合使用对MTX引起的肠黏膜炎的治疗效果更好。Zhou等[29]在小鼠和小鼠类肠团上的研究表明,L-天冬氨酸锌能提高肠道干细胞的增殖和分化活性,加速呕吐毒素暴露下肠上皮再生。此外,Cario等[41]通过细胞划痕试验发现,培养基中添加ZnSO4可以提高肠上皮细胞的迁移能力,并促进伤口愈合。
在畜牧生产实践中,仔猪的高腹泻率是影响我国养猪生产效率的关键因素。其中细菌性腹泻的机理很大程度上是因为包括肠毒素在内的一系列细菌分泌的毒素或5-羟色胺(5-hydroxytryptamine,5-HT)激活了肠上皮细胞膜上相关受体,诱导氯离子(Cl-)过量分泌,进一步引起水的分泌,导致仔猪分泌性腹泻。而缺Zn使机体对有害菌更敏感[47],因此补充Zn对预防和治疗仔猪腹泻具有重要意义[12, 48]。研究表明,饲粮中添加高水平Zn(2 500 mg/kg)会减少断奶仔猪肠道样品中5-HT的含量,降低腹泻发生率[49-50]。而Ou等[51]则证明,ZnO通过下调肠道肥大细胞中干细胞因子(stem cell factor,SCF)基因mRNA和蛋白质表达水平,抑制其释放组胺,从而降低仔猪腹泻。此外,有研究报道,Zn以抵御肠道病原菌的侵害和激活免疫系统等形式预防和治疗急性和慢性腹泻疾病[52]。尽管目前相关研究已揭示了Zn抑制腹泻的部分机理,但是其中还存在一些疑点等待研究人员的进一步解答,如Zn如何影响肠道微生物代谢,以及Zn通过什么途径影响肠道免疫系统。对这些问题的回答,有助于深入认识Zn缓解仔猪腹泻的机制,为Zn的精准调控指明方向。
4 Zn对肠道干细胞调控机制研究ISC的增殖分化受到Wnt/β-连环蛋白(Wnt/β-catenin)和哺乳动物雷帕霉素靶蛋白复合物1(mammalian target of rapamycin complex 1,mTORC1)信号通路等信号通路的调控[53-54]。Wnt/β-catenin通路是维护肠道干细胞增殖分化所必需的[55-57],mTORC1信号通路通过调节肠道蛋白质代谢,增强肠道干细胞活性[58-60]。它们参与了Zn促进肠道干细胞自更新和再生过程(图 2)。
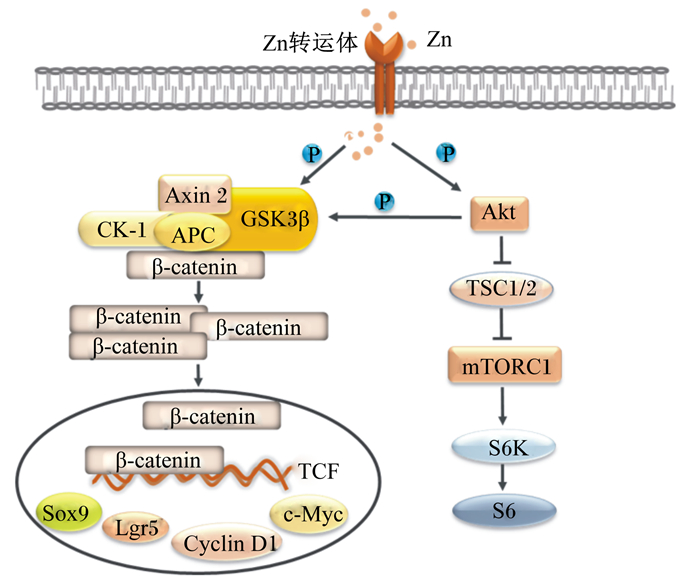
|
Zn:锌zinc;GSK3β:糖原合酶激酶3β glycogen synthase kinase 3β;APC:结肠腺瘤性息肉病蛋白adenomatous polyposis coli protein;CK-1:肌酸激酶-1 creatine kinase-1;Axin 2:轴抑制蛋白2 axis inhibition protein 2;S6K:核糖体蛋白S6激酶ribosomal protein S6 kinase;S6:S6核糖体蛋白S6 ribosomal protein;TCF:T细胞特异性转录因子T cell-specific transcription factor;Cyclin D1:细胞周期蛋白D1 cell-cycle protein cyclin D1;Lgr5:亮氨酸重复单位的G蛋白偶联受体5 leucinerich-repeat-containing G-protein-coupled receptor 5;TSC1/2:结节性硬化1/2 tuberous sclerosis 1/2;mTORC1:哺乳动物雷帕霉素靶蛋白复合体1 mammalian target of rapamycin complex 1;Sox9:性别决定区Y框蛋白质9 sex determining region Y-box 9;Akt:蛋白激酶B protein kinase B;β-catenin:β-连环蛋白。 图 2 Zn介导Wnt/β-catenin和mTORC1信号转导机制 Fig. 2 Regulatory mechanisms of Wnt/β-catenin and mTORC1 signaling pathways by Zn[55, 64] |
肠道中Wnt信号梯度沿隐窝-绒毛轴逐渐减弱[61-62]。当存在Wnt配体时,它会与细胞膜上的受体结合,引起β-catenin去磷酸化,使得β-catenin在细胞质中富集并转移至细胞核内,与T细胞特异性转录因子(T cell-specific transcription factor,TCF)结合,调控c-Myc基因、细胞周期蛋白D1(cell-cycle protein cyclin D1,Cyclin D1)和亮氨酸重复单位的G蛋白偶联受体5(leucinerich-repeat-containing G-protein-coupled receptor 5,Lgr5)等关键靶基因转录,从而促进细胞增殖[63]。研究表明,Zn能够提高糖原合酶激酶3β(glycogen synthase kinase 3β,GSK3β)磷酸化水平,诱导β-catenin上游的负调控因子GSK3β、肌酸激酶-1(creatine kinase-1,CK-1)、轴抑制蛋白2(axis inhibition protein 2,Axin 2)和结肠腺瘤性息肉病蛋白(adenomatous polyposis coli protein,APC)等组成的降解复合物失活,从而激活Wnt/β-catenin信号通路[55]。Zhou等[29]发现,口服Zn-Asp可激活小鼠肠道中Wnt/β-catenin信号通路,上调肠道干细胞标志Lgr5的表达,促进小鼠干细胞体外扩增为类肠团。有趣的是,在干细胞培养基中缺乏Wnt配体的情况下,补充ZFP转录因子——多形性腺瘤基因样蛋白2(pleiomorphic adenoma gene-like protein 2,PLAGL2)同样能激活Wnt/β-catenin信号通路,提高类肠团的出芽率[61]。
4.2 Zn参与mTORC1信号通路调节肠道发育蛋白质代谢主要受到mTORC1信号通路的调节[60]。mTORC1是磷脂酰肌醇3-激酶相关激酶家族的成员,其通过磷酸化翻译起始因子eIF4和核糖体蛋白S6激酶1调控mRNA的翻译,进而调节细胞增殖。p70S6激酶是mTORC1信号通路下游重要的靶标之一,Lynch等[65]发现,Zn可通过激活mTORC1信号通路,上调p70S6激酶的表达,促进细胞蛋白质的合成。Nimmanon等[64]进一步研究证实,Zn通过提高蛋白激酶B(protein kinase B,PKB/Akt)磷酸化水平促进mTORC1信号通路的激活,同时抑制了GSK3β的表达。此外,Geiser等[16]敲除小鼠肠道中的ZIP4基因后,mTORC1活性也随之降低,导致蛋白质代谢紊乱,潘氏细胞分泌溶菌酶和α-防御素的功能发生障碍,肠道干细胞增殖分化异常,肠上皮功能性细胞数量显著降低。
