2. 华南农业大学海洋学院, 广州 510000
2. College of Marine Sciences, South China Agricultural University, Guangzhou 510000, China
高不饱和脂肪酸(highly unsaturated fatty acids,HUFA)指碳原子数≥20且双键数量≥3的直链脂肪酸,其中二十碳五烯酸(eicosapentaenoic acid,EPA)和二十二碳六烯酸(docosahexaenoic acid,DHA)等n-3 HUFA在促进人类和动物的大脑与视神经系统发育,以及防治炎症反应、肿瘤、心血管和代谢性疾病等方面具有重要作用[1-8]。尽管人类可利用亚麻酸(18 : 3n-3)作为底物经脂肪酸去饱和酶和延长酶的系列催化,生物合成n-3 HUFA,但该途径合成内源性n-3 HUFA的效率低,难以满足人类特殊生理情况下的需求[9]。根据世界卫生组织和联合国粮食及农业组织推荐,人对n-3 HUFA的最低需要量为250 mg/d[10],因此,人类每年对n-3 HUFA的需求量高达127万t(250 mg/d×365 d×70亿人)。作为粮食供应的重要组成部分,鱼类不仅是一种优质“蛋白质源”,更是人类获取n-3 HUFA的主要食物来源。人类每年消耗20万t n-3 HUFA,其中有98.5%来源于鱼类资源[11]。由于过度捕捞、环境恶化等原因,天然渔业资源呈现衰退的趋势。2010—2018年,我国捕捞鱼年均产量1 009.79万t,同上个10年比下降1.26%;而养殖鱼年均产量2 593.60万t,同比增加50.18%[12]。自2013年来,全球水产养殖量对人类水产品消费的贡献已超过捕捞量,且养殖产量逐年递增。因此,未来消费者获取n-3 HUFA的食物源主要依赖于产量日益增长的养殖鱼类[9]。
随着水产养殖业的迅猛发展,鱼粉和鱼油(富含n-3 HUFA)等优质饲料源供不应求,价格飙升。为了降低对渔源饲料的依赖,大量的植物源饲料(缺乏n-3 HUFA)在各种养殖鱼类中被广泛应用。研究结果表明,大量添加的植物源饲料对鱼类生长性能的影响较小,但显著降低了鱼类n-3 HUFA含量,使鱼肉的营养价值大打折扣[13-33](表 1)。为了降低植物原料大量替代鱼粉和鱼油对鱼肉营养品质的负面影响,目前,水产科技工作者从营养策略、功能性饲料添加剂、新品种选育和基因工程等方面对提升养殖鱼类n-3 HUFA含量做了大量工作。本文将对上述研究进展予以简要综述,以期为提高养殖鱼类营养价值和促进水产养殖业的健康发展提供参考。
|
|
表 1 植物油完全或部分替代鱼油对鱼类肌肉EPA和DHA含量的影响(占肌肉总脂肪酸的比例) Table 1 Effects of complete or partial replacement of fish oil with vegetable oils on fish muscle EPA and DHA contents (percentage of total fatty acids) |
鱼体内n-3 HUFA的来源可分为内源性合成和外源性摄入。大部分鱼类缺乏Δ12和Δ15脂肪酸去饱和酶(fatty acid desaturase,Fad),不能从头合成多不饱和脂肪酸(ploy-unsaturated fatty acids,PUFA),因此,需要以亚油酸(linoleic acid,LA,C18 : 2n-6)或亚麻酸(α-linolenic acid,ALA,C18 : 3n-3)为必需脂肪酸(essential fatty acids,EFA)。然而,一些具有HUFA合成能力的鱼类(如淡水鱼、鲑鳟类和植食性的海水鱼)可利用一系列Fad和长链脂肪酸延长酶(elongase of very long-chain fatty acids,Elovl)以ALA底物转化合成EPA和DHA等n-3 HUFA(图 1)。
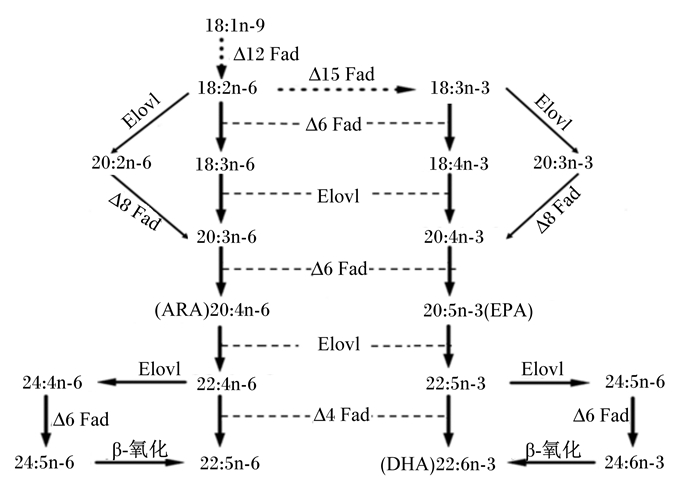
|
Fad:脂肪酸去饱和酶fatty acid desaturase;Elovl:长链脂肪酸延长酶elongase of very long-chain fatty acids;ARA:花生四烯酸arachidonic acid;EPA:二十碳五烯酸eicosapentaenoic acid; DHA:二十二碳六烯酸docosahexaenoic acid。 去饱和反应由Fad催化,用“Δ”表示起始双键位于脂肪酰基链羧基末端(Δ)内的碳位置。延伸反应,用“Elovl”表示,由长链脂肪酸延长酶催化。虚线箭头表示硬骨鱼该路径缺少,实线箭头表示该路径存。Desaturation reactions were catalyzed by Fad and were denoted with “Δ” to indicate the carbon position at which the incipient double bond locates within the carboxylic group end (Δ) of the fatty acyl chain. Elongation reactions, denoted with “Elovl”, were catalyzed by elongase of very long-chain fatty acids (Elovl). The dotted arrow indicated that the pathway lacked in teleost, and the solid arrow indicated that the pathway existed. 图 1 硬骨鱼体内HUFA的生物合成途径 Fig. 1 Biosynthetic pathways of HUFA in teleost[19, 34-36] |
从食物摄入的外源性n-3 HUFA经小肠上皮细胞吸收,再酯化,与载脂蛋白形成乳糜微粒(chylomicrons,CM),CM经淋巴系统进入血液。当CM流经各组织时,在脂蛋白脂肪酶(lipoprotein lipase,LPL)水解作用下,n-3 HUFA被释放,然后被组织细胞吸收利用。同时,被肝脏组织所吸收的n-3 HUFA,可在肝细胞中重新组装成极低密度脂蛋白(very low density lipoprotein,VLDL)释放到血液,运至肌肉、心脏、脂肪等等肝外组织。
然而,目前有关鱼类肌肉组织中n-3 HUFA积累的机制尚不明确,但哺乳动物肌肉组织中n-3 HUFA的吸收机制已逐渐清晰(图 2):n-3 HUFA在脂肪酸移位酶(fatty acid translocase,FAT/CD36)、膜脂肪酸结合蛋白(plasma membrane fatty acid binding protein,FABPpm)和脂肪酸转运蛋白(fatty acid transport proteins,FATPs)的作用下进入细胞,然后在长链脂酰辅酶A合成酶(long-chain acyl-CoA synthetases,ACSLs)的催化下激活形成脂酰辅酶A,最后再经二酰基甘油酰基转移酶(diacylglycerol acyltransferase,DGAT)的作用,生成甘油三酯沉积于脂滴,或生成磷脂整合于膜上[37-39]。
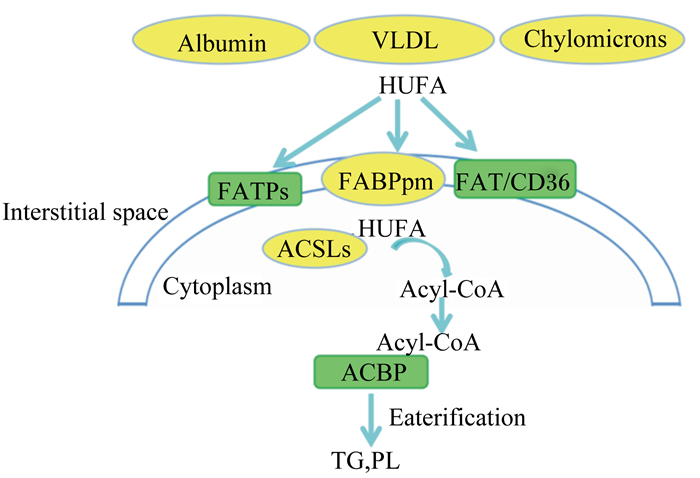
|
Interstitial space:细胞间隙;Cytoplasm:细胞质;Albumin:白蛋白;Chylomicrons:乳糜微粒;Acyl-CoA:酰基辅酶A;Eaterification:酯化;VLDL:极低密度脂蛋白very low density lipoprotein;HUFA:高不饱和脂肪酸highly unsaturated fatty acids;FATPs:脂肪酸转运蛋白fatty acid transport proteins;FABPpm:膜脂肪酸结合蛋白plasma membrane fatty acid binding protein;FAT/CD36:脂肪酸移位酶fatty acid translocase;ACSLs:长链脂酰辅酶A合成酶long-chain acyl-CoA synthetases;ACBP:酰基辅酶A结合蛋白acyl-CoA-binding protein。 图 2 肌肉中HUFA跨膜转运及沉积 Fig. 2 'HUFA transmembrane transport and deposition in muscle[40] |
