奶牛围产期包括围产前期(产前3周)和围产后期(产后3周),是泌乳周期中重要的转折期。奶牛此时面临妊娠后期胎儿快速生长和产后泌乳启动,营养和能量需求增加;激素水平也会随生理快速转变而变化,适应并调节机体的生理转变过程,围产期将面临巨大挑战[1-2]。精准营养调控有助于改善围产期奶牛健康[3-4]。围产前能量负平衡(negative energy balance,NEB)是主要生理特征之一,是奶牛免疫抑制、机体脂质代谢紊乱和氧化应激等诱因。为满足母体和胎儿在围产期营养需求,奶牛将动员自身体脂提供能量,体脂动员产生大量的游离脂肪酸(non-esterified fatty acid,NEFA)进入组织和器官供能;氧化供能过程中产生大量的氧自由基,超出机体清除能力时将处于氧化应激状态。围产期营养需要量增加而供给匮乏,抗氧化酶体系和非酶体系(硒、维生素A、维生素C、维生素E等)易受机体微量元素含量的影响,围产期奶牛氧化应激与该时期营养供给和能量供应存在必然联系。
1 围产期氧化应激氧化应激是机体内氧化系统和抗氧化系统的一种失衡状态,氧化产物增加或抗氧化能力下降都将引发氧化应激。氧化应激发生与氧化产物生成(脂质氧化、氧化磷酸化和细胞有氧呼吸等过程)和抗氧化能力(抗氧化酶体系和非酶体系)等息息相关,围产期奶牛糖脂代谢状况见图 1,泌乳启动后,大量葡萄糖用于乳糖合成,产后奶牛泌乳高峰期提早于采食量高峰期,将导致机体缺乏肝脏糖异生底物,糖异生能力降低是奶牛围产期处于NEB因素之一[5]。围产期NEB是奶牛机体脂质代谢紊乱、免疫抑制和氧化应激等的诱因。围产期因特殊的生理需求和代谢,机体内的脂质代谢和能量供应等均发生变化,氧化产物生成受到以上代谢途径的影响。
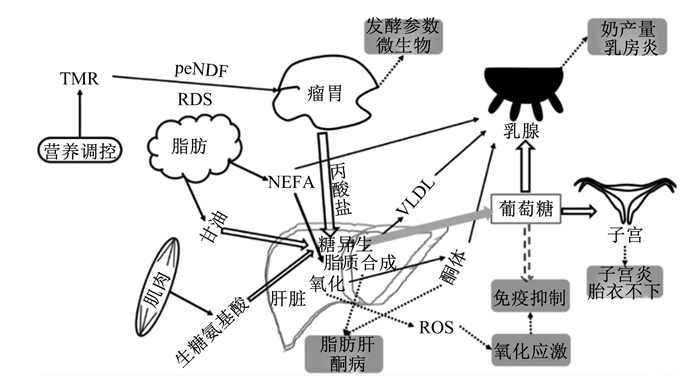
|
TMR:全混合日粮total mixed ration;RDS:瘤胃可降解淀粉rumen degradable starch;peNDF:物理中性有效洗涤纤维physical neutral effective washing fiber;VLDL:极低密度脂蛋白very low density lipoprotein;NEFA:游离脂肪酸non-esterified fatty acid;ROS:活性氧reactive oxygen species。 图 1 围产期奶牛糖脂代谢 Fig. 1 Glucolipid metabolism of dairy cow during transition period[2, 5-9] |
奶牛围产期能量需求增加将加快肝脏糖异生和脂质动员过程,氧化产物生成使自由基产生,处于一种氧化应激状态,氧化应激是机体代谢适应NEB状态的结果[10]。奶牛通过动员体脂以满足自身能量需求,缓解围产期NEB,脂肪动员的结果是血液NEFA含量增加,随着血液NEFA含量升高,奶牛外周血免疫球蛋白M合成下降,新陈代谢加快,将导致活性氧(reactive oxygen species,ROS)产量增加[11-12]。高NEFA含量可降低奶牛原代肝细胞抗氧化酶超氧化物歧化酶(superoxide dismutase,SOD)、过氧化氢酶(catalase,CAT)和谷胱甘肽过氧化物酶(glutathion peroxidase,GSH-Px)基因表达,增加丙二醛(MDA)和过氧化氢含量,加剧机体氧化应激[13];NEB将导致奶牛体脂持续动员,机体将长期处于高代谢状态[14],机体持续脂质动员可诱发氧化应激发生[1]。机体处于氧化应激状态可引发炎症反应,严重时可损坏DNA,造成细胞凋亡[15];妊娠、分娩和泌乳启动相关的代谢将增加ROS的产生,ROS可引发脂质过氧化,造成组织细胞损伤[16-17]。机体产生ROS过量时,可上调氧化还原信号通路,进而影响炎症因子表达,引发机体发生胰岛素抵抗[18]。当体内抗氧化能力过强时,机体将处于还原应激,目前有关氧化还原稳态的评定还没有明确的指标界定范围[19],有关氧化应激的探究处于相对水平的评价,尚无法实现绝对水平的评价。目前关于抗氧化能力的评价多基于单一抗氧化酶活性变化进行评估,单一抗氧酶活性变化无法呈现机体整体的抗氧化能力[20],抗氧化酶活性之间的比值可辅助判断机体清除自由基的能力[21]。氧化应激因氧化产物生成与清除不平衡所引起,使用抗氧化能力与氧化产物含量间的比值进行氧化应激评定可能更合理。未来有关氧化应激的研究应深入探讨其相关指标间的绝对比值,以便于更好地评估氧化应激状态。
氧化应激是奶牛围产期多种疾病的诱因[22];氧化应激与奶牛的酮病、乳房炎和胎衣不下等疾病相关[23-25]。围产期高发疾病如乳房炎和子宫炎等均伴随体温升高[26],但氧化应激发生无明显的临床表现,在动物生产易被忽视,奶牛围产期的氧化应激是机体潜在的危害[9, 27-28],缓解围产期的氧化应激可降低产后相关疾病发病率。添加抗氧化剂是目前生产中缓解氧化应激最常用的方式,已被证实具有抗氧化功能的添加剂包括维生素E、硒和蛋氨酸等。
