仔猪断奶后消化系统和免疫器官尚没有发育完全,加之断奶过程中环境改变和饲粮摄入改变所带来的应激,断奶导致仔猪对养分消化吸收下降、抗病能力下降、腹泻率增加,最终导致仔猪产生“断奶仔猪应激综合征”[1-2]。饲粮纤维在小肠内不被消化吸收,但可在大肠被发酵,可降低血糖、血脂,预防高血压、高血脂、高血糖及结肠癌等疾病。随着对饲粮纤维结构和功能的深入研究,发现饲粮纤维可改善肠道微生物群落结构及其代谢产物,维持肠道内环境稳态,从而促进营养物质的吸收和改善肠道健康[3-4]。菊粉是一种绿色天然的膳食纤维。菊粉的聚合度一般在2~60,平均聚合度为10[5]。大量水果蔬菜中可以分离出菊粉,如香蕉、芦笋、韭菜和洋葱。目前工业生产菊粉大多数是从菊苣和菊芋中加工提取[6]。作为一种膳食纤维,菊粉在哺乳动物小肠内不能被消化吸收,但在后肠可以被微生物发酵分解[7]。研究表明,肠道细菌发酵膳食纤维会产生大量挥发性脂肪酸(VFA),如乙酸、丙酸和丁酸,可作为肠上皮细胞的能量来源,并可预防各种炎症[8-9]。此外,菊粉可通过增加有益菌群的数量和代谢活性来改善断奶仔猪肠道功能和胃肠道环境[10]。然而到目前为止,菊粉对畜禽抗氧化的研究应用效果还鲜有报道。本试验通过在断奶仔猪基础饲粮中添加不同水平的菊粉,研究菊粉对断奶仔猪生长性能、养分消化率和抗氧化能力的影响,以期为断奶仔猪中菊粉的应用提供理论依据。
1 材料与方法 1.1 试验设计试验采用单因子设计,选取32头平均体重为(7.10±0.20) kg的杜长大(DLY)断奶仔猪,随机分为4组,每组8个重复,每个重复1头猪。对照组饲喂基础饲粮,试验组分别在基础饲粮中添加2.5、5.0和10.0 g/kg的菊粉(等量替代基础饲粮中的玉米)。试验期21 d。
1.2 基础饲粮玉米-豆粕型基础饲粮参照NRC(2012)中7~11 kg断奶仔猪的营养需要进行配制,基础饲粮组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
本试验在四川农业大学动物营养研究所试验场进行。试验使用的圈舍和代谢笼多次冲洗后,用消毒剂消毒,24 h后再次冲洗,然后用2 : 1比例的甲醛与高锰酸钾熏蒸圈室,熏蒸持续24 h,全程门窗密闭,结束后通风3 d。试验期间严格按照断奶仔猪饲养管理饲养,每日饲喂3次(08:00、14:00和20:00),每次喂食遵循多次少量原则,自由饮水。圈舍卫生每日清扫,每周消毒。试验期间早晚观察仔猪精神状态,并记录每日采食量与腹泻情况。
1.4 样品采集 1.4.1 饲粮与粪便样品收集采用四分法收集饲粮样品,每个组取500 g左右,装入样品袋,记号,放入-20 ℃冰箱保存,用于常规养分的分析。在试验第18~21天对所有仔猪采用内源指示剂法进行消化试验,采用部分收粪法收集粪便。
1.4.2 血清样品收集于试验开始后第21天早上,对空腹仔猪前腔静脉采血15 mL,置于普通离心管,室温下放置30 min后,4 ℃、3 000×g离心10 min,分离血清,置于-20 ℃保存,待测血清抗氧化指标。
1.4.3 组织样品收集于试验第21天早上仔猪采血后,立即进行屠宰,取出肝脏、小肠等内脏。采集多份肝脏样品于冻存管中,用锡箔纸包严放入液氮,然后转至-80 ℃冻存。使用生理盐水对小肠段进行冲洗,待洗净残留在肠段上的食糜后,用载玻片将十二肠、空肠、回肠黏膜刮下来,然后置于无菌EP管中,迅速用锡箔纸包严放入液氮,然后转至-80 ℃冻存。
1.5 指标测定 1.5.1 生长性能测定在试验第1天和第21天08:00对仔猪进行空腹称重,统计仔猪每日采食量,分别计算平均日采食量(ADFI)和平均日增重(ADG)。
1.5.2 养分表观消化率测定消化试验采用内源指示剂法,利用盐酸不溶灰分(AIA)为指示剂。饲粮养分表观消化率计算公式为:
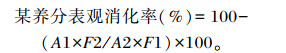
|