鱼类体内脂肪含量的多少最终取决于脂肪沉积的多少。研究表明,饲料中过高的可消化糖含量可提高虹鳟布氏体和下丘脑中脂肪酸合成酶(fatty acid synthetase,FAS)mRNA的表达量,促进脂肪的内源性合成并最终引起体脂肪的沉积[41]。作为脂肪代谢途径中的关键酶,LPL可以通过调节其在不同组织中的活性和表达量来决定机体中不同组织器官间的脂质配比,从而间接决定脂肪的代谢用途,并最终对机体的脂质蓄积产生决定性影响[42]。除此之外,脂肪转运也是影响脂肪沉积的一个重要因素[43]。与哺乳动物类似,鱼类的脂肪转运也是通过脂蛋白来完成的。当前,在鱼类中已发现的参与脂蛋白代谢过程中有载脂蛋白(apolipoprotein,Apo)(ApoA、ApoB、ApoC等)、酶[微粒体甘油三酯转移蛋白(microsmal triglyceride transfer protein,MTP)、LPL、LCAT、胆固醇酯转移蛋白(cholesterol ester transfer protein,CETP)等]、脂蛋白受体[低密度脂蛋白受体(LDL receptor,LDLR)、低密度脂蛋白受体相关蛋白-1(LDLR related protein-1,LRP-1)、B族Ⅰ型清道夫受体(scavenger receptor B type Ⅰ,SRBⅠ)][44]。同时,鱼类的代谢并不是恒定的,而是随着时间的变化而变化的[45]。研究表明,虹鳟鱼体内脂肪酸沉积存在着周期性的昼夜节律模式,在晚餐期间饮食脂肪酸更有可能沉积[46],这可能是受到其内源性循环节律的影响[47-48]。
2 影响养殖鱼类n-3 HUFA含量的因素如图 1和图 2所示,鱼类n-3 HUFA含量受HUFA合成和转运吸收途径中的关键酶基因表达量及活性调控。上述酶基因表达及活性主要受内在的遗传因素以及外在的营养与环境因素的影响。
2.1 遗传发育因素鱼类是否具有一套完整的HUFA合成关键酶体系,是影响鱼类HUFA生物合成强弱的主要因素之一。一般认为,淡水硬骨鱼和鲑鳟类,以及植食性海水鱼,如黄斑蓝子鱼和叉牙鲷(Pegusa lascaris)具有完整的HUFA合成酶系,可利用富含ALA的植物油在体内合成n-3 HUFA,以满足生长需求;而绝大多数海水鱼HUFA合成酶系不完整或这些酶活性很低,缺乏n-3 HUFA合成能力,需从食物摄取[49-50]。
同时,鱼体HUFA含量受鱼类发育阶段影响,施培松[51]分别对不同发育阶段的匙吻鲟(Polyodon spathula)和鳙(Aristichthys nobilis)肌肉脂肪酸组成进行分析发现,EPA和DHA含量在匙吻鲟和鳙鱼3个时期中变化一致:均为稚鱼期较低,幼鱼期升高,而亚成鱼期又降低;n-6/n-3 PUFA在匙吻鲟稚鱼期较高,幼鱼期和亚成鱼期较低,而鳙鱼则是幼鱼期最高,亚成鱼期最低。
2.2 营养因素影响鱼类n-3 HUFA含量的营养因素包括饲料脂肪酸组成、矿物质和维生素等,其作用机理主要是通过影响HUFA合成途径中关键酶基因的表达及活性,详细见本团队前期综述[52]。富含C18 PUFA的植物油可促进鱼类n-3 HUFA的生物合成,而富含HUFA的鱼油则反馈抑制其合成;n-3、n-6 C18 PUFA同为Fad和Elovl的作用底物,造成2种底物之间存在HUFA合成代谢的竞争性作用。大量研究发现,Fad和Elovl对n-3 C18 PUFA底物的亲和力高于n-6 C18 PUFA,高比例的n-3/n-6 C18 PUFA提高Fad基因表达及活性,促进n-3 HUFA合成[53]。除了影响鱼类HUFA合成代谢途径外,饲料脂肪酸组成还影响肌肉脂肪酸的吸收转运。研究表明,与富含鱼油的饲料相比,富含植物油的饲料显著降低了金头鲷肌肉LPL活性,影响了肌细胞对胞外脂质的水解;同时,植物油也影响了与脂肪酸转运相关基因的表达[54]。与鱼油组相比,植物油组大西洋鲑鱼肌肉FAT/CD36的表达量降低了2.5倍[55]。以上研究表明,饲料脂肪酸组成也可能通过影响LPL和FAT/CD36等脂肪酸转运吸收相关基因的表达,影响组织n-3 HUFA的沉积。
此外,作为HUFA合成代谢关键酶Fad和Elovl的重要辅酶,微量元素锌、镁等矿物质也影响n-3 HUFA的合成;维生素E等过氧化清除分子的存在可以促进组织HUFA的保留量。在成年人中,维生素B6可增强Elovl的作用;然而,维生素A、β-胡萝卜素和反式脂肪酸似乎能够抑制Fad的活性,降低体内HUFA的合成[56]。
2.3 环境因素鱼类组织脂肪酸组成受到季节和盐度等环境因素的影响。研究发现春季的鳀鱼(Engraulis japonicus)和沙丁鱼(Sardina pilchardus)n-3 HUFA含量显著高于秋季;鲭鱼(Horse mackerel)DHA含量在春季显著高于秋季[57-58]。在另一项研究中,与秋季捕获的黑鲷(Acanthopagrus schlegelii)和拟棘鲷(Centroberyx affinis)相比,春季捕获的黑鲷和拟棘鲷的体脂肪中含有更多的HUFA,以及更高的n-3/n-6 PUFA[59]。相似的是,冬季的罗非鱼体内EPA和DHA含量之和占总脂肪酸的12.5%,显著高于夏季的7.46%[60]。Sushchik等[61]研究发现,3月份的鲈鱼(Perca fluviatilis)n-3 HUFA含量显著高于7月份。季节变化影响鱼类脂肪酸组成,可能存在以下2个主要原因:1)浮游生物、藻类等初级生产者的脂肪酸组成。大量研究证明春冬季节水环境初级生产者的n-3 PUFA含量高于夏秋季节[62-65],从而导致下游捕食者——鱼类的n-3 PUFA含量相应提高。2)细胞膜脂流动性。膜脂正常的流动性对维持细胞和细胞器的正常生理功能是必需的。为了维持膜脂的正常流动性,低温提高了鱼体Fad和Elovl的活性,促进HUFA的合成[66-67],从而影响机体内HUFA含量。
相似的是,为适应环境盐度的变化,鱼类可以通过提高细胞膜中HUFA的含量,以增加膜的离子通透性和保持细胞正常的渗透压。研究表明,在海水鱼欧洲鲈鱼[68-69]、黄斑蓝子鱼[19]、真鲷(Pagrus major)[70]养殖过程中,它们肌肉中PUFA含量随着盐度的降低呈增高趋势,尤其以EPA、DHA和AA含量的变化最为显著。但研究也发现,适当增加水体盐度可在一定程度上提高咸淡水鱼花鲈(Lateolabrax japonocus)[71],以及淡水鱼罗非鱼[72-73]、虹鳟[74]肌肉中EPA和DHA等n-3 HUFA的含量。
3 养殖鱼类n-3 HUFA含量的提升策略 3.1 鱼油饲料复饲策略为降低成本,减少养殖业对鱼油的依赖,过去几十年研究者们在用植物油替代鱼油方面进行了大量研究。然而,配合饲料中植物油过量替代鱼油会显著降低鱼体内的n-3 HUFA含量(表 1)。针对这一现象,有研究学者发现,通过复饲鱼油饲料营养策略可缓解这一现状。
在海水鱼的相关研究中,Woitel等[75]采用100%牛油饲料、33%牛油+67%鱼油饲料以及67%牛油+33%鱼油饲料养殖军曹鱼幼鱼8周后,再采用100%鱼油饲料复饲6周,可显著提高肌肉组织中n-3 HUFA含量。采用混合油(植物油+鱼油)饲料养殖欧洲鲈幼鱼(初始体重约为75 g)至中成鱼后,再投喂鱼油饲料150 d,复饲组肌肉DHA含量可完全恢复至鱼油组水平,EPA含量可恢复至鱼油组的65%~87%[33]。摄食植物油饲料的塞内加尔鳎(Solea senegalensis)复饲鱼油饲料26 d后,其肌肉EPA含量可完全恢复至鱼油组的水平[76]。Izquierdo等[77]研究发现,以植物油替代鱼油饲喂金头鲷一段时间后采用鱼油饲料复饲,其EPA和DHA的含量随着鱼油饲料的复饲期增加而逐渐增加,在复饲60 d后达到鱼油组的水平。
在鲑鳟类的相关研究中,Bell等[78]发现大西洋鲑复饲鱼油饲料后,肌肉中HUFA含量可恢复到鱼油组的80%。在另一篇相关报道中发现,大西洋鲑复饲鱼油饲料4周后,其EPA含量可恢复至鱼油组的水平,而其DHA含量在复饲12周后才得以完全恢复[79]。Stone等[80]前期以菜籽油饲料饲喂186 g的虹鳟8周,再以100%鱼油复饲9周后,其肌肉中EPA和DHA含量比一直摄食菜籽油饲料的虹鳟高1倍。虹鳟幼鱼(初始体重约为15.7 g)饲喂棉籽油或菜籽油饲料12周后,再以鱼油饲料复饲12周,其组织中EPA和DHA含量随着复饲时间的延长而逐渐升高[81]。在淡水鱼相关研究中,于若梦[82]采用植物油饲料养殖鲤8周后,再以含1%DHA的饲料分别复饲10、20和30 d,结果发现,与全程植物油饲料投喂组相比,复饲10 d后,鱼体肌肉DHA含量显著提高,随着复饲时间的延长,肌肉DHA含量也随之增加。
综上所述,在生长前中期,以植物性原料为主的饲料养殖鱼类,既不影响生长,又可降低养殖饲料成本;后期采用鱼油饲料复饲的策略可降低植物油饲料对鱼类n-3 HFUA含量的负面影响,维持养殖鱼的营养价值和感官特征。