围产期氧化应激可通过外源添加营养素缓解,围产期补饲15 g/d过瘤胃蛋氨酸或15 g/d过瘤胃胆碱可提高奶牛产后采食量和产后泌乳性能,同时可调节肝脏脂质代谢缓解NEB,进而改善抗氧化状态及免疫功能[29]。烟酰胺(nicotinamide,NAM)作为辅酶Ⅰ烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD+)和辅酶Ⅱ烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide-adenine dinucleotide phosphate,NADP+)的前体物质,已被证实可参与多条氧化应激通路,前期研究发现奶山羊围产期补饲NAM(5 g/d)可调控糖脂代谢及能量供应,改善机体抗氧化功能,且母体的调控效应在子代中有所体现,改善子代的健康状况[30-31]。NAM可改善围产期的氧化应激状态,下文将具体阐述其调控机理。
2 NAM缓解奶牛氧化应激的调控机理 2.1 调节糖代谢奶牛能量供应的70%来自肝脏糖异生,围产期糖异生作用减弱,能量供应不足,易引发氧化应激,缓解NEB可改善围产期应激状态[32],增强糖异生途径缓解围产期NEB,可通过以下4种方式:1)外源提供糖异生底物;2)驱使瘤胃趋向丙酸型发酵;3)外源提供葡萄糖;4)提高内源能量供应效率。奶牛围产期能量优先供给胎儿及泌乳,同时肝脏对胰岛素的敏感性降低。围产期多发生胰岛素抵抗现象用于维持机体血糖平衡,分娩前后,高胰岛素水平将干扰强氧化酶还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶4的信号传递,增加ROS生成。同时ROS通过促进葡萄糖转运载体4(GLUT4)转位到溶酶体,并诱导细胞应激途径,促进胰岛素抵抗,氧化应激与胰岛素抵抗之间相互作用[33]。
围产期奶牛外源添加NAM增加血浆葡萄糖浓度;奶牛血清代谢组学发现补充NAM(45 g/d)可通过调节氨基酸和碳水化合物等代谢途径,促进氨基酸代谢,提高糖异生供能效率,进而改善机体氧化应激状态[34-35]。NAM可作为NAD+和NADP+的前体物质发挥其生理功能。高浓度NAM处理大鼠试验中发现,肝脏组织中烟酰胺磷酸核糖转移酶、NAD+和沉默调节蛋白(SIRT)1、2、3、6 mRNA表达以及NAD+/还原型烟酰胺腺嘌呤二核苷酸(NADH)比值、SIRT1活性均升高,进而增加辅激活因子1、增殖体激活受体和线粒体DNA表达水平,NAM可能通过改变线粒体生物功能调节葡萄糖代谢和NAD+-SIRT通路[36]。NAM可能通过调节线粒体功能、葡萄糖代谢和减缓胰岛素抵抗等途径缓解氧化应激。过氧化氢诱导的人皮肤成纤维细胞氧化应激模型中发现,NAM能保护糖酵解和氧化磷酸化,NAM通过加快糖酵解速率保护细胞代谢免受氧化应激的损伤,其保护机制可能与NAD+合成相关[37]。NAD+作为辅酶在氧化应激反应中发挥重要作用,氧化应激严重时将导致细胞核内聚腺苷二磷酸-核糖聚合酶-1(PARP-1)过度活化,NAD+在此过程被消耗,导致凋亡诱导因子释放。NAM是机体内合成NAD+的主要来源,在人原代角质细胞培养中加入烟酰胺磷酸核糖转移酶的抑制剂(FK866),NAM可缓解因此引起的糖酵解和氧化磷酸化[38]。补饲NAM可预防由于氧化应激造成的NAD+缺乏。
NAM通过调解葡萄糖代谢改善机体的能量供应,进而缓解氧化应激,奶牛围产期NAM的补充可能通过加快线粒体代谢、氨基酸代谢、糖异生途径等提高内源能量供应效率,提高内源葡萄糖的供能效率,缓解NEB。但线粒体氧化磷酸化供能过程中伴有超氧阴离子的泄露,NAM添加量增强此过程,因此导致的氧化产物累积是否将被动引发机体抗氧化能力的提升或超出机体的清除能力还需要进一步观测和探讨。
2.2 调节脂质代谢脂肪组织动员产生甘油和NEFA可进入肝脏和其他组织供能,大量的NEFA进入肝脏代谢,少量的NEFA可被乳腺利用合成乳脂,脂肪酸可间接修饰细胞内的信号,改变脂质生物合成,诱导氧化应激,围产期反刍动物维护肝脏健康有助于脂质代谢的调控[39]。奶牛原代肝细胞培养研究发现高浓度的NEFA可诱发酮病,增加肝细胞内ROS含量并诱导细胞氧化损伤,通过激活c-Jun N端激酶(c-jun N-terminal kinases,JNK)并抑制胞外信号调控激酶(extracellular signal-regulated kinases,ERK)的表达,介导线粒体途径ROS-JNK/ERK信号通路造成肝细胞凋亡[40]。NEFA进入肝脏可参与不同代谢途径,可作为细胞器(内质网等)合成代谢途径和线粒体氧化的能量底物[8]。线粒体中NEFA可减缓电子传递链中的电子流通量;作为能量底物参与β氧化过程,均可增加ROS生成[41-42]。