式中:F1是饲粮中该养分含量(%);F2是粪中该养分含量(%);A1是饲粮中AIA含量(%);A2是粪中AIA含量(%)。
1.5.3 血清、肝脏和肠道抗氧化指标测定使用南京建成生物工程研究所试剂盒测定仔猪血清、肝脏和肠道中总抗氧化能力(T-AOC)和谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)、总超氧化物歧化酶(T-SOD)活性及丙二醛(MDA)含量,严格按照说明书操作。
1.5.4 肝脏和肠道抗氧化相关基因表达测定总RNA的提取:按照RNA提取试剂盒(Trizol Reagent TaKaRa,日本)严格操作,得到总RNA后,在核酸蛋白仪上取RNA样品测出相应的吸光度(OD)260 nm和OD280 nm,样品检测数值处在1.8≤OD260 nm/OD280 nm≤2.0,用1%琼脂糖凝胶电泳测定RNA的质量和完整性。
反转录:利用反转录得到cDNA,所有步骤严格按照反转录试剂盒[TaKaRa,宝生物工程(大连)有限公司]说明书进行。反转录所用仪器是PCR仪(Bio-Rad DNA Engine,美国),反应共有2步,分别为:反转录反应37 ℃,15 min;反转录酶失活反应85 ℃,5 s。
引物设计:肝脏和肠道抗氧化相关基因[核因子E2相关因子2(nuclear factor-E2-related factor 2,Nrf2)、Kelch样环氧氯丙烷相关蛋白1(Kelch-like epichlorohydrin-associated protein 1,Keap1)和血红素氧合酶-1(heme oxygenase-1,HO-1)]引物序列见表 2。
|
|
表 2 肝脏和肠道抗氧化相关基因引物序列 Table 2 Primer sequences of antioxidant related genes in liver and intestine |
实时荧光定量PCR:使用染料为SYBR Green PCR Mix[TaKaRa,宝生物工程(大连)有限公司],使用仪器是实时荧光定量PCR仪(CFX-96 Real-Time PCR System,Bio-Rad公司,美国)。以β-肌动蛋白(β-actin)为内参基因。用2-ΔΔCt法[11]计算目的基因的mRNA相对表达量。
1.6 数据统计及分析所有试验数据经Excel 2016初步分析后,使用SPSS 22.0统计软件对4组的试验数据进行单因素方差分析,差异显著时进行Duncan氏多重比较,所有试验数据均以“平均值±标准误”表示,P<0.05为差异显著,0.05≤P≤0.10为有趋势。
2 结果与分析 2.1 菊粉对断奶仔猪生长性能的影响由表 3可见,与对照组相比,饲粮添加2.5、5.0和10.0 g/kg菊粉对断奶仔猪ADFI和ADG无显著影响(P>0.05),但饲粮添加2.5 g/kg菊粉后断奶仔猪ADFI和ADG分别提高了12.2%和20.1%。
|
|
表 3 菊粉对断奶仔猪生长性能的影响 Table 3 Effects of inulin on growth performance of weaned piglets |
由表 4可见,与对照组相比,饲粮添加2.5、5.0和10.0 g/kg菊粉可显著提高断奶仔猪粗灰分表观消化率(P < 0.05),饲粮添加2.5和5.0 g/kg菊粉可显著提高断奶仔猪粗蛋白质表观消化率(P < 0.05),饲粮添加2.5、5.0和10.0 g/kg菊粉对断奶仔猪干物质、粗脂肪和能量表观消化率无显著影响(P>0.05)。
|
|
表 4 菊粉对断奶仔猪养分表观消化率的影响 Table 4 Effects of inulin on nutrient apparent digestibilities of weaned piglets |