3.2 维生素E和磷脂的添加维生素E是鱼类不可或缺的微量营养物质,具有重要的生理功能[83]。在斜带石斑鱼亲鱼饲料中添加维生素E不仅可以增大亲鱼受精卵的卵径、油球直径和仔鱼全长,而且受精卵中DHA和EPA含量得到增加[84]。用添加适量维生素E的鱼油饲料饲喂波斯鲟鱼[85](Acipenser persicus)、比目鱼[86](Paralichthys olivaceus)和红鲷[87](Pagrus major),也一定程度上增加了其机体内n-3 HUFA的含量。丁兆坤等[88]以添加不同剂量维生素E的饲料饲喂军曹鱼稚鱼,均显著提高了军曹鱼稚鱼肌肉中n-3 HUFA的含量。维生素E作为一种过氧化清除分子,其存在可以避免细胞膜上的PUFA受过氧化氢自由基的攻击,阻止自由基的进一步增殖和自由基介导的PUFA降解,从而提高了机体内HUFA的保留量[89-90];同时,作为过氧化物酶体增殖物激活受体α(peroxisome proliferator-activated receptor α,PPARα)的激动剂,维生素E可通过促进PPARα的表达,促进脂肪酸的氧化,影响组织脂肪酸组成[91]。
另有研究表明,饲料中添加磷脂能够影响鱼类的脂质代谢与沉积[92]。用添加大豆卵磷脂的饲料投喂草鱼,可显著提高其肝胰脏中n-3 HUFA含量[93]。卢素芳[94]发现在黄颡鱼仔稚鱼人工微粒饲料中添加6%磷脂时,14日龄黄颡鱼n-3 HUFA含量显著增加。作为乳化剂,磷脂可以促进体内脂质转运与吸收[95-100],提高脂类利用效率[101];同时,饲料中磷脂的含量与组成可影响肠道内脂蛋白的含量与组成[102-103],提高水生动物肝脏、肠部脂类运输能力,从而间接影响机体内n-3 HUFA的积累[101]。
3.3 微藻类的应用微藻是一类广泛分布于海洋,光合利用度高的自养植物,且富含n-3 HUFA(特别是EPA和DHA),水产中研究较多的有裂壶藻(Schizochytrium sp.)、微绿球藻(Nannochloropsis sp.)、寇氏隐甲藻(Crypthecodinium cohnii)、三角褐指藻(Phaeodactylum tricornutum),尤其是裂壶藻、微绿球藻,其总体的脂肪酸组成与鱼油相似,被认为是鱼油的良好替代品[104-106]。在杂交条纹鲈(Morone saxatilis)幼鱼(初始体重约为10.6 g)配合饲料中添加50%微藻粉[裂壶藻+节螺旋藻(Arthrospira sp.)],显著提高了肌肉组织中EPA和DHA含量[107]。相似的是,与鱼油饲料相比,含有裂壶藻、微绿球藻的饲料显著提高了牙鲆(Paralichthys olivaceus)稚鱼的成活率及其DHA含量[108]。在罗非鱼相关研究中,与鱼油饲料相比,藻粉饲料不仅可以提高其肌肉中HUFA含量,同时也能降低n-6/n-3 PUFA[109]。以添加90 mg/g寇氏隐甲藻粉的植物源饲料饲喂虹鳟鱼12周,显著增强了鱼肉的香气,且与鱼油组相比,鱼肉中DHA含量更高[110]。研究表明,以富含EPA的微绿球藻富集轮虫后饲喂高体能够有效地促进其生长,提高存活率[111]。
3.4 功能性添加剂的应用作为脂质调节剂,芝麻素和共轭亚油酸(conjugated linoleic acid,CLA)在哺乳动物中已得到广泛的研究,但在鱼类中的研究较少。为了降低鱼油替代过程中HUFA含量降低的负面影响,Vestergren等[112]以添加0.58%芝麻素的植物油饲料饲喂虹鳟幼鱼(初始体重约为36.5 g)58 d,促进了鱼体内ALA向DHA的转化。Trattner等[113]发现,在植物油饲料中添加0.58%芝麻素饲喂虹鳟幼鱼(初始体重约为34 g)35 d后,其肌肉中磷脂和甘油三酯中DHA的比例增加37%。在芝麻素对大西洋鲑鱼肝细胞脂肪酸代谢影响的研究中发现,与对照组相比,芝麻素的添加显著促进了从ALA向DHA转化,其DHA含量增加了32.35%[114]。此外,CLA通过调节多种脂质代谢转录因子的表达,影响体内脂肪酸的摄取、氧化及脂质的合成代谢,从而改变肌肉中脂肪含量和脂肪酸组成,改善肌肉品质[115]。欧洲鲈幼鱼摄食不同CLA含量的饲料12周后,其内脏脂肪含量显著降低,肌肉n-3 HUFA含量显著提高[116]。在大黄鱼相关研究中,随着饲料中CLA添加量的增加,鱼体肌肉中单不饱和脂肪酸(monounsaturated fatty acids,MUFA)含量显著减少,PUFA(尤其是EPA和DHA)含量显著增加[117]。在饲料中添加0.5%~1.0% CLA或0.5%十四烷基硫代乙酸,饲养大西洋鳕鱼90 d,发现各添加剂组肌肉DHA含量均显著高于对照组[118]。相似的是,分别采用添加0.50%、0.75%、1.00%、2.00% CLA的饲料饲养虹鳟幼鱼12周,各添加CLA组肌肉中MUFA含量显著降低,且0.75%、1.00%和2.00% CLA添加组肌肉中PUFA含量显著增加[119]。由此可见,芝麻素、CLA和十四烷基硫代乙酸等功能性饲料添加剂可促进鱼类组织中n-3 HUFA的积累,提高其营养价值。
3.5 品种选育技术选择育种是指利用现有品种和类型在繁殖过程中自然产生的变异,通过人工选择和淘汰的手段,以改变群体的遗传组成,从中选出新的品种。孙中武等[120]对丹麦虹鳟、道氏虹鳟、挪威虹鳟、芬兰虹鳟、美国虹鳟5个品系进行肌肉营养成分分析发现,道氏虹鳟肌肉中EPA和DHA含量最高,显著高于其他4个品系的虹鳟,而美国虹鳟肌肉中EPA和DHA含量最低。李明云等[121]通过对比岱衢洋家系、官井洋家系以及它们的正交和反交家系这4个家系的大黄鱼的营养成分发现,正交、反交家系含有较高的PUFA及n-3 HUFA含量,且与另外2个家系有显著差异。以野生大黄鱼作为对照,对同等养殖条件下所养成的3个不同家系WW家系[野生F1(♀)×野生F1()]、WC家系[野生F1(♀)×养殖F8()]和CC家系[养殖F8(♀)×养殖F8()]的大黄鱼肌肉营养成分进行测定分析,发现WC家系成鱼肌肉中EPA与DHA的总含量为25.8%,高于WW家系(21.0%)、CC家系(20.0%)和野生大黄鱼(16.0%)[122]。通过对杂交新品长丰鲫(Carassius auratus gibelio)、彭泽鲫(Carassius auratus pengzesis)、异育银鲫(Carassius auratus gibelio)和红鲫(Carassius auratus red var. Xiangjiang)的肌肉营养品质进行比较分析,发现长丰鲫肌肉中HUFA含量高达24.2%,而彭泽鲫、异育银鲫和红鲫则分别为14.4%、11.3%和8.3%[123]。除了在同一品种的不同家系之间进行杂交育种,通过个体差异亦可有效地选育出HUFA合成能力强的鱼种,提高其n-3 HUFA含量。Le Boucher等[124]利用植物性饲料培育大西洋鲑鱼1代后,显著提高了子代利用植物油饲料合成n-3 HUFA的能力。综上所述,通过选育手段,可以在一定程度上提高鱼类的n-3 HUFA合成能力。
3.6 转基因技术转基因技术可以实现对基因的定向高效转移,效率更高,针对性更强。相对传统的鱼类选育种而言,转基因育种具有育种目的性强、周期短、优良性状遗传稳定、跨越生殖隔离、拓宽遗传资源利用范围等优点。
3.6.1 转基因油料作物在饲料加工业中,来源广泛、产量稳定、价格较低的菜籽油、大豆油、亚麻油等植物油是比较理想的饲料脂肪源。但是,植物油缺乏EPA、DHA等n-3 HUFA,长期摄食植物油饲料会导致养殖鱼类n-3 HUFA含量显著降低。采用转基因技术将与n-3 HUFA合成相关酶基因转到油菜、大豆、亚麻荠等油料作物中,使之获得合成n-3 HUFA的能力,从而有效地提高植物油替代鱼油的利用率,该技术的应用和推广有望摆脱植物油因缺乏n-3 HUFA而不能大量添加的限制。
为了使亚麻荠获得EPA的合成能力,Betancor等[125-126]用葡萄球菌(Ostococcus tauri)的Δ6 Fad、小立碗藓(Physcomitrella patens)的Δ6 Fad、海洋真菌(Thraustochytrium sp.)的Δ5 Fad、大豆疫霉菌(Phytophthora sojae)的Δ12 Fad、马铃薯晚疫病菌(Phytophthora infestans)ω-3 Fad基因构建了一个共表达载体,然后将其导入到亚麻荠中,能产生出富含EPA的亚麻油(EPA-亚麻油);然后,采用含鱼油、亚麻油或EPA-亚麻油的3种饲料饲喂大西洋鲑(初始体重约为82.5 g)7周,与亚麻油组相比,EPA-亚麻油组肌肉中二十二碳五稀酸(docosapentaenoic acid,DPA)和DHA的含量显著提高。随之,该团队在此基础上再加入葡萄球菌Δ5 Fad和海洋球石藻(Emiliania huxleyi)Δ4 Fad的2个基因,培养出能产出富含EPA和DHA亚麻油(EPA/DHA-亚麻油)的亚麻荠。通过用含鱼油、亚麻油或EPA/DHA-亚麻油的3种饲料饲喂大西洋鲑鱼(初始体重约为256.2 g)11周,结果发现EPA/DHA-亚麻油组肌肉中EPA含量是亚麻油组的2倍,并且其DHA和总HUFA含量与鱼油组相似[127]。
以添加不同比例的鱼油与菜籽油混合油、亚麻油或EPA/DHA-亚麻油的饲料饱食饲喂大西洋鲑鱼12周,结果发现,EPA/DHA-亚麻油组每份鱼片中EPA+DHA含量几乎是混合油(鱼油+菜籽油)组和亚麻油组的2倍[128-129]。采用鱼油、野生亚麻籽油、EPA-亚麻籽油或EPA/DHA-亚麻籽油为脂肪源的饲料养殖金头鲷幼鱼(初始体重约为55.5 g)11周,发现2种转基因植物油饲料对鱼体健康和生长速率无任何负面影响,同时均显著提高了鱼体肌肉中HUFA的含量,改善了鱼肉的营养品质[130]。以上研究结果表明,转基因植物油在为养殖鱼类提供HUFA方面非常有效,是饲料加工业中鱼油的有效替代品。