围产期奶牛补饲烟酸可降低血浆NEFA含量,缓解NEB并提高初乳品质[43]。围产期添加NAM(45 g/d)可调控奶牛脂质代谢;奶牛血清代谢组学发现补充NAM可通过碳水化合物和脂肪酸等代谢途径,改善氧化应激状态[34-35]。前期研究发现,NAM可通过代谢提高NAD+/NADH比值和诱导SIRT1活化实现线粒体自噬,促进细胞健康[44]。棕榈酸酯诱导肝细胞内质网应激模型中,补充NAM可通过上调SIRT1缓解内质网应激,NAM抑制磷酸二酯酶(PDE)活性激活环磷酸腺苷/蛋白激酶A/环磷酸腺苷反应元件结合蛋白(cAMP/PKA/CREB)途径刺激SIRT1活化,NAM可通过充当NAD+增强剂或SIRT1激活剂抗脂毒性[45-46]。NAM在线粒体功能及线粒体氧化磷酸化过程中均发挥一定作用,NAD+的补充可预防或逆转非酒精性脂肪肝相关表型,如肝脂质蓄积和肝纤维化等,通过上调过氧化物酶体增殖剂激活受体γ(PPARγ)mRNA水平及其下游转录基因,线粒体激活和抗氧化能力增强或氧化磷酸化的继发性作用导致脂肪生成基因的变化[47]。NAM抑制脂质氧化在医学领域已被广泛证明,围产期补饲NAM可通过抑制脂肪组织的动员,减少NEFA的生成,缓解氧化应激。NAM可能通过调节线粒体功能、改变脂质代谢和氧化磷酸化水平缓解氧化应激潜在因素,其具体的机制需进一步探究。
2.3 参与氧化应激代谢通路NAM是烟酸(维生素B3)的酰胺形式,分子式C6H6N2O,在体内来源于烟酸转化和色氨酸转化途径补充。烟酸与NAM的生理作用存在差异,NAM作为NAD+和NADP+氧化还原组分,催化不同代谢过程的电子转移,NAD+/NADH比值可作为监测氧化还原的敏感指标之一,影响线粒体的转化通透性[48]。NADH是许多氧化还原反应中辅酶,氧化应激是机体内的综合反映,细胞凋亡是氧化应激的主要特征之一,NAM与视黄醇协同可诱导ERK活化,阻滞氧化代谢和G0细胞周期[49],NAM与线粒体功能和衰老相关[50],依赖SIRT介导细胞凋亡,SIRT活性不足是NAD+水平降低的主要因素[51],促进细胞衰老的p53基因乙酰化可由NAD+依赖性的去乙酰化酶SIRT1调节,NAM不会增加肝脏NAD+或NADP+水平,但是通过饮食和NAM剂量依赖性的方式补充NAM可以增强某些SIRT1靶标的乙酰化作用[52]。同时NAM可通过维持细胞内钙离子(Ca2+)水平和稳定线粒体膜电位,进而抑制细颗粒物(PM2.5)诱导的人原代角质细胞凋亡,同时可参与氧化应激通路,通过降低NADP/NADPH比值抑制ROS生成,并阻止氧化应激诱导的分子损伤(脂质过氧化、蛋白质羰基化和DNA修饰)[53]。NADPH是细胞质和线粒体抗氧化剂系统的主要电子供体,补饲NAM的高脂饮食喂养的小鼠肝脏中,NAM通过糖酵解和磷酸戊糖途径(PP途径)增强葡萄糖的摄取和糖原内部贮藏物的降解进而增加葡萄糖分解代谢。在肝细胞(HepG2)中,约50%的葡萄糖通过氧化磷酸化途径被氧化,其余的作为乳酸排泄出去,通过PP途径的葡萄糖量含量显着提高。NAM对葡萄糖稳态调节进而改善细胞质氧化还原稳态,可能通过增加的PP途径衍生的NADPH[52]。一氧化氮(nitric oxide,NO)作为活性氮簇的重要组成,是引发机体内氧化应激的因素之一,PARP-1可以直接通过结合到某些基因,如同种型一氧化氮合酶(inducible nitric oxide synthase,iNOS)和趋化因子(chemokine,CXC)配体1的启动子影响基因表达[54-55],增加NO产量,引发氧化应激,NAM可能具有自身抗氧化特性或通过抑制PARP-1间接改善细胞氧化还原环境[56]。NAM具有传递氢的作用,可参与体内多条氧化应激通路,高剂量NAM保护细胞免受氧化应激的损害[57]。NAM对促进NAD+氧化还原平衡至关重要[48],体外研究发现人纤维细胞添加NAM可显著降低ROS产生[58]。氧化应激状态是氧化产物含量与抗氧化酶的平衡状态,许多病理模型中NAM均可通过降低氧化产物含量或提高抗氧化酶活性缓解氧化应激:NAM处理对乙酰氨基酚诱导大鼠肝脏损伤模型,可降低氧化产物MDA含量并提高CAT、GSH-Px活性[59];阿尔兹海默症大鼠注射NAM可降低MDA含量并提高CAT、SOD和GSH-Px活性[60];妊娠期糖尿病大鼠补饲NAM可提高其抗氧化酶活性,进而缓解氧化应激[61]。
关于维生素B3在奶牛围产期的应用,多以饲粮中补饲烟酸的形式进行探究[62-66]。NAM与烟酸在前期的研究中均表明具有提高围产期抗氧化的作用(表 1)。烟酸进入机体可转化为NAM,二者部分作用功能相同,均可参与多种生化反应,如组织呼吸的氧化作用、脂质代谢和糖原分解等。但NAM进入不同组织转化为NAD+的程度不一致[67],目前关于检测牛奶中NAD+含量的技术也有所关注[68]。NAM进入机体转化为NAD+的程度和其过瘤胃效率还需要进一步检测。在以往的研究中关于维生素B3的添加形式和添加剂量存在很大差别,过瘤胃烟酸的添加剂量甚至高于未经瘤胃保护的烟酸,维生素B3的应用形式及添加剂量还需进一步探索和规范。
|
|
表 1 围产期补饲烟酸或NAM对奶牛抗氧化功能的影响 Table 1 Effects of supplement of nicotinic acid or NAM on antioxidant function of dairy cows during transition period |