由表 5可见,与对照组相比,饲粮添加2.5 g/kg菊粉可显著提高血清中T-SOD活性(P < 0.05),但饲粮添加2.5、5.0和10.0 g/kg菊粉对血清中CAT和GSH-Px活性、T-AOC及MDA含量无显著影响(P>0.05);饲粮添加2.5、5.0和10.0 g/kg菊粉可显著降低肝脏中MDA含量(P < 0.05),但饲粮添加2.5、5.0和10.0 g/kg菊粉对肝脏中T-AOC及CAT、T-SOD和GSH-Px活性无显著影响(P>0.05);饲粮添加2.5和5.0 g/kg菊粉可显著提高空肠中CAT活性(P < 0.05),饲粮添加2.5和5.0 g/kg菊粉可显著降低回肠中MDA含量(P < 0.05),饲粮添加2.5 g/kg菊粉可显著提高十二指肠中CAT活性(P < 0.05),但饲粮添加2.5、5.0和10.0 g/kg菊粉对十二指肠、空肠和回肠中T-AOC及T-SOD和GSH-Px活性无显著影响(P>0.05)。
|
|
表 5 菊粉对断奶仔猪血清、肝脏和肠道抗氧化指标的影响 Table 5 Effects of inulin on serum, liver and intestine antioxidant indexes of weaned piglets |
由表 6可见,与对照组相比,饲粮添加2.5、5.0和10.0 g/kg菊粉可显著提高肝脏中Nrf2的mRNA相对表达量(P < 0.05),饲粮添加5.0和10.0 g/kg菊粉可显著提高肝脏中HO-1的mRNA相对表达量(P < 0.05),饲粮添加2.5、5.0和10.0 g/kg菊粉对肝脏中Keap1的mRNA相对表达量无显著影响(P>0.05)。
|
|
表 6 菊粉对断奶仔猪肝脏抗氧化相关基因表达的影响 Table 6 Effects of inulin on expression of liver antioxidant related genes of weaned piglets |
由表 7可见,与对照组相比,饲粮添加2.5、5.0和10.0 g/kg菊粉可显著提高十二指肠中HO-1的mRNA相对表达量和空肠中Nrf2的mRNA相对表达量(P < 0.05),饲粮添加2.5和5.0 g/kg菊粉可显著提高回肠中HO-1和Nrf2的mRNA相对表达量(P < 0.05),饲粮添加2.5 g/kg菊粉可显著提高空肠中Keap1的mRNA相对表达量(P < 0.05),饲粮添加10.0 g/kg菊粉可显著提高空肠中HO-1和Keap1的mRNA相对表达量(P < 0.05)。
|
|
表 7 菊粉对断奶仔猪小肠抗氧化相关基因表达的影响 Table 7 Effects of inulin on expression of intestinal antioxidant-related genes of weaned piglets |
菊粉是一种从菊苣和菊芋等天然植物中提取的可溶性膳食纤维,是一种潜在的绿色饲料添加剂,可用来提高动物的生长性能肠道健康。研究发现,饲粮添加10.0、15.0和20.0 g/kg菊粉均可显著断奶仔猪ADG[12]。周锡红等[13]将含有5%菊粉的饲粮喂给仔猪,结果发现,菊粉可显著提高ADG,并显著降低料重比。在本试验中,饲粮添加不同水平菊粉对断奶仔猪ADFI和ADG无显著影响。与前人试验相比,本试验饲粮中添加菊粉水平较低可能是造成断奶仔猪ADFI和ADG无显著变化的原因。本试验中,饲粮添加菊粉可提高粗蛋白质和粗灰分表观消化率,与前人研究结果[14-16]一致。