3.6.2 转基因鱼大多数海水鱼缺乏Δ5 Fad及Elovl 2基因或其酶活性很低,因此不具有将C18 PUFA合成为HUFA的能力,或HUFA合成能力很弱。Alimuddin等[131]通过将马苏麻哈鱼(Oncorhynchus masou)Δ5 Fad基因转移至斑马鱼(Barchydanio rerio var.)体内,发现斑马鱼EPA含量提高了1.21倍,DHA含量提高了1.24倍。相似的是,将马苏麻哈鱼Δ6 Fad基因转移至斑马鱼体内后发现,与非转基因鱼相比,鱼体总脂质含量保持不变,但Δ6 Fad的作用底物——ALA含量下降,EPA和DHA含量显著升高,最终转基因鱼体内EPA含量增加了1.4倍,DHA增加了2.1倍[132]。研究发现,从马苏麻哈鱼中克隆到一种类似延长酶的基因MELO,并将其转入斑马鱼中,与非转基因鱼相比,转基因鱼EPA和DHA含量分别提高了1.30和1.33倍[133]。同样,在双转基因斑马鱼中,来自秀丽线虫(Caenorhabditis elegans)的Δ15 Fad和Δ12 Fad的共表达分别使EPA和DHA含量提高了1.7和2.8倍[134]。将马苏麻哈鱼Elovl 2基因转入鮸鱼(Miichthys miiuy)体内,转基因鱼肝脏中EPA含量较非转基因鱼低,但其EPA的转化物——DPA含量提高了1.33倍[135]。
4 小结与展望如今,水产配合饲料中植物饲料原料添加比例越来越高,显著降低了养殖鱼类n-3 HUFA含量,影响人类对n-3 HUFA的获取。因此,在不影响养殖鱼类n-3 HUFA含量的前提下,如何有效地降低或摆脱对鱼源饲料的依赖,已成为水产业可持续发展亟待解决的问题。为此,研究者已从营养策略、新型饲料原料、功能性饲料添加剂、品种选育和基因工程等方面开展了系列研究。然而,以上方面研究均有不足之处。鱼油饲料复饲策略虽然在一定程度上改善植物源饲料对鱼类n-3 HUFA含量的负面影响,但仍然无法彻底摆脱对渔源饲料的依赖。新型饲料原料——微藻可高效地替代鱼油,提高鱼类n-3 HUFA含量,然而其产量低且价格较高,无法在水产养殖业中广泛应用。功能性添加剂——芝麻素和CLA等脂代谢调节剂通过促进鱼体饱和脂肪酸(saturated fatty acids,SFA)和MUFA分解,以提高HUFA的比例,但促进脂质分解过程中也随之降低了HUFA总含量[115, 136]。品种选育技术可提高鲑鳟类和淡水鱼等具有HUFA合成能力的鱼类对植物油饲料的利用率[123-124],但对无HUFA合成能力的海水鱼的改善效率不理想。同时,品种选育耗时长,目的性差,杂交品种不易控制。转基因技术在鱼类上应用可高效地提高养殖鱼类n-3 HUFA含量,可是转基因食品的潜在安全问题一直被消费者所顾虑。近几年来,苏格兰Tocher团队将转基因技术成功应用到亚麻荠、油菜等油料作物中,为植物油赋予新的HUFA源,有望使水产养殖业彻底摆脱对鱼油的依赖[125-129, 137]。然而,目前我国在该方面尚无相关研究报道。
为了提高养殖鱼类n-3 HUFA含量,除上述研究方向外,如下2个方面有待于研究:1)转基因昆虫。相比于油料作物,蚕蛹、黄粉虫等昆虫不仅含有可观的油脂,而且可为水产动物提供优质的蛋白质[138-140]。蚕蛹和黄粉虫的油脂含量高达30%,其中不饱和脂肪酸(主要为棕榈油酸、亚麻酸、亚油酸)占总脂肪酸的比例为70%~80%,但均不含n-3 HUFA[141-142]。若采用转基因技术将HUFA合成代谢关键酶基因导入上述昆虫中,得到富含n-3 HUFA的昆虫蛋白产品,则可为水产养殖业摆脱鱼油和鱼粉的依赖提供可能。2)鱼类肌肉n-3 HUFA沉积机制。外源性和内源性的n-3 HUFA经血液循环运送到肌肉、脑、心脏等组织细胞中,但是其在鱼类组织细胞中的吸收转运机制尚不清晰。鱼类肌肉中n-3 HUFA吸收和沉积机制的阐明,可为定向提高鱼类肌肉n-3 HUFA含量奠定理论基础。在当前鱼油资源紧缺、养殖鱼类营养价值下降的情况下,上述研究技术的应用和科学问题的突破可改善目前的窘境,促进水产养殖业健康、可持续发展。
| [1] |
WU F C, TING Y Y, CHEN H Y. Dietary docosahexaenoic acid is more optimal than eicosapentaenoic acid affecting the level of cellular defence responses of the juvenile grouper Epinephelus malabaricus[J]. Fish & Shellfish Immunology, 2003, 14(3): 223-238. |
| [2] |
HUDSON E A, TISDALE M J. Comparison of the effectiveness of eicosapentaenoic acid administered as either the free acid or ethyl ester as an anticachectic and antitumour agent[J]. Prostaglandins, Leukotrienes and Essential Fatty Acids, 1994, 51(2): 141-145. DOI:10.1016/0952-3278(94)90090-6 |
| [3] |
DAS U N. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory[J]. Nutrition, 2003, 19(1): 62-65. DOI:10.1016/S0899-9007(02)00852-3 |
| [4] |
CRAWFORD M A, COSTELOE K, GHEBREMESKEL K, et al. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies?[J]. The American Journal of Clinical Nutrition, 1997, 66(4): 1032S-1041S. DOI:10.1093/ajcn/66.4.1032S |
| [5] |
SPITE M. Deciphering the role of n-3 polyunsaturated fatty acid-derived lipid mediators in health and disease[J]. Proceedings of the Nutrition Society, 2013, 72(4): 441-450. DOI:10.1017/S0029665113003030 |
| [6] |
钱兴国, 苏宜香, 苗丽曼, 等. 孕妇、乳母二十二碳六烯酸摄入水平对婴儿早期运动发育和视功能的影响[J]. 中国儿童保健杂志, 2004(2): 123-126. |
| [7] |
DELGADO-LISTA J, PEREZ-MARTINEZ P, LOPEZ-MIRANDA J, et al. Long chain omega-3 fatty acids and cardiovascular disease:a systematic review[J]. The British Journal of Nutrition, 2012, 107(Suppl.2): S201-S213. |
| [8] |
SARAVANAN P, DAVIDSON N C, SCHMIDT E B, et al. Cardiovascular effects of marine omega-3 fatty acid[J]. The Lancet, 2010, 376(9740): 540-550. DOI:10.1016/S0140-6736(10)60445-X |
| [9] |
TOCHER D R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective[J]. Aquaculture, 2015, 449: 94-107. DOI:10.1016/j.aquaculture.2015.01.010 |
| [10] |
RICHTER C K, SKULAS-RAY A C, KRIS-ETHERTON P M.Recommended intake of fish and fish oils worldwide[M]//RAATZ S K, BIBUS D M.Fish and fish oil in health and disease prevention.Commonwealth of Pennsylvania: Academic Press, 2016: 27-48.