围产期是奶牛生产中非常重要的时期,包括自身健康和犊牛的生长发育状况,此时期奶牛面临巨大的生理变化、NEB、免疫抑制和氧化应激等因素,导致产后疾病高发,精准的营养调控有助于改善该时期的健康状况。NAM可调控奶牛肝脏健康并缓解NEB,且在改善氧化应激状态过程中发挥重要作用,其具体的添加形式及添加量需要进一步探讨。未来的研究可集中于以下几点:1)NAM形式及添加量的规范。基础饲粮中精准其添加形式、过瘤胃效率和与烟酸的作用效果的区别。2)解析NAM调节肝脏代谢的信号通路。探讨其作用于肝脏的关键信号通路,系统解析其具体的作用机理,以氧化磷酸化为核心出发点研究其调控机体氧化应激的作用途径及机制。3)挖掘NAM在母子营养一体化中的潜力。进一步探讨母体供应NAM对犊牛发育、生理代谢及健康状况的调节,探究其潜在的作用及机制。
| [1] |
KUHLA B. Review: pro-inflammatory cytokines and hypothalamic inflammation: implications for insufficient feed intake of transition dairy cows[J]. Animal, 2020, 14(S1): s65-s77. |
| [2] |
ESPOSITO G, IRONS P C, WEBB E C, et al. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows[J]. Animal Reproduction Science, 2014, 144(3/4): 60-71. |
| [3] |
BOGADO PASCOTTINI O, PROBO M, LEBLANC S J, et al. Assessment of associations between transition diseases and reproductive performance of dairy cows using survival analysis and decision tree algorithms[J]. Preventive Veterinary Medicine, 2020, 176: 104908. DOI:10.1016/j.prevetmed.2020.104908 |
| [4] |
CARDOSO F C, KALSCHEUR K F, DRACKLEY J K. Symposium review: nutrition strategies for improved health, production, and fertility during the transition period[J]. Journal of Dairy Science, 2020, 103(6): 5684-5693. DOI:10.3168/jds.2019-17271 |
| [5] |
LARSEN M, KRISTENSEN N B. Precursors for liver gluconeogenesis in periparturient dairy cows[J]. Animal, 2013, 7(10): 1640-1650. DOI:10.1017/S1751731113001171 |
| [6] |
SORDILLO L M. Nutritional strategies to optimize dairy cattle immunity[J]. Journal of Dairy Science, 2016, 99(6): 4967-4982. DOI:10.3168/jds.2015-10354 |
| [7] |
WULLEPIT N, HOSTENS M, GINNEBERGE C, et al. Influence of a marine algae supplementation on the oxidative status of plasma in dairy cows during the periparturient period[J]. Preventive Veterinary Medicine, 2012, 103(4): 298-303. DOI:10.1016/j.prevetmed.2011.09.007 |
| [8] |
CONTRERAS G A, SORDILLO L M. Lipid mobilization and inflammatory responses during the transition period of dairy cows[J]. Comparative Immunology Microbiology and Infectious Diseases, 2011, 34(3): 281-289. DOI:10.1016/j.cimid.2011.01.004 |
| [9] |