通常情况下机体内自身产生的活性氧(ROS)可以被自身酶类清除,因此对机体并无太大影响。但机体受到刺激后产生的活性氧数量会远远超过机体酶类清除能力,机体会出现应激状态[17-19],最终对机体造成不可逆的伤害[20]。断奶仔猪早期断奶破坏了仔猪的机体抗氧化防御系统,导致机体进入应激状态[21]。机体产生的超氧化物歧化酶(SOD)、GSH-Px、CAT等抗氧化酶能够有效清除机体内的自由基[22]。机体自身产生的这些抗氧化酶的活性高低直接反映出机体的抗氧化能力,也关系到机体的健康状况[23]。研究发现,在断奶仔猪饲粮中添加菊粉可以提高血清SOD活性[24]。尚红梅[25]研究发现,饲粮添加菊粉可显著提高血清中SOD活性,显著降低血清中MDA含量。魏轶男等[26]研究发现,肉仔鸡饲粮中添加菊粉可以显著提高肉仔鸡肝脏和血清中SOD、GSH-Px活性,并显著降低肉仔鸡肝脏和血清中MDA含量。本试验中,饲粮添加2.5 g/kg菊粉可显著提高血清中T-SOD活性和十二指肠CAT活性;饲粮添加2.5、5.0和10.0 g/kg菊粉可显著降低肝脏中MDA含量;饲粮添加2.5和5.0 g/kg菊粉可显著提高空肠中CAT活性,降低回肠中MDA含量。这表明饲粮添加菊粉可增加断奶仔猪血清、肝脏和肠道黏膜系统抗氧化酶活性,从而提高断奶仔猪的抗氧化能力。
Nrf2/Keap1是机体细胞内维持氧化还原平衡的重要信号通路之一,同时Nrf2作为一种应激基本表达的关键转录因子,存在于机体多个器官,Nrf2的缺失或激活障碍直接导致细胞对应激源的敏感性变化[27-28]。应激可以损坏细胞内氧化还原平衡,进而激活或抑制一些信号通路和许多信号介导分子,例如Nrf2/Keap1信号通路[29]。HO-1作为Nrf2信号通路下游的重要因子[30],是反映细胞应激状态敏感性的关键指标[31]。前人的研究发现,Nrf2的mRNA表达水平上升,可以提高机体抗氧化酶的活性[32]。本试验研究发现,饲粮添加菊粉可提高肝脏HO-1和Nrf2、十二指肠HO-1和空肠Nrf2的mRNA相对表达量。这表明饲粮添加菊粉可以通过活化Nrf2通路增强断奶仔猪抗氧化酶活性,进而提高断奶仔猪的抗氧化能力。
4 结论饲粮添加菊粉对断奶仔猪生长性能无显著影响,但可改善养分表观消化率,提高抗氧化能力。本试验条件下,断奶仔猪饲粮中菊粉适宜添加水平为2.5 g/kg。
| [1] |
TAN B, LI X G, KONG X F, et al. Dietary L-arginine supplementation enhances the immune status in early-weaned piglets[J]. Amino Acids, 2009, 37(2): 323-331. DOI:10.1007/s00726-008-0155-1 |
| [2] |
张振斌, 蒋宗勇, 林映才, 等. 超早期断奶应激对仔猪消化酶活性的影响初报[J]. 中国畜牧杂志, 1999, 35(6): 6-7. ZHANG Z B, JIANG Z Y, LIN Y C, et al. Effect of stress from early weaning pig on its digestive enzymes activity[J]. Chinese Journal of Animal Science, 1999, 35(6): 6-7 (in Chinese). DOI:10.3969/j.issn.0258-7033.1999.06.002 |
| [3] |
KNUDSEN K E B, HEDEMANN M S, LÆRKE H N. The role of carbohydrates in intestinal health of pigs[J]. Animal Feed Science and Technology, 2012, 173(1/2): 41-53. |
| [4] |
KNUDSEN K B.Nutritional and functional properties of fiber in swine diets[C]//Proceedings of the midwest swine nutrition conference.Indianapolis: [s.n.], 2014: 35.