5 小结综上所述,Zn不仅作为微量元素维持机体正常需要,也是肠上皮发育和再生的控制因子;其可通过增强肠道干细胞增殖分化能力,提高肠道屏障和抗氧化功能,进而促进肠道损伤后修复,这些过程依赖于Wnt/β-catenin和mTORC1等信号通路的调控作用。从目前的研究来看,GSK3β可能是联系Zn与Wnt/β-catenin和mTORC1之间桥梁,然而,Zn与它们之间是否还存在其他直接或间接的作用仍不清楚。未来在分子生物学的基础上,结合分子动力学,可能有助于揭示胞外Zn和胞内Zn调节肠道干细胞活性的机制,为畜牧生产中Zn制剂调控动物肠道发育的生产应用以及其在药理学方面对肠炎和腹泻等疾病的治疗提供理论依据。
| [1] |
MCCALL K A, HUANG C C, FIERKE C A. Function and mechanism of zinc metalloenzymes[J]. The Journal of Nutrition, 2000, 130(5): 1437S-1446S. DOI:10.1093/jn/130.5.1437S |
| [2] |
陈良妹, 董晓明, 杨帆, 等. 锌缺乏症对慢性肾脏病发展的影响及其作用机制的研究进展[J]. 吉林大学学报(医学版), 2018, 44(1): 195-199. |
| [3] |
TANEJA S K, JAIN M, MANDAL R, et al. Excessive zinc in diet induces leptin resistance in Wistar rat through increased uptake of nutrients at intestinal level[J]. Journal of Trace Elements in Medicine and Biology, 2012, 26(4): 267-272. DOI:10.1016/j.jtemb.2012.03.002 |
| [4] |
罗治彬, 吴嘉惠, 徐采朴. 过量锌对大鼠小肠粘膜SIgA免疫的影响[J]. 细胞与分子免疫学杂志, 1998(4): 295-296. |
| [5] |
于昱, 罗绪刚, 吕林, 等. 动物小肠锌吸收特点及其机制的研究进展[J]. 肠外与肠内营养, 2006, 13(3): 179-183, 187. |
| [6] |
高建伟, 王林枫, 杨改青, 等. 锌的消化吸收机制研究进展[J]. 安徽农业科学, 2010, 38(1): 33-34, 67. |
| [7] |
KREBS N F, HAMBIDGE K M, WESTCOTT J E, et al. Exchangeable zinc pool size in infants is related to key variables of zinc homeostasis[J]. The Journal of Nutrition, 2003, 133(5): 1498S-1501S. DOI:10.1093/jn/133.5.1498S |
| [8] |
JOU M Y, PHILIPPS A F, KELLEHER S L, et al. Effects of zinc exposure on zinc transporter expression in human intestinal cells of varying maturity[J]. Journal of Pediatric Gastroenterology and Nutrition, 2010, 50(6): 587-595. DOI:10.1097/MPG.0b013e3181d98e85 |
| [9] |
GAITHER L A, EIDE D J. Functional expression of the human hZIP2 zinc transporter[J]. Journal of Biological Chemistry, 2000, 275(8): 5560-5564. DOI:10.1074/jbc.275.8.5560 |
| [10] |
HUANG L P, GITSCHIER J. A novel gene involved in zinc transport is deficient in the lethal milk mouse[J]. Nature Genetics, 1997, 17(3): 292-297. DOI:10.1038/ng1197-292 |
| [11] |
KAMBE T, YAMAGUCHI-IWAI Y, SASAKI R, et al. Overview of mammalian zinc transporters[J]. Cellular and Molecular Life Sciences, 2004, 61(1): 49-68. DOI:10.1007/s00018-003-3148-y |
| [12] |
COUSINS R J, LIUZZI J P, LICHTEN L A. Mammalian zinc transport, trafficking, and signals[J]. Journal of Biological Chemistry, 2006, 281(34): 24085-24089. DOI:10.1074/jbc.R600011200 |
| [13] |
于昱, 王福俤. 锌转运蛋白家族SLC39A/ZIP和SLC30A/ZnT的研究进展[J]. 中国细胞生物学学报, 2010, 32(2): 176-188. |
| [14] |