|
| [11] |
SALEM J N, EGGERSDORFER M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition?[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2015, 18(2): 147-154. DOI:10.1097/MCO.0000000000000145 |
| [12] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 中国渔业统计年鉴2019[M]. 北京: 中国农业出版社, 2019.
|
| [13] |
TOYES-VARGAS E A, PARRISH C C, VIANA M T, et al. Replacement of fish oil with camelina (Camelina sativa) oil in diets for juvenile tilapia (var. GIFT Oreochromis niloticus) and its effect on growth, feed utilization and muscle lipid composition[J]. Aquaculture, 2020, 523: 735177. DOI:10.1016/j.aquaculture.2020.735177 |
| [14] |
AYISI C L, ZHAO J L, WU J W. Replacement of fish oil with palm oil:effects on growth performance, innate immune response, antioxidant capacity and disease resistance in Nile tilapia (Oreochromis niloticus)[J]. PLoS One, 2018, 13(4): e0196100. DOI:10.1371/journal.pone.0196100 |
| [15] |
陆游, 金敏, 袁野, 等. 不同脂肪源对黄颡鱼幼鱼生长性能、体成分、血清生化指标、体组织脂肪酸组成及抗氧化能力的影响[J]. 水产学报, 2018, 42(7): 1094-1110. |
| [16] |
李经奇, 李学山, 姬仁磊, 等. 亚麻籽油和豆油替代鱼油对大黄鱼肝脏和肌肉脂肪酸组成及Δ6Fad基因表达的影响[J]. 水生生物学报, 2018, 42(2): 232-239. |
| [17] |
陈涛, 杨艳, 卢航, 等. 不同脂肪源对红罗非鱼稚鱼生长及肌肉脂肪酸组成的影响[J]. 饲料工业, 2017, 38(4): 29-35. |
| [18] |
WANG S Q, LIU X B, XU S D, et al. Total replacement of dietary fish oil with a blend of vegetable oils in the marine herbivorous teleost, Siganus canaliculatus[J]. Journal of the World Aquaculture Society, 2018, 49(4): 692-702. DOI:10.1111/jwas.12434 |
| [19] |
LI Y Y, HU C B, ZHENG Y J, et al. The effects of dietary fatty acids on liver fatty acid composition and Δ6-desaturase expression differ with ambient salinities in Siganus canaliculatus[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2008, 151(2): 183-190. DOI:10.1016/j.cbpb.2008.06.013 |
| [20] |
MONGE-ORTIZ R, TOMÁS-VIDAL A, RODRIGUEZ-BARRETO D, et al. Replacement of fish oil with vegetable oil blends in feeds for greater amberjack (Seriola dumerili) juveniles:effect on growth performance, feed efficiency, tissue fatty acid composition and flesh nutritional value[J]. Aquaculture Nutrition, 2018, 24(1): 605-615. DOI:10.1111/anu.12595 |
| [21] |
ROMBENSO A N, TRUSHENSKI J T, SCHWARZ M H. Fish oil replacement in feeds for juvenile florida pompano:composition of alternative lipid influences degree of tissue fatty acid profile distortion[J]. Aquaculture, 2016, 458: 177-186. DOI:10.1016/j.aquaculture.2016.03.009 |
| [22] |
黄裕, 王际英, 李宝山, 等. 小麦胚芽油替代鱼油对半滑舌鳎幼鱼生长、体成分、血清生化指标及脂肪代谢酶的影响[J]. 中国水产科学, 2015, 22(6): 1195-1208. |
| [23] |
彭墨.饲料脂肪水平和脂肪酸组成对大菱鲆幼鱼脂沉积的影响[D].博士学位论文.青岛: 中国海洋大学, 2014.
|
| [24] |
HIXSON S M, PARRISH C C, ANDERSON D M. Effect of replacement of fish oil with camelina (Camelina sativa) oil on growth, lipid class and fatty acid composition of farmed juvenile Atlantic cod (Gadus morhua)[J]. Fish Physiology and Biochemistry, 2013, 39(6): 1441-1456. DOI:10.1007/s10695-013-9798-2 |
| [25] |
HIXSON S M, PARRISH C C, ANDERSON D M. Full substitution of fish oil with camelina (Camelina sativa) oil, with partial substitution of fish meal with camelina meal, in diets for farmed Atlantic salmon (Salmo salar) and its effect on tissue lipids and sensory quality[J]. Food Chemistry, 2014, 157: 51-61. DOI:10.1016/j.foodchem.2014.02.026 |
| [26] |
TRUSHENSKI J, SCHWARZ M, LEWIS H, et al. Effect of replacing dietary fish oil with soybean oil on production performance and fillet lipid and fatty acid composition of juvenile cobia Rachycentron canadum[J]. Aquaculture Nutrition, 2011, 17(2): e437-e447. DOI:10.1111/j.1365-2095.2010.00779.x |
| [27] |
SUN S, YE J, CHEN J, et al. Effect of dietary fish oil replacement by rapeseed oil on the growth, fatty acid composition and serum non-specific immunity response of fingerling black carp, Mylopharyngodon piceus[J]. Aquaculture Nutrition, 2011, 17(4): 441-450. DOI:10.1111/j.1365-2095.2010.00822.x |
| [28] |
周秋白, 朱长生, 吴华东, 等. 饲料中不同脂肪源对黄鳝生长和组织中脂肪酸含量的影响[J]. 水生生物学报, 2011, 35(2): 246-255. |
| [29] |
FOUNTOULAKI E, VASILAKI A, HURTADO R, et al. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile:recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures[J]. Aquaculture, 2009, 289(3/4): 317-326. |
| [30] |
DU Z Y, CLOUET P, HUANG L M, et al. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella):mechanism related to hepatic fatty acid oxidation[J]. Aquaculture Nutrition, 2008, 14(1): 77-92. DOI:10.1111/j.1365-2095.2007.00507.x |
| [31] |
BAHURMIZ O M, NG W K. Effects of dietary palm oil source on growth, tissue fatty acid composition and nutrient digestibility of red hybrid tilapia, Oreochromis sp., raised from stocking to marketable size[J]. Aquaculture, 2007, 262(2/3/4): 382-392. |
| [32] |
BALLESTRAZZI R, RAINIS S, MAXIA M. The replacement of fish oil with refined coconut oil in the diet of large rainbow trout (Oncorhynchus mykiss)[J]. Italian Journal of Animal Science, 2006, 5(2): 155-164. DOI:10.4081/ijas.2006.155 |
| [33] |
MONTERO D, ROBAINA L, CABALLERO M J, et al. Growth, feed utilization and flesh quality of European sea bass (Dicentrarchus labrax) fed diets containing vegetable oils:a time-course study on the effect of a re-feeding period with a 100% fish oil diet[J]. Aquaculture, 2005, 248(1/2/3/4): 121-134. |
| [34] |
LI Y Y, MONROIG O, ZHANG L, et al. Vertebrate fatty acyl desaturase with Δ4 activity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(39): 16840-16845. DOI:10.1073/pnas.1008429107 |
| [35] |
MONROIG O, WANG S Q, ZHANG L, et al. Elongation of long-chain fatty acids in rabbitfish Siganus canaliculatus:cloning, functional characterisation and tissue distribution of Elovl5-and Elovl4-like elongases[J]. Aquaculture, 2012, 350/351/352/353: 63-70. |
| [36] |
XIE D Z, WANG S Q, YOU C H, et al. Characteristics of LC-PUFA biosynthesis in marine herbivorous teleost Siganus canaliculatus under different ambient salinities[J]. Aquaculture Nutrition, 2015, 21(5): 541-551. DOI:10.1111/anu.12178 |
| [37] |
CLARKE D C, MISKOVIC D, HAN X X, et al. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism[J]. Physiological Genomics, 2004, 17(1): 31-37. DOI:10.1152/physiolgenomics.00190.2003 |
| [38] |
GUO J, SHU G, ZHOU L, et al. Selective transport of long-chain fatty acids by FAT/CD36 in skeletal muscle of broilers[J]. Animal, 2013, 7(3): 422-429. DOI:10.1017/S1751731112001619 |
| [39] |
LARQUÉ E, KRAUSS-ETSCHMANN S, CAMPOY C, et al. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins[J]. The American Journal of Clinical Nutrition, 2006, 84(4): 853-861. DOI:10.1093/ajcn/84.4.853 |
| [40] |
KOONEN D P Y, GLATZ J F C, BONEN A, et al. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle[J]. Biochimica et Biophysica Acta:Molecular and Cell Biology of Lipids, 2005, 1736(3): 163-180. DOI:10.1016/j.bbalip.2005.08.018 |
| [41] |
POLAKOF S, PANSERAT S, PLAGNES-JUAN E, et al. Altered dietary carbohydrates significantly affect gene expression of the major glucosensing components in brockmann bodies and hypothalamus of rainbow trout[J]. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology, 2008, 295(4): R1077-R1088. DOI:10.1152/ajpregu.90476.2008 |
| [42] |
ZECHNER R. The tissue-specific expression of lipoprotein lipase:implications for energy and lipoprotein metabolism[J]. Current Opinion in Lipidology, 1997, 8(2): 77-88. DOI:10.1097/00041433-199704000-00005 |
| [43] |
TOCHER D R. Metabolism and functions of lipids and fatty acids in teleost fish[J]. Reviews in Fisheries Science, 2003, 11(2): 107-184. DOI:10.1080/713610925 |
| [44] |
艾庆辉, 严晶, 麦康森. 鱼类脂肪与脂肪酸的转运及调控研究进展[J]. 水生生物学报, 2016, 40(4): 859-868. |
| [45] |
DE SILVA S S. Performance of Oreochromis niloticus (L.) fry maintained on mixed feeding schedules of differing protein content[J]. Aquaculture Research, 1985, 16(4): 331-340. DOI:10.1111/j.1365-2109.1985.tb00075.x |
| [46] |
BROWN T D, FRANCIS D S, TURCHINI G M. Can dietary lipid source circadian alternation improve omega-3 deposition in rainbow trout?[J]. Aquaculture, 2010, 300(1/2/3/4): 148-155. |
| [47] |
TURCHINI G M, FRANCIS D S, DE SILVA S S. Finishing diets stimulate compensatory growth:results of a study on Murray cod, Maccullochella peelii peelii[J]. Aquaculture Nutrition, 2007, 13(5): 351-360. DOI:10.1111/j.1365-2095.2007.00483.x |
| [48] |
FRANCIS D S, TURCHINI G M, SMITH B K, et al. Effects of alternate phases of fish oil and vegetable oil-based diets in Murray cod[J]. Aquaculture Research, 2009, 40(10): 1123-1134. DOI:10.1111/j.1365-2109.2009.02208.x |
| [49] |
GARRIDO D, MONROIG O, GALINDO A, et al.Molecular and functional characterization and expression of desaturase and elongase genes involved in ω3 LC-PUFA biosynthesis in Sarpa salpa and Pegusa lascaris[C]. Cork: Aquaculture Europe, 2017.