|
| [5] |
SMITS G, DE LEENHEER L.Process for the manufacture of chicory inulin, hydrolysates and derivatives of inulin, and improved chicory inulin products, hydrolysates and derivatives: EP, 99802251.9[P]. 1999-01-13.
|
| [6] |
VAN LOO J, COUSSEMENT P, DE LEENHEER L, et al. On the presence of inulin and oligofructose as natural ingredients in the western diet[J]. Critical Reviews in Food Science and Nutrition, 1995, 35(6): 525-552. DOI:10.1080/10408399509527714 |
| [7] |
ROBERFROID M B, VAN LOO J A, GIBSON G R. The bifidogenic nature of chicory inulin and its hydrolysis products[J]. The Journal of Nutrition, 1998, 128(1): 11-19. DOI:10.1093/jn/128.1.11 |
| [8] |
FLINT H J, SCOTT K P, LOUIS P, et al. The role of the gut microbiota in nutrition and health[J]. Nature Reviews Gastroenterology & Hepatology, 2012, 9(10): 577-589. |
| [9] |
GAO Z G, YIN J, ZHANG J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice[J]. Diabetes, 2009, 58(7): 1509-1517. DOI:10.2337/db08-1637 |
| [10] |
MAIR C, PLITZNER C, DOMIG K J, et al. Original article:impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets[J]. Journal of Animal Physiology and Animal Nutrition, 2010, 94(5): e164-e177. DOI:10.1111/j.1439-0396.2010.01000.x |
| [11] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [12] |
王中华, 周德忠. 菊粉对断奶仔猪生长性能和免疫功能的作用研究[J]. 饲料工业, 2011, 32(24): 36-38. WANG Z H, ZHOU D Z. Effects of inulin on growth performance and immune function of weaned piglets[J]. Feed Industry, 2011, 32(24): 36-38 (in Chinese). DOI:10.3969/j.issn.1001-991X.2011.24.011 |
| [13] |
周锡红, 吴信, 燕富永, 等. 菊粉和刺五加提取物对早期断奶仔猪生长性能、血液指标和肠道形态的影响[J]. 天然产物研究与开发, 2010, 22(6): 1103-1108. ZHOU X H, WU X, YAN F Y, et al. Effects of inulin and Acanthopanax senticosus extract on growth performance, serum biochemical parameters and intestinal morphology of early-weaned piglets[J]. Natural Product Research and Development, 2010, 22(6): 1103-1108 (in Chinese). DOI:10.3969/j.issn.1001-6880.2010.06.046 |
| [14] |
吕知谦, 黄冰冰, 李藏兰, 等. 日粮纤维组成对生长猪净能和营养物质消化率的影响[J]. 中国畜牧杂志, 2017, 53(2): 65-69. LV Z Q, HUANG B B, LI Z S. Effects of dietary fiber on net energy value and total tract digestibility of nutrients of growing pigs[J]. Chinese Journal of Animal Science, 2017, 53(2): 65-69 (in Chinese). |
| [15] |
CHEN L, ZHANG H F, GAO L X, et al. Effect of graded levels of fiber from alfalfa meal on intestinal nutrient and energy flow, and hindgut fermentation in growing pigs[J]. Journal of Animal Science, 2013, 91(10): 4757-4764. DOI:10.2527/jas.2013-6307 |
| [16] |
HESTA M, JANSSENS G P J, DEBRAEKELEER J, et al. The effect of oligofructose and inulin on faecal characteristics and nutrient digestibility in healthy cats[J]. Journal of Animal Physiology and Animal Nutrition, 2001, 85(5/6): 135-141. |
| [17] |
MIYAMOTO H, DOITA M, NISHIDA K, et al. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro[J]. Spine, 2006, 31(1): 4-9. DOI:10.1097/01.brs.0000192682.87267.2a |
| [18] |