KELLEHER S L, LOPEZ V, LÖNNERDAL B, et al. Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland[J]. American Journal of Physiology:Regulatory Integrative and Comparative Physiology, 2009, 297(1): R194-R201. DOI:10.1152/ajpregu.00162.2009 |
| [15] |
LICHTEN L A, COUSINS R J. Mammalian zinc transporters:nutritional and physiologic regulation[J]. Annual Review of Nutrition, 2009, 29: 153-176. DOI:10.1146/annurev-nutr-033009-083312 |
| [16] |
GEISER J, VENKEN K J T, DE LISLE R C, et al. A mouse model of acrodermatitis enteropathica:loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity[J]. PLoS Genetics, 2012, 8(6): e1002766. DOI:10.1371/journal.pgen.1002766 |
| [17] |
DUFNER-BEATTIE J, KUO Y M, GITSCHIER J, et al. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5[J]. Journal of Biological Chemistry, 2004, 279(47): 49082-49090. DOI:10.1074/jbc.M409962200 |
| [18] |
乔珏, 夏海滨. 锌离子转运蛋白LIV-1的研究进展[J]. 现代肿瘤医学, 2014, 22(6): 1454-1458. |
| [19] |
OLLIG J, KLOUBERT V, TAYLOR K M, et al. B cell activation and proliferation increase intracellular zinc levels[J]. The Journal of Nutritional Biochemistry, 2019, 64: 72-79. DOI:10.1016/j.jnutbio.2018.10.008 |
| [20] |
PALMITER R D. Protection against zinc toxicity by metallothionein and zinc transporter[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(14): 4918-4923. DOI:10.1073/pnas.0401022101 |
| [21] |
YU Y Y, KIRSCHKE C P, HUANG L P. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract[J]. Journal of Histochemistry & Cytochemistry, 2007, 55(3): 223-234. |
| [22] |
YAMAJI S, TENNANT J, TANDY S, et al. Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells[J]. FEBS Letters, 2001, 507(2): 137-141. DOI:10.1016/S0014-5793(01)02953-2 |
| [23] |
YASUNO T, OKAMOTO H, NAGAI M, et al. In vitro study on the transport of zinc across intestinal epithelial cells using Caco-2 monolayers and isolated rat intestinal membranes[J]. Biological and Pharmaceutical Bulletin, 2012, 35(4): 588-593. DOI:10.1248/bpb.35.588 |
| [24] |
TAYLOR K M, NICHOLSON R I. The LZT proteins; the LIV-1 subfamily of zinc transporters[J]. Biochimica et Biophysica Acta:Biomembranes, 2003, 1611(1/2): 16-30. |
| [25] |
SHEN H, QIN H H, GUO J S. Cooperation of metallothionein and zinc transporters for regulating zinc homeostasis in human intestinal Caco-2 cells[J]. Nutrition Research, 2008, 28(6): 406-413. DOI:10.1016/j.nutres.2008.02.011 |
| [26] |
HOLLAND T C, KILLILEA D W, SHENVI S V, et al. Acute changes in cellular zinc alters zinc uptake rates prior to zinc transporter gene expression in Jurkat cells[J]. BioMetals, 2015, 28(6): 987-996. DOI:10.1007/s10534-015-9883-3 |
| [27] |
TAKO E, FERKET P R, UNI Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration[J]. The Journal of Nutritional Biochemistry, 2005, 16(6): 339-346. DOI:10.1016/j.jnutbio.2005.01.002 |