CEIL P. Biomarkers of oxidative stress in ruminant medicine[J]. Immunopharmacology and Immunotoxicology, 2011, 33(2): 233-240. DOI:10.3109/08923973.2010.514917 |
| [10] |
REUTER S, GUPTA S C, CHATURVEDI M M. Oxidative stress, inflammation, and cancer: how are they linked?[J]. Free Radical Biology & Medicine, 2010, 49(11): 1603-1616. |
| [11] |
LACETERA N, SCALIA D, BERNABUCCI U. Lymphocyte functions in overconditioned cows around parturition[J]. Journal of Dairy Science, 2005, 88(6): 2010-2016. DOI:10.3168/jds.S0022-0302(05)72877-0 |
| [12] |
ABUELO A, HERNÁNDEZ J, BENEDITO J L, et al. The importance of the oxidative status of dairy cattle in the periparturient period: revisiting antioxidant supplementation[J]. Journal of Animal Physiology and Animal Nutrition, 2015, 99(6): 1003-1016. DOI:10.1111/jpn.12273 |
| [13] |
刘兆喜. NEFA和BHBA对酮病奶牛氧化应激状态的影响[D]. 硕士学位论文. 长春: 吉林大学, 2013. LIU Z X. Effects of NEFA and BHBA on oxidative stress status of ketosis in dairy cows[D]. Master's Thesis. Changchun: Jilin University, 2013. (in Chinese) |
| [14] |
孙博非, 余超, 曹阳春, 等. 奶牛围产期饲粮营养平衡和机体营养生理状况评价体系[J]. 动物营养学报, 2018, 30(1): 14-21. SUN B F, YU C, CAO Y C, et al. A comprehensive evaluation system of dietary nutrient balance and characteristics of nutrition and physiology in transition dairy cows[J]. Chinese Journal of Animal Nutrition, 2018, 30(1): 14-21 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.01.003 |
| [15] |
SCHIEBER M, CHANDEL N S. ROS function in redox signaling and oxidative stress[J]. Current Biology, 2014, 24(10): R453-R462. DOI:10.1016/j.cub.2014.03.034 |
| [16] |
MCFADDEN J W. Review: lipid biology in the periparturient dairy cow: contemporary perspectives[J]. Animal, 2020, 14(S1): s165-s175. |
| [17] |
MASSCHELIN P M, COX A R, CHERNIS N, et al. The impact of oxidative stress on adipose tissue energy balance[J]. Frontiers in Physiology, 2019, 10: 1638. DOI:10.3389/fpls.2019.01638 |
| [18] |
EVANS J L, GOLDFINE I D, MADDUX B A, et al. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes[J]. Endocrine Reviews, 2002, 23(5): 599-622. |
| [19] |
ZINI A, AGARWAL A. A clinician's guide to sperm DNA and chromatin damage[M]. Springer Cham: Switzerland, 2018.