|
| [50] |
GARRIDO D, KABEYA N, BETANCOR M B, et al. Functional diversification of teleost Fads2 fatty acyl desaturases occurs independently of the trophic level[J]. Scientific Reports, 2019, 9: 11199. DOI:10.1038/s41598-019-47709-0 |
| [51] |
施培松.匙吻鲟和鳙的生长、肌肉品质比较及FAS基因克隆与表达[D].博士学位论文.武汉: 华中农业大学, 2013.
|
| [52] |
谢帝芝, 王树启, 游翠红, 等. 鱼类高度不饱和脂肪酸合成的影响因素及其机理[J]. 中国水产科学, 2013, 20(2): 456-466. |
| [53] |
BELL J G, HENDERSON R J, TOCHER D R, et al. Substituting fish oil with crude palm oil in the diet of atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism[J]. The Journal of Nutrition, 2002, 132(2): 222-230. DOI:10.1093/jn/132.2.222 |
| [54] |
BOURAOUI L, SÁNCHEZ-GURMACHES J, CRUZ-GARCIA L, et al. Effect of dietary fish meal and fish oil replacement on lipogenic and lipoprotein lipase activities and plasma insulin in gilthead sea bream (Sparus aurata)[J]. Aquaculture Nutrition, 2011, 17(1): 54-63. DOI:10.1111/j.1365-2095.2009.00706.x |
| [55] |
TORSTENSEN B E, NANTON D A, OLSVIK P A, et al. Gene expression of fatty acid-binding proteins, fatty acid transport proteins (CD36 and FATP) and β-oxidation-related genes in Atlantic salmon (Salmo salar L.) fed fish oil or vegetable oil[J]. Aquaculture Nutrition, 2009, 15(4): 440-451. DOI:10.1111/j.1365-2095.2008.00609.x |
| [56] |
DAS U N.Long-chain polyunsaturated fatty acids[M]//UNDURTI N D.A Perinatal strategy for preventing adult disease: the role of long-chain polyunsaturated fatty acids.New York: Springer, 2002: 136-174.
|
| [57] |
PIRINI M, TESTI S, VENTRELLA V, et al. Blue-back fish:fatty acid profile in selected seasons and retention upon baking[J]. Food Chemistry, 2010, 123(2): 306-314. DOI:10.1016/j.foodchem.2010.04.036 |
| [58] |
FARABEGOLI F, NESCI S, VENTRELLA V, et al. Season and cooking may alter fatty acids profile of polar lipids from blue-back fish[J]. Lipids, 2019, 54(11/12): 741-753. |
| [59] |
ARMSTRONG S G, WYLLIE S G, LEACH D N. Effects of season and location of catch on the fatty acid compositions of some Australian fish species[J]. Food Chemistry, 1994, 51(3): 295-305. DOI:10.1016/0308-8146(94)90030-2 |
| [60] |
LUZIA L A, SAMPAIO G R, CASTELLUCCI C M N, et al. The influence of season on the lipid profiles of five commercially important species of Brazilian fish[J]. Food Chemistry, 2003, 83(1): 93-97. DOI:10.1016/S0308-8146(03)00054-2 |
| [61] |
SUSHCHIK N N, RUDCHENKO A E, GLADYSHEV M I. Effect of season and trophic level on fatty acid composition and content of four commercial fish species from Krasnoyarsk Reservoir (Siberia, Russia)[J]. Fisheries Research, 2017, 187: 178-187. DOI:10.1016/j.fishres.2016.11.016 |
| [62] |
HONEYFIELD D C, MALONEY K O. Seasonal patterns in stream periphyton fatty acids and community benthic algal composition in six high-quality headwater streams[J]. Hydrobiologia, 2015, 744(1): 35-47. DOI:10.1007/s10750-014-2054-7 |
| [63] |
SCHMID M, GUIHÉNEUF F, STENGEL D B. Fatty acid contents and profiles of 16 macroalgae collected from the Irish coast at two seasons[J]. Journal of Applied Phycology, 2014, 26(1): 451-463. DOI:10.1007/s10811-013-0132-2 |
| [64] |
LEE R F. Lipid composition of the copepod Calanus hyperboreas from the Arctic ocean.Changes with depth and season[J]. Marine Biology, 1974, 26(4): 313-318. DOI:10.1007/BF00391515 |
| [65] |
MAYZAUD P, BOUTOUTE M, GASPARINI S, et al. Lipids and fatty acid composition of particulate matter in the North Atlantic:importance of spatial heterogeneity, season and community structure[J]. Marine Biology, 2014, 161(9): 1951-1971. DOI:10.1007/s00227-014-2476-9 |
| [66] |
VAGNER M, ROBIN J H, INFANTE J L Z, et al. Combined effects of dietary HUFA level and temperature on sea bass (Dicentrarchus labrax) larvae development[J]. Aquaculture, 2007, 266(1/2/3/4): 179-190. |
| [67] |
SOMERVILLE C. Direct tests of the role of membrane lipid composition in low-temperature induced photoinhibition and chilling sensitivity in plants and cyanobacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 1995, 92(14): 6215-6218. DOI:10.1073/pnas.92.14.6215 |
| [68] |
王艳, 胡先成, 罗颖. 盐度对鲈鱼稚鱼的生长及脂肪酸组成的影响[J]. 重庆师范大学学报(自然科学版), 2007, 24(2): 62-66. |
| [69] |
CORDIER M, BRICHON G, WEBER J M, et al. Changes in the fatty acid composition of phospholipids in tissues of farmed sea bass (Dicentrarchus labrax) during an annual cycle.Roles of environmental temperature and salinity[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2002, 133(3): 281-288. DOI:10.1016/S1096-4959(02)00149-5 |
| [70] |
SARKER M A A, YAMAMOTO Y, HAGA Y, et al. Influences of low salinity and dietary fatty acids on fatty acid composition and fatty acid desaturase and elongase expression in red sea bream Pagrus major[J]. Fisheries Science, 2011, 77(3): 385-396. DOI:10.1007/s12562-011-0342-y |
| [71] |
许建和, 徐加涛, 林永健, 等. 海水和淡水养殖花鲈肌肉脂肪酸组成和含量分析[J]. 食品科学, 2010, 31(14): 209-211. |
| [72] |
甘雷.不同盐度下尼罗罗非鱼幼鱼的脂肪营养生理研究[D].硕士学位论文.上海: 华东师范大学, 2016.