| [28] |
LIU H W, LIU D S, ZHENG L X. Study on Zn relative concentration and chemical state in broilers duodenum by micro-X-ray fluorescence and micro-X-ray absorption fine structure[J]. Livestock Science, 2014, 161: 101-108. DOI:10.1016/j.livsci.2013.12.023 |
| [29] |
ZHOU J Y, LIN H L, WANG Z, et al. Zinc L-Aspartate enhances intestinal stem cell activity to protect the integrity of the intestinal mucosa against deoxynivalenol through activation of the Wnt/β-catenin signaling pathway[J]. Environmental Pollution, 2020, 262: 114290. DOI:10.1016/j.envpol.2020.114290 |
| [30] |
YUE M, FANG S L, ZHUO Z, et al. Zinc glycine chelate absorption characteristics in sprague dawley rat[J]. Journal of Animal Physiology and Animal Nutrition, 2015, 99(3): 457-464. DOI:10.1111/jpn.12255 |
| [31] |
HUANG D P, ZHUO Z, FANG S L, et al. Different zinc sources have diverse impacts on gene expression of zinc absorption related transporters in intestinal porcine epithelial cells[J]. Biological Trace Element Research, 2016, 173(2): 325-332. DOI:10.1007/s12011-016-0655-x |
| [32] |
YEUNG T M, CHIA L A, KOSINSKI C M, et al. Regulation of self-renewal and differentiation by the intestinal stem cell niche[J]. Cellular and Molecular Life Sciences, 2011, 68(15): 2513-2523. DOI:10.1007/s00018-011-0687-5 |
| [33] |
AMCHESLAVSKY A, NIE Y C, LI Q, et al. Gene expression profiling identifies the zinc-finger protein Charlatan as a regulator of intestinal stem cells in Drosophila[J]. Development, 2014, 141(13): 2621-2632. DOI:10.1242/dev.106237 |
| [34] |
OHASHI W, KIMURA S, IWANAGA T, et al. Zinc transporter SLC39A7/ZIP7 promotes intestinal epithelial self-renewal by resolving ER stress[J]. PLoS Genetics, 2016, 12(10): e1006349. DOI:10.1371/journal.pgen.1006349 |
| [35] |
BLANCHARD R K, COUSINS R J. Differential display of intestinal mRNAs regulated by dietary zinc[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(14): 6863-6868. DOI:10.1073/pnas.93.14.6863 |
| [36] |
DUFF M, ETTARH R R. Crypt cell production rate in the small intestine of the zinc-supplemented mouse[J]. Cells Tissues Organs, 2002, 172(1): 21-28. |
| [37] |
郑立鑫, 刘华伟, 吴鹏华, 等. 不同锌源对肉仔鸡肠道形态及金属硫蛋白表达的影响[J]. 中国饲料, 2013(20): 7-10. |
| [38] |
ZHANG B K, GUO Y M. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets[J]. British Journal of Nutrition, 2009, 102(5): 687-693. DOI:10.1017/S0007114509289033 |
| [39] |
SHAO Y X, WOLF P G, GUO S S, et al. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells[J]. The Journal of Nutritional Biochemistry, 2017, 43: 18-26. DOI:10.1016/j.jnutbio.2017.01.013 |
| [40] |