|
| [20] |
BERNABUCCI U, RONCHI B, LACETERA N, et al. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season[J]. Journal of Dairy Science, 2002, 85(9): 2173-2179. DOI:10.3168/jds.S0022-0302(02)74296-3 |
| [21] |
RADIN L, ŠIMPRAGA M, VINCE S, et al. Metabolic and oxidative status of Saanen goats of different parity during the peripartum period[J]. The Journal of Dairy Research, 2015, 82(4): 426-433. DOI:10.1017/S0022029915000552 |
| [22] |
ABUELO A, HERNÁNDEZ J, BENEDITO J L, et al. Redox biology in transition periods of dairy cattle: role in the health of periparturient and neonatal animals[J]. Antioxidants, 2019, 8(1): 20. DOI:10.3390/antiox8010020 |
| [23] |
DU X L, CHEN L, HUANG D, et al. Elevated apoptosis in the liver of dairy cows with ketosis[J]. Cellular Physiology and Biochemistry, 2017, 43(2): 568-578. DOI:10.1159/000480529 |
| [24] |
SILANIKOVE N, MERIN U, SHAPIRO F, et al. Subclinical mastitis in goats is associated with upregulation of nitric oxide-derived oxidative stress that causes reduction of milk antioxidative properties and impairment of its quality[J]. Journal of Dairy Science, 2014, 97(6): 3449-3455. DOI:10.3168/jds.2013-7334 |
| [25] |
SORDILLO L M. Factors affecting mammary gland immunity and mastitis susceptibility[J]. Livestock Production Science, 2005, 98(1/2): 89-99. |
| [26] |
LOMB J, KEYSERLINGK M A G V, WEARY D M. Behavioral changes associated with fever in transition dairy cows[J]. Journal of Dairy Science, 2020, 103(8): 7331-7338. DOI:10.3168/jds.2018-15969 |
| [27] |
PUTMAN A K, BROWN J L, GANDY J C, et al. Changes in biomarkers of nutrient metabolism, inflammation, and oxidative stress in dairy cows during the transition into the early dry period[J]. Journal of Dairy Science, 2018, 101(10): 9350-9359. DOI:10.3168/jds.2018-14591 |
| [28] |
SORDILLO L M, RAPHAEL W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders[J]. The Veterinary Clinics of North America. Food Animal Practice, 2013, 29(2): 267-278. DOI:10.1016/j.cvfa.2013.03.002 |
| [29] |
SUN F F, CAO Y C, CAI C J, et al. Regulation of nutritional metabolism in transition dairy cows: energy homeostasis and health in response to post-ruminal choline and methionine[J]. PLOS One, 2016, 11(8): e0160659. DOI:10.1371/journal.pone.0160659 |
| [30] |
WEI X S, ZHAO H H, HE J J, et al. Maternal nicotinamide supplementation during the perinatal period modifies the small intestine morphology and antioxidative status of offspring kids[J]. Animal Feed Science and Technology, 2019, 252: 41-50. DOI:10.1016/j.anifeedsci.2019.04.003 |
| [31] |
赵会会, 尹清艳, 何家俊, 等. 奶山羊围产期添加烟酰胺对羔羊腹脂脂质代谢的影响[J]. 农业生物技术学报, 2018, 26(9): 1527-1534. ZHAO H H, YIN Q Y, HE J J, et al. Effect of nicotinamide supplementation in perinatal dairy goat (Capra hircus) on lipid metabolism in abdominal adipose tissue of lambs[J]. Journal of Agricultural Biotechnology, 2018, 26(9): 1527-1534 (in Chinese). |
| [32] |
弓剑, 晓敏. 围产期奶牛炎症反应及其与免疫和能量代谢的关系[J]. 动物营养学报, 2016, 28(9): 2667-2672. GONG J, XIAO M. Inflammation in periparturient dairy cows and its relationship with immunity and energy metabolism[J]. Chinese Journal of Animal Nutrition, 2016, 28(9): 2667-2672 (in Chinese). DOI:10.3969/j.issn.1006-267x.2016.09.001 |
| [33] |
WALTON E L. Oxidative stress and diabetes: glucose response in the cROSsfire[J]. Biomedical Journal, 2017, 40(5): 241-244. DOI:10.1016/j.bj.2017.10.001 |
| [34] |
WEI X S, CAI C J, HE J J, et al. Effects of biotin and nicotinamide supplementation on glucose and lipid metabolism and milk production of transition dairy cows[J]. Animal Feed Science and Technology, 2018, 237(1): 106-117. |
| [35] |
WEI X S, YIN Q Y, ZHAO H H, et al. Metabolomics for the effect of biotin and nicotinamide on transition dairy cows[J]. Journal of Agricultural and Food Chemistry, 2018, 66(22): 5723-5732. DOI:10.1021/acs.jafc.8b00421 |
| [36] |
YANG S J, CHOI J M, KIM L, et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes[J]. The Journal of Nutritional Biochemistry, 2014, 25(1): 66-72. DOI:10.1016/j.jnutbio.2013.09.004 |
| [37] |