MADSEN K G, OLSEN J, SKONBERG C, et al. Development and evaluation of an electrochemical method for studying reactive phase-Ⅰ metabolites:correlation to in vitro drug metabolism[J]. Chemical Research in Toxicology, 2007, 20(5): 821-831. DOI:10.1021/tx700029u |
| [19] |
PI J B, ZHANG Q, FU J Q, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function[J]. Toxicology and Applied Pharmacology, 2010, 244(1): 77-83. DOI:10.1016/j.taap.2009.05.025 |
| [20] |
REUTER S, GUPTA S C, CHATURVEDI M M, et al. Oxidative stress, inflammation, and cancer:how are they linked[J]. Free Radical Biology and Medicine, 2010, 49(11): 1603-1616. |
| [21] |
TSANG A H K, CHUNG K K K. Oxidative and nitrosative stress in Parkinson's disease[J]. Biochimica et Biophysica Acta:Molecular Basis of Disease, 2009, 1792(7): 643-650. |
| [22] |
VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radicals and antioxidants in normal physiological functions and human disease[J]. The International Journal of Biochemistry & Cell Biology, 2007, 39(1): 44-84. |
| [23] |
ANDREAZZA A C, KAUER-SANT'ANNA M, FREY B N, et al. Oxidative stress markers in bipolar disorder:a meta-analysis[J]. Journal of Affective Disorders, 2008, 111(2/3): 135-144. |
| [24] |
花城, 陈立祥, 燕富永, 等. 菊粉对断奶仔猪血液生化指标的影响[J]. 中国饲料, 2008(14): 27-29. HUA C, CHEN L X, WU F Y. Effects of inulin on blood biochemical indexes of weaned piglets[J]. China Feed, 2008(14): 27-29 (in Chinese). DOI:10.3969/j.issn.1004-3314.2008.14.010 |
| [25] |
尚红梅.菊苣菊粉的纯化与活性研究[D].硕士学位论文.咸阳: 西北农林科技大学, 2007. SHANG H M.Purification and activity of chicory inulin[D]. Master's Thesis.Xianyang: Northwest Agricultural and Forestry University, 2007.(in Chinese) |
| [26] |
魏轶男, 黄倩倩, 吕亚军, 等. 菊粉对肉仔鸡生长性能、免疫器官指数及抗氧化指标的影响[J]. 西北农林科技大学学报(自然科学版), 2013, 41(11): 13-18. WEI Y N, HUANG Q Q, LYU Y J, et al. Effects of inulin on growth performance, immune organs indexes and antioxidantion of broilers[J]. Journal of Northwest A & F University (Natural Science Edition), 2013, 41(11): 13-18 (in Chinese). |
| [27] |
STEPKOWSKI T M, KRUSZEWSKI M K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis[J]. Free Radical Biology and Medicine, 2011, 50(9): 1186-1195. DOI:10.1016/j.freeradbiomed.2011.01.033 |
| [28] |
CHENG L, JIN Z X, ZHAO R, et al. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury:role of Nrf2/ARE pathway[J]. International Journal of Clinical and Experimental Medicine, 2015, 8(7): 10420-10428. |
| [29] |
SYKIOTIS G P, HABEOS I G, SAMUELSON A V, et al. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2011, 14(1): 41-48. DOI:10.1097/MCO.0b013e32834136f2 |
| [30] |
BAO L P, LI J S, ZHA D Q, et al. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-κB pathways[J]. International Immunopharmacology, 2018, 54: 245-253. |
| [31] |
KIM Y M, PAE H O, PARK J E, et al. Heme oxygenase in the regulation of vascular biology:from molecular mechanisms to therapeutic opportunities[J]. Antioxidants & Redox Signaling, 2011, 14(1): 137-167. |
| [32] |
WEN H L, FENG L, JIANG W D, et al. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella)[J]. Fish & Shellfish Immunology, 2014, 40(1): 275-287. |