|
| [73] |
郭振, 梁拥军, 杨广. 改变水体盐度对吉富罗非鱼肌肉营养和呈味的影响[J]. 淡水渔业, 2014, 44(4): 77-82, 95. |
| [74] |
刘文娟, 董亢, 刘骋跃, 等. 盐度驯化对虹鳟鳃、肌肉和肝脏非极性脂脂肪酸的影响[J]. 中国海洋大学学报(自然科学版), 2019, 49(3): 72-78. |
| [75] |
WOITEL F R, TRUSHENSKI J T, SCHWARZ M H, et al. More judicious use of fish oil in cobia feeds:Ⅱ.Effects of graded fish oil sparing and finishing[J]. North American Journal of Aquaculture, 2014, 76(3): 232-241. DOI:10.1080/15222055.2014.893470 |
| [76] |
REIS B, CABRAL E M, FERNANDES T J R, et al. Long-term feeding of vegetable oils to senegalese sole until market size:effects on growth and flesh quality.Recovery of fatty acid profiles by a fish oil finishing diet[J]. Aquaculture, 2014, 434: 425-433. DOI:10.1016/j.aquaculture.2014.09.002 |
| [77] |
IZQUIERDO M S, MONTERO D, ROBAINA L, et al. Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period.Recovery of fatty acid profiles by fish oil feeding[J]. Aquaculture, 2005, 250(1/2): 431-444. |
| [78] |
BELL J G, TOCHER D R, HENDERSON R J, et al. Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet[J]. The Journal of Nutrition, 2003, 133(9): 2793-2801. DOI:10.1093/jn/133.9.2793 |
| [79] |
BELL J G, MCGHEE F, CAMPBELL P J, et al. Rapeseed oil as an alternative to marine fish oil in diets of post-smolt Atlantic salmon (Salmo salar):changes in flesh fatty acid composition and effectiveness of subsequent fish oil "wash out"[J]. Aquaculture, 2003, 218(1/2/3/4): 515-528. |
| [80] |
STONE D A J, OLIVEIRA A C M, ROSS C F, et al. The effects of phase-feeding rainbow trout (Oncorhynchus mykiss) with canola oil and Alaskan pollock fish oil on fillet fatty acid composition and sensory attributes[J]. Aquaculture Nutrition, 2011, 17(2): E521-E529. DOI:10.1111/j.1365-2095.2010.00792.x |
| [81] |
YILDIZ M, EROLDOĞAN T O, OFORI-MENSAH S, et al. The effects of fish oil replacement by vegetable oils on growth performance and fatty acid profile of rainbow trout:re-feeding with fish oil finishing diet improved the fatty acid composition[J]. Aquaculture, 2018, 488: 123-133. DOI:10.1016/j.aquaculture.2017.12.030 |
| [82] |
于若梦.饲料DHA水平对鲤肌肉脂质代谢及其品质的影响[D].硕士学位论文.新乡: 河南师范大学, 2019.
|
| [83] |
HORWITT M K. The promotion of vitamin E[J]. The Journal of Nutrition, 1986, 116(7): 1371-1377. DOI:10.1093/jn/116.7.1371 |
| [84] |
肖伟平, 刘永坚, 田丽霞, 等. 维生素E和维生素C对斜带石斑鱼亲鱼产卵质量的影响[J]. 中山大学学报(自然科学版), 2003, 42(增刊2): 214-217. |
| [85] |
KENARI A A, NADERI M. Effects of enriched Artemia by fish and soybean oils supplemented with vitamin E on growth performance, lipid peroxidation, lipase activity and fatty acid composition of Persian sturgeon (Acipenser persicus) larvae[J]. Aquaculture Nutrition, 2016, 22(2): 382-391. DOI:10.1111/anu.12260 |
| [86] |
MONIRUZZAMAN M, LEE J H, LEE J H, et al. Interactive effect of dietary vitamin E and inorganic mercury on growth performance and bioaccumulation of mercury in juvenile olive flounder, Paralichthys olivaceus treated with mercuric chloride[J]. Animal Nutrition, 2017, 3(3): 276-283. DOI:10.1016/j.aninu.2017.07.001 |
| [87] |
GAO J, KOSHIO S, ISHIKAWA M, et al. Effects of dietary oxidized fish oil with vitamin E supplementation on growth performance and reduction of lipid peroxidation in tissues and blood of red sea bream Pagrus major[J]. Aquaculture, 2012, 356/357: 73-79. DOI:10.1016/j.aquaculture.2012.05.034 |
| [88] |
丁兆坤, 黄金华, 李伟峰, 等. 维生素E和/或柠檬酸添加剂对军曹鱼稚幼鱼ACO和PPARα基因、抗氧化酶活力和多不饱和脂肪酸代谢的影响[J]. 饲料工业, 2018, 39(8): 1-12. |
| [89] |
WANG X Y, QUINN P J. The location and function of vitamin E in membranes (review)[J]. Molecular Membrane Biology, 2000, 17(3): 143-156. DOI:10.1080/09687680010000311 |
| [90] |
LEBOLD K M, JUMP D B, MILLER G W, et al. Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio)[J]. The Journal of Nutrition, 2011, 141(12): 2113-2118. DOI:10.3945/jn.111.144279 |
| [91] |
DING Z K, LI W F, HUANG J H, et al. Dietary alanyl-glutamine and vitamin E supplements could considerably promote the expression of GPx and PPARα genes, antioxidation, feed utilization, growth, and improve composition of juvenile cobia[J]. Aquaculture, 2017, 470: 95-102. DOI:10.1016/j.aquaculture.2016.12.015 |
| [92] |
SHERIDAN M A. Lipid dynamics in fish:aspects of absorption, transportation, deposition and mobilization[J]. Comparative Biochemistry and Physiology Part B:Comparative Biochemistry, 1988, 90(4): 679-690. DOI:10.1016/0305-0491(88)90322-7 |
| [93] |
曹俊明, 林鼎, 劳彩玲, 等. 饲料中添加大豆卵磷脂对草鱼肝胰脏脂质脂肪酸组成的影响[J]. 水产学报, 1997, 21(1): 32-38. |
| [94] |
卢素芳.磷脂在黄颡鱼仔稚鱼人工微粒饲料中应用及其作用机理的研究[D].博士学位论文.武汉: 华中农业大学, 2008.
|
| [95] |
COUTTEAU P, GEURDEN I, CAMARA M R, et al. Review on the dietary effects of phospholipids in fish and crustacean larviculture[J]. Aquaculture, 1997, 155(1/2/3/4): 149-164. |
| [96] |
SALHI M, HERNÁNDEZ-CRUZ C, BESSONART M, et al. Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut and liver histological structure of gilthead seabream (Sparus aurata) larvae[J]. Aquaculture, 1999, 179(1/2/3/4): 253-263. |
| [97] |
LIU J L, CABALLERO M J, IZQUIERDO M S, et al. Necessity of dietary lecithin and eicosapentaenoic acid for growth, survival, stress resistance and lipoprotein formation in gilthead sea bream Sparus aurata[J]. Fisheries Science, 2002, 68(6): 1165-1172. DOI:10.1046/j.1444-2906.2002.00551.x |
| [98] |
HUNG S S O, BERGE G M, STOREBAKKEN T. Growth and digestibility effects of soya lecithin and choline chloride on juvenile Atlantic salmon[J]. Aquaculture Nutrition, 1997, 3(2): 141-144. DOI:10.1046/j.1365-2095.1997.00080.x |
| [99] |
CRAIG S R, GATLIN Ⅲ D M. Growth and body composition of juvenile red drum (Sciaenops ocellatus) fed diets containing lecithin and supplemental choline[J]. Aquaculture, 1997, 151(1/2/3/4): 259-267. |
| [100] |
KASPER C S, BROWN P B. Growth improved in juvenile Nile tilapia fed phosphatidylcholine[J]. North American Journal of Aquaculture, 2003, 65(1): 39-43. DOI:10.1577/1548-8454(2003)065<0039:GIIJNT>2.0.CO;2 |
| [101] |
TOCHER D R, BENDIKSEN E A, CAMPBELL P J, et al. The role of phospholipids in nutrition and metabolism of teleost fish[J]. Aquaculture, 2008, 280(1/2/3/4): 21-34. |
| [102] |
NELSON G J, SHORE V G. Characterization of the serum high density lipoprotein and apolipoproteins of pink salmon[J]. Journal of Biological Chemistry, 1974, 249(2): 536-542. |
| [103] |
CHAPMAN M J. Animal lipoproteins:chemistry, structure, and comparative aspects[J]. Journal of Lipid Research, 1980, 21(7): 789-853. |
| [104] |
佘隽, 田华, 陈涛, 等. 高产DHA寇氏隐甲藻突变株的筛选[J]. 食品科学, 2013, 34(17): 230-235. |
| [105] |
孙春晓, 乔洪金, 王际英, 等. 鱼油与微藻和植物油脂肪酸成分比较及其替代策略分析[J]. 广西科学, 2016, 23(2): 125-130. |
| [106] |
ATALAH E, CRUZ C M H, IZQUIERDO M, et al. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata)[J]. Aquaculture, 2007, 270(1/2/3/4): 178-185. |
| [107] |
PEREZ-VELAZQUEZ M, GATLIN Ⅲ D M, GONZÁLEZ-FÉLIX M L, et al. Effect of fishmeal and fish oil replacement by algal meals on biological performance and fatty acid profile of hybrid striped bass (Morone crhysops♀×M.saxatilis)[J]. Aquaculture, 2019, 507: 83-90. DOI:10.1016/j.aquaculture.2019.04.011 |
| [108] |
乔洪金, 王际英, 张利民, 等. 微粒饲料中以微藻粉替代鱼油对牙鲆(Paralichthys olivaceus)稚鱼生长存活和脂肪酸组成的影响[J]. 渔业科学进展, 2016, 37(5): 56-63. |
| [109] |
STONEHAM T R, KUHN D D, TAYLOR D P, et al. Production of omega-3 enriched tilapia through the dietary use of algae meal or fish oil:improved nutrient value of fillet and offal[J]. PLoS One, 2018, 13(4): e0194241. DOI:10.1371/journal.pone.0194241 |