金美林, 岳小婧, 莫才红, 等. 日粮添加黄芪和锌对肉鸡生产性能、肠道微生物及抗氧化能力的影响[J]. 中兽医医药杂志, 2017, 36(4): 55-58. |
| [41] |
CARIO E, JUNG S, D'HEUREUSE J H, et al. Effects of exogenous zinc supplementation on intestinal epithelial repair in vitro[J]. European Journal of Clinical Investigation, 2000, 30(5): 419-428. DOI:10.1046/j.1365-2362.2000.00618.x |
| [42] |
HAN X Y, MA Y F, LV M Y, et al. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs[J]. British Journal of Nutrition, 2014, 111(8): 1405-1411. DOI:10.1017/S0007114513004042 |
| [43] |
HOLODOVA M, COBANOVA K, SEFCIKOVA Z, et al. Dietary zinc and fibre source can influence the mineral and antioxidant status of piglets[J]. Animals, 2019, 9(8): 497. DOI:10.3390/ani9080497 |
| [44] |
TRAN C D, HAWKES J, GRAHAM R D, et al. Zinc-fortified oral rehydration solution improved intestinal permeability and small intestinal mucosal recovery[J]. Clinical Pediatrics, 2015, 54(7): 676-682. DOI:10.1177/0009922814562665 |
| [45] |
MAHMOOD A, FITZGERALD A J, MARCHBANK T, et al. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes[J]. Gut, 2007, 56(2): 168-175. DOI:10.1136/gut.2006.099929 |
| [46] |
TRAN C D, HOWARTH G S, COYLE P, et al. Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine[J]. The American Journal of Clinical Nutrition, 2003, 77(5): 1296-1303. DOI:10.1093/ajcn/77.5.1296 |
| [47] |
ZHANG B K, GUO Y M. Beneficial effects of tetrabasic zinc chloride for weanling piglets and the bioavailability of zinc in tetrabasic form relative to ZnO[J]. Animal Feed Science and Technology, 2007, 135(1/2): 75-85. |
| [48] |
POULSEN H D. Zinc oxide for weanling piglets[J]. Acta Agriculturae Scandinavica, SectionA:Animal Sciences, 1995, 45(3): 159-167. DOI:10.1080/09064709509415847 |
| [49] |
CARLSON D, POULSEN H D, SEHESTED J. Influence of weaning and effect of post weaning dietary zinc and copper on electrophysiological response to glucose, theophylline and 5-HT in piglet small intestinal mucosa[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2004, 137(4): 757-765. |
| [50] |
CARLSON D, SEHESTED J, FENG Z, et al. Serosal zinc attenuate serotonin and vasoactive intestinal peptide induced secretion in piglet small intestinal epithelium in vitro[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2008, 149(1): 51-58. |
| [51] |
OU D Y, LI D F, CAO Y H, et al. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs[J]. The Journal of Nutritional Biochemistry, 2007, 18(12): 820-826. DOI:10.1016/j.jnutbio.2006.12.022 |
| [52] |
张崇远. 葡萄糖酸锌治疗儿童腹泻作用机制研究进展[J]. 中国处方药, 2017, 15(10): 18-19. |
| [53] |
ZHU M, WANG X Q. Regulation of mTORC1 by small GTPases in response to nutrients[J]. The Journal of Nutrition, 2020, 150(5): 1004-1011. DOI:10.1093/jn/nxz301 |