ROVITO, H A, OBLONG J E. Nicotinamide preferentially protects glycolysis in dermal fibroblasts under oxidative stress conditions[J]. The British Journal of Dermatology, 2013, 169(S2): 15-24. |
| [38] |
TAN C L, CHIN T, TAN C Y R, et al. Nicotinamide metabolism modulates the proliferation/differentiation balance and senescence of human primary keratinocytes[J]. The Journal of Investigative Dermatology, 2019, 139(8): 1638-1647. DOI:10.1016/j.jid.2019.02.005 |
| [39] |
孙博非, 余超, 曹阳春, 等. 奶牛围产期典型代谢特征及其营养调控的技术途径[J]. 动物营养学报, 2018, 30(2): 428-436. SUN B F, YU C, CAO Y C, et al. Typical metabolic characteristics and potential technical approaches of nutritional regulation in transition dairy cows: a review[J]. Chinese Journal of Animal Nutrition, 2018, 30(2): 428-436 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.02.004 |
| [40] |
李玉. 奶牛酮病氧化应激致肝细胞凋亡的信号转导机制研究[D]. 博士学位论文. 长春: 吉林大学, 2014. LI Y. The signaling transduction mechanism of hepatocytes apoptosis induced by oxidative stress in ketotic dairy cows[D]. Ph. D. Thesis. Changchun: Jilin University, 2014. (in Chinese) |
| [41] |
MELENDEZ P, RISCO C A. Management of transition cows to optimize reproductive efficiency in dairy herds[J]. The Veterinary Clinics of North America.Food Animal Practice, 2005, 21(2): 485-501. DOI:10.1016/j.cvfa.2005.02.008 |
| [42] |
WEI T, TIAN W L, XIE G H. Non-esterified fatty acids induce apoptosis via a ROS-dependent mechanism involving the mitochondrial pathway in bovine abomasal smooth muscle cells[J]. European Journal of Lipid Science and Technology, 2014, 116(11): 1477-1484. DOI:10.1002/ejlt.201400137 |
| [43] |
ARAGONA K M, CHAPMAN C E, PEREIRA A B D, et al. Prepartum supplementation of nicotinic acid: effects on health of the dam, colostrum quality, and acquisition of immunity in the calf[J]. Journal of Dairy Science, 2016, 99(5): 3529-3538. DOI:10.3168/jds.2015-10598 |
| [44] |
JANG S Y, KANG H T, HWANG E S. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation[J]. Journal of Biological Chemistry, 2012, 287(23): 19304-19314. DOI:10.1074/jbc.M112.363747 |
| [45] |
SHEN C, DOU X B, MA Y, et al. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction[J]. Nutrition Research, 2017, 40: 40-47. DOI:10.1016/j.nutres.2017.03.005 |
| [46] |
LI J X, DOU X B, LI S T, et al. Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent SIRT1 upregulation[J]. Biochimica et Biophysica acta, 2015, 1853(11 Pt A): 2929-2936. |
| [47] |
GARIANI K, MENZIES K J, RYU D, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice[J]. Hepatology, 2016, 63(4): 1190-1204. DOI:10.1002/hep.28245 |
| [48] |
BOGAN K L, BRENNER C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition[J]. Annual Review of Nutrition, 2008, 28: 115-130. DOI:10.1146/annurev.nutr.28.061807.155443 |
| [49] |
SHEN M, YEN A. Nicotinamide cooperates with retinoic acid and 1, 25-dihydroxyvitamin D (3) to regulate cell differentiation and cell cycle arrest of human myeloblastic leukemia cells[J]. Oncology, 2009, 76(2): 91-100. DOI:10.1159/000188664 |
| [50] |
FINKEL T, HOLBROOK N J. Oxidants, oxidative stress and the biology of ageing[J]. Nature, 2000, 408(6809): 239-247. DOI:10.1038/35041687 |
| [51] |
FURUKAWA A, TADA-OIKAWA S, KAWANISHI S, et al. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion[J]. Cellular Physiology and Biochemistry, 2007, 20(1/4): 45-54. |
| [52] |
MITCHELL S J, BERNIER M, AON M A, et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice[J]. Cell Metabolism, 2018, 27(3): 667-676. DOI:10.1016/j.cmet.2018.02.001 |
| [53] |
ZHEN A X, PIAO M J, KANG K A, et al. Niacinamide protects skin cells from oxidative stress induced by particulate matter[J]. Biomolecules & Therapeutics, 2019, 27(6): 562-569. |
| [54] |
YU Z Y, KUNCEWICZ T, DUBINSKY W P, et al. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene[J]. The Journal of Biological Chemistry, 2006, 281(14): 9101-9109. DOI:10.1074/jbc.M511049200 |
| [55] |
AMIRI K I, HA H C, SMULSON M E, et al. Differential regulation of CXC ligand 1 transcription in melanoma cell lines by poly (ADP-ribose) polymerase-1[J]. Oncogene, 2006, 25(59): 7714-7722. DOI:10.1038/sj.onc.1209751 |
| [56] |