| [110] |
BETIKU O C, BARROWS F T, ROSS C, et al. The effect of total replacement of fish oil with DHA-Gold and plant oils on growth and fillet quality of rainbow trout (Oncorhynchus mykiss) fed a plant-based diet[J]. Aquaculture Nutrition, 2016, 22(1): 158-169. DOI:10.1111/anu.12234 |
| [111] |
MATSUNARI H, HASHIMOTO H, ODA K, et al. Effect of different algae used for enrichment of rotifers on growth, survival, and swim bladder inflation of larval amberjack Seriola dumerili[J]. Aquaculture International, 2012, 20(5): 981-992. DOI:10.1007/s10499-012-9522-8 |
| [112] |
VESTERGREN A S, TRATTNER S, PAN J F, et al. The effect of combining linseed oil and sesamin on the fatty acid composition in white muscle and on expression of lipid-related genes in white muscle and liver of rainbow trout (Oncorhynchus mykiss)[J]. Aquaculture International, 2013, 21(4): 843-859. DOI:10.1007/s10499-012-9511-y |
| [113] |
TRATTNER S, KAMAL-ELDIN A, BRÄNNAS E, et al. Sesamin supplementation increases white muscle docosahexaenoic acid (DHA) levels in rainbow trout (Oncorhynchus mykiss) fed high alpha-linolenic acid (ALA) containing vegetable oil:metabolic actions[J]. Lipids, 2008, 43(11): 989-997. DOI:10.1007/s11745-008-3228-8 |
| [114] |
TRATTNER S, RUYTER B, ØSTBYE T, et al. Sesamin increases alpha-linolenic acid conversion to docosahexaenoic acid in Atlantic salmon (Salmo salar L.) hepatocytes:role of altered gene expression[J]. Lipids, 2008, 43(11): 999-1008. DOI:10.1007/s11745-008-3229-7 |
| [115] |
杨媚, 马杰, 杨泰, 等. 共轭亚油酸的生物学功能及其在动物生产中的应用[J]. 中国畜牧兽医, 2019, 46(11): 3216-3224. |
| [116] |
MAKOL A, TORRECILLAS S, FERNÁNDEZ-VAQUERO A, et al. Effect of conjugated linoleic acid on dietary lipids utilization, liver morphology and selected immune parameters in sea bass juveniles (Dicentrarchus labrax)[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2009, 154(2): 179-187. DOI:10.1016/j.cbpb.2009.06.001 |
| [117] |
ZHAO Z Y, WU T X, TANG H G, et al. Influence of dietary conjugated linoleic acid on growth, fatty acid composition and hepatic lipogenesis in large yellow croaker (Pseudosciaena crocea R.)[J]. Journal of Zhejiang University:Science B, 2008, 9(9): 691-700. DOI:10.1631/jzus.B0820181 |
| [118] |
KENNEDY S R, BICKERDIKE R, BERGE R K, et al. Influence of dietary conjugated linoleic acid (CLA) and tetradecylthioacetic acid (TTA) on growth, lipid composition and key enzymes of fatty acid oxidation in liver and muscle of Atlantic cod (Gadus morhua L.)[J]. Aquaculture, 2007, 264(1/2/3/4): 372-382. |
| [119] |
BANDARRA N M, NUNES M L, ANDRADE A M, et al. Effect of dietary conjugated linoleic acid on muscle, liver and visceral lipid deposition in rainbow trout juveniles (Oncorhynchus mykiss)[J]. Aquaculture, 2005, 25(1/2/3/4): 496-505. |
| [120] |
孙中武, 李超, 尹洪滨, 等. 不同品系虹鳟的肌肉营养成分分析[J]. 营养学报, 2008, 30(3): 298-302. |
| [121] |
李明云, 郑岳夫, 管丹东, 等. 大黄鱼四家系肌肉营养成分差异及品质选育分析[J]. 水产学报, 2009, 33(4): 632-638. |
| [122] |
林利民, 王秋荣, 王志勇, 等. 不同家系大黄鱼肌肉营养成分的比较[J]. 中国水产科学, 2006, 13(2): 286-291. |
| [123] |
李忠, 梁宏伟, 王忠卫, 等. 四倍体异育银鲫新品种"长丰鲫"肌肉品质和营养成分分析[J]. 水生生物学报, 2016, 40(4): 853-858. |
| [124] |
LE BOUCHER R, DUPONT-NIVET M, VANDEPUTTE M, et al. Selection for adaptation to dietary shifts:towards sustainable breeding of carnivorous fish[J]. PLoS One, 2012, 7(9): e44898. DOI:10.1371/journal.pone.0044898 |
| [125] |
BETANCOR M B, SPRAGUE M, SAYANOVA O, et al. Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.):effects on tissue fatty acid composition, histology and gene expression[J]. Aquaculture, 2015, 444: 1-12. DOI:10.1016/j.aquaculture.2015.03.020 |
| [126] |
BETANCOR M B, SPRAGUE M, USHER S, et al. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish[J]. Scientific Reports, 2015, 5: 8104. DOI:10.1038/srep08104 |
| [127] |
BETANCOR M B, SPRAGUE M, SAYANOVA O, et al. Nutritional evaluation of an EPA-DHA oil from transgenic Camelina sativa in feeds for post-smolt Atlantic salmon (Salmo salar L.)[J]. PLoS One, 2016, 11(7): e0159934. DOI:10.1371/journal.pone.0159934 |
| [128] |
BETANCOR M B, LI K H, BUCERZAN V S, et al. Oil from transgenic Camelina sativa containing over 25% n-3 long-chain PUFA as the major lipid source in feed for Atlantic salmon (Salmo salar)[J]. British Journal of Nutrition, 2018, 119(12): 1378-1392. DOI:10.1017/S0007114518001125 |
| [129] |
BETANCOR M B, LI K H, SPRAGUE M, et al. An oil containing EPA and DHA from transgenic Camelina sativa to replace marine fish oil in feeds for Atlantic salmon (Salmo salar L.):effects on intestinal transcriptome, histology, tissue fatty acid profiles and plasma biochemistry[J]. PLoS One, 2017, 12(4): e0175415. DOI:10.1371/journal.pone.0175415 |
| [130] |
BETANCOR M B, SPRAGUE M, MONTERO D, et al. Replacement of marine fish oil with de novo omega-3 oils from transgenic Camelina sativa in feeds for gilthead sea bream (Sparus aurata L.)[J]. Lipids, 2016, 51(10): 1171-1191. DOI:10.1007/s11745-016-4191-4 |
| [131] |
ALIMUDDIN, YOSHIZAKI G, KIRON V, et al. Expression of masu salmon Δ5-desaturase-like gene elevated EPA and DHA biosynthesis in zebrafish[J]. Marine Biotechnology, 2007, 9(1): 92-100. |
| [132] |
ALIMUDDIN, YOSHIZAKI G, KIRON V, et al. Enhancement of EPA and DHA biosynthesis by over-expression of masu salmon Δ6-desaturase-like gene in zebrafish[J]. Transgenic Research, 2005, 14(2): 159-165. DOI:10.1007/s11248-004-7435-7 |
| [133] |
ALIMUDDIN, KIRON V, SATOH S, et al. Cloning and over-expression of a masu salmon (Oncorhynchus masou) fatty acid elongase-like gene in zebrafish[J]. Aquaculture, 2008, 282(1/2/3/4): 13-18. |
| [134] |
PANG S C, WANG H P, LI K Y, et al. Double transgenesis of humanized fat1 and fat2 genes promotes omega-3 polyunsaturated fatty acids synthesis in a zebrafish model[J]. Marine Biotechnology, 2014, 16(5): 580-593. DOI:10.1007/s10126-014-9577-9 |
| [135] |
KABEYA N, TAKEUCHI Y, YAMAMOTO Y, et al. Modification of the n-3 HUFA biosynthetic pathway by transgenesis in a marine teleost, nibe croaker[J]. Journal of Biotechnology, 2014, 172: 46-54. DOI:10.1016/j.jbiotec.2013.12.004 |
| [136] |
王毅.芝麻素防治非酒精性脂肪肝的作用及机理研究[D].硕士学位论文.合肥: 合肥工业大学, 2012.
|
| [137] |
NAPIER J A, OLSEN R E, TOCHER D R. Update on GM canola crops as novel sources of omega-3 fish oils[J]. Plant Biotechnology Journal, 2019, 17(4): 703-705. DOI:10.1111/pbi.13045 |
| [138] |
KRÖNCKE N, GREBENTEUCH S, KEIL C, et al. Effect of different drying methods on nutrient quality of the yellow mealworm (Tenebrio molitor L.)[J]. Insects, 2019, 10(4): 84. DOI:10.3390/insects10040084 |
| [139] |
PICCOLO G, IACONISI V, MARONO S, et al. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata)[J]. Animal Feed Science and Technology, 2017, 226: 12-20. DOI:10.1016/j.anifeedsci.2017.02.007 |
| [140] |
XU X X, JI H, YU H B, et al. Influence of replacing fish meal with enzymatic hydrolysates of defatted silkworm pupa (Bombyx mori L.) on growth performance, body composition and non-specific immunity of juvenile mirror carp (Cyprinus carpio var. Specularis)[J]. Aquaculture Research, 2018, 49(4): 1480-1490. DOI:10.1111/are.13603 |
| [141] |
段爱莉, 高贵田, 潘静. 气相色谱-质谱联用法对蚕蛹油中脂肪酸成分分析[J]. 蚕桑通报, 2011, 42(1): 14-16. |
| [142] |
李福伟, 王文亮, 李恩霞, 等. GC-MS法测定黄粉虫脂肪酸组成及含量的研究[J]. 食品研究与开发, 2008, 29(10): 92-94. |