| [54] |
朱秋杰, 周加义, 梁少杰, 等. Wnt/β-连环蛋白信号驱动小肠上皮更新和再生机制的研究进展[J]. 动物营养学报, 2019, 31(11): 4995-5002. |
| [55] |
ZHOU J Y, WANG Z, ZHANG S W, et al. Methionine and its hydroxyl analogues improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating Wnt/β-catenin signaling[J]. Journal of Agricultural and Food Chemistry, 2019, 67(41): 11464-11473. DOI:10.1021/acs.jafc.9b04442 |
| [56] |
LI X G, ZHU M, CHEN M X, et al. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway[J]. Toxicology Letters, 2019, 305: 19-31. DOI:10.1016/j.toxlet.2019.01.008 |
| [57] |
ZHOU J Y, ZHANG S W, LIN H L, et al. Hydrolyzed wheat gluten alleviates deoxynivalenol-induced intestinal injury by promoting intestinal stem cell proliferation and differentiation via upregulation of Wnt/β-catenin signaling in mice[J]. Food and Chemical Toxicology, 2019, 131: 110579. DOI:10.1016/j.fct.2019.110579 |
| [58] |
ZHU M, QIN Y C, GAO C Q, et al. L-glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway[J]. Food & Function, 2020, 11(3): 2714-2724. |
| [59] |
ZHU M, QIN Y C, GAO C Q, et al. Extracellular glutamate-induced mTORC1 activation via the IR/IRS/PI3K/Akt pathway enhances the expansion of porcine intestinal stem cells[J]. Journal of Agricultural and Food Chemistry, 2019, 67(34): 9510-9521. DOI:10.1021/acs.jafc.9b03626 |
| [60] |
ZHOU J Y, HUANG D G, QIN Y C, et al. mTORC1 signaling activation increases intestinal stem cell activity and promotes epithelial cell proliferation[J]. Journal of Cellular Physiology, 2019, 234(10): 19028-19038. DOI:10.1002/jcp.28542 |
| [61] |
STRUBBERG A M, PANIAGUA D A V, ZHAO T T, et al. The zinc finger transcription factor PLAGL2 enhances stem cell fate and activates expression of ASCL2 in intestinal epithelial cells[J]. Stem Cell Reports, 2018, 11(2): 410-424. |
| [62] |
LI X G, WANG Z, CHEN R Q, et al. Lgr5 and Bmi1 increase pig intestinal epithelial cell proliferation by stimulating Wnt/β-catenin signaling[J]. International Journal of Molecular Sciences, 2018, 19(4): 1036-1048. DOI:10.3390/ijms19041036 |
| [63] |
MACDONALD B T, TAMAI K, HE X. Wnt/β-catenin signaling:components, mechanisms, and diseases[J]. Developmental Cell, 2009, 17(1): 9-26. DOI:10.1016/j.devcel.2009.06.016 |
| [64] |
NIMMANON T, ZILIOTTO S, MORRIS S, et al. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling[J]. Metallomics, 2017, 9(5): 471-481. DOI:10.1039/C6MT00286B |
| [65] |
LYNCH C J, PATSON B J, GOODMAN S A, et al. Zinc stimulates the activity of the insulin- and nutrient-regulated protein kinase mTOR[J]. American Journal of Physiology:Endocrinology and Metabolism, 2001, 281(1): E25-E34. DOI:10.1152/ajpendo.2001.281.1.E25 |