SIDHU A, DIWAN V, KAUR H, et al. Nicotinamide reverses behavioral impairments and provides neuroprotection in 3-nitropropionic acid induced animal model of Huntington's disease: implication of oxidative stress-poly (ADP-ribose) polymerase pathway[J]. Metabolic Brain Disease, 2018, 33(6): 1911-1921. DOI:10.1007/s11011-018-0297-0 |
| [57] |
TRAMMELL S A, SCHMIDT M S, WEIDEMANN B J, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans[J]. Nature Communications, 2016, 7: 12948. DOI:10.1038/ncomms12948 |
| [58] |
SONG S B, JANG S Y, KANG H T. Modulation of mitochondrial membrane potential and ROS generation by nicotinamide in a manner independent of SIRT1 and mitophagy[J]. Molecules and Cells, 2017, 40(7): 503-514. |
| [59] |
MAHMOUD Y I, MAHMOUD A A. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: nicotinamide effect in acetaminophen-damged liver[J]. Experimental and Toxicologic Pathology, 2016, 68(6): 345-354. DOI:10.1016/j.etp.2016.05.003 |
| [60] |
TURUNC BAYRAKDAR E, UYANIKGIL Y, KANIT L, et al. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ (1-42)-induced rat model of Alzheimer's disease[J]. Free Radical Research, 2014, 48(2): 146-158. DOI:10.3109/10715762.2013.857018 |
| [61] |
JOHN C M, RAMASAMY R, AL NAQEEB G, et al. Nicotinamide supplementation protects gestational diabetic rats by reducing oxidative stress and enhancing immune responses[J]. Current Medicinal Chemistry, 2012, 19(30): 5181-5186. DOI:10.2174/092986712803530449 |
| [62] |
CHEN J C, YANG Z G, DONG G Z. Niacin nutrition and rumen-protected niacin supplementation in dairy cows: an updated review[J]. The British Journal of Nutrition, 2019, 122(10): 1103-1112. DOI:10.1017/S0007114519002216 |
| [63] |
PESCARA J B, PIRES J A, GRUMMER R R. Antilipolytic and lipolytic effects of administering free or ruminally protected nicotinic acid to feed-restricted Holstein cows[J]. Journal of Dairy Science, 2010, 93(11): 5385-5396. DOI:10.3168/jds.2010-3402 |
| [64] |
RINGSEIS R, ZEITZ J O, WEBER A, et al. Hepatic transcript profiling in early-lactation dairy cows fed rumen-protected niacin during the transition from late pregnancy to lactation[J]. Journal of Dairy Science, 2019, 102(1): 365-376. DOI:10.3168/jds.2018-15232 |
| [65] |
ZEITZ J O, WEBER A, MOST E, et al. Effects of supplementing rumen-protected niacin on fiber composition and metabolism of skeletal muscle in dairy cows during early lactation[J]. Journal of Dairy Science, 2018, 101(9): 8004-8020. DOI:10.3168/jds.2018-14490 |
| [66] |
PIRES, J A A, GRUMMER R R. The use of nicotinic acid to induce sustained low plasma nonesterified fatty acids in feed-restricted Holstein cows[J]. Journal of Dairy Science, 2007, 90(8): 3725-3732. DOI:10.3168/jds.2006-904 |
| [67] |
HWANG E S, SONG S B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells[J]. Cellular and Molecular Life Sciences, 2017, 74(18): 3347-3362. DOI:10.1007/s00018-017-2527-8 |
| [68] |
UMMARINO S, MOZZON M, ZAMPORLINI F, et al. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay[J]. Food Chemistry, 2017, 221: 161-168. |
| [69] |
ARAGONA K M, RICE E M, ENGSTROM M, et al. Supplementation of nicotinic acid to prepartum Holstein cows increases colostral immunoglobulin G, excretion of urinary purine derivatives, and feed efficiency in calves[J]. Journal of Dairy Science, 2020, 103(3): 2287-2302. |
| [70] |
BÜHLER S, FRAHM J, TIENKEN R, et al. Effects of energy supply and nicotinic acid supplementation on serum anti-oxidative capacity and on expression of oxidative stress-related genes in blood leucocytes of periparturient primi-and pluriparous dairy cows[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(1): e87-e98. |
| [71] |
HRISTOVSKA T, CINCOVIC M, STOJANOVIC D, et al. Influence of niacin supplementation on the metabolic parameters and lipolysis in dairy cows during early lactation[J]. Kafkas Vniversitesi Veteriner Fakültesi Dergisi, 2017, 23(5): 773-778. |
| [72] |
YUAN K, SHAVER R D, BERTICS S J, et al. Effect of rumen-protected niacin on lipid metabolism, oxidative stress, and performance of transition dairy cows[J]. Journal of Dairy Science, 2012, 95(5): 2673-2679. |




