2. 中国农业科学院北京畜牧兽医研究所, 动物营养学国家重点实验室, 北京 100193
2. State Key Laboratory of Animal Nutrition, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China
瘤胃微生物蛋白合成量的估测方法主要分为2类,包括外源标记物和内源标记物[1]。外源标记物主要包括磷-32(32P)、硫-35(35S)、氮-15(15N),内源标记物主要包括三磷酸腺苷(ATP)、嘧啶、嘌呤和核酸。嘌呤衍生物法相比于其他方法不仅节约成本,且方法简单、快速、不需瘘管动物。饲粮中核酸进入瘤胃被微生物降解成核苷酸、核苷、碱基,这些底物作为前体物质合成微生物核酸,微生物核酸进入小肠后被降解成嘌呤核苷和碱基,其中一小部分被利用合成组织核酸,而未被利用的嘌呤则以嘌呤衍生物形式进入血液,最终随尿液、唾液和奶排出体外。嘌呤衍生物包括次黄嘌呤、黄嘌呤、尿囊素和尿酸,其含量受动物品种、采食量和饲粮蛋白质水平的影响[2]。由于奶牛肠道黏膜内含有较高活性的黄嘌呤氧化酶,因此奶牛的嘌呤衍生物只有尿囊素和尿酸。目前,人们普遍认为血浆、乳或尿中嘌呤衍生物含量是衡量十二指肠可利用微生物蛋白的指标[3-6]。虽然在嘌呤衍生物含量与微生物蛋白产量关系的研究已获得很大进步,但影响嘌呤衍生物含量的因素仍然没有弄清楚,尤其是奶牛个体生理因素对嘌呤衍生物含量影响的相关研究尚未报道[7]。由于血液更容易采集,通过研究血浆嘌呤衍生物含量的变化,可为基于嘌呤衍生物的微生物蛋白合成量预测模型提供优化依据。鉴于此,本试验以284头荷斯坦奶牛为研究对象,探讨了奶牛的产奶性能、瘤胃发酵参数以及血浆生化指标对血浆嘌呤衍生物含量的影响,为提高嘌呤衍生物评估微生物蛋白产量的准确度提供建议。
1 材料与方法 1.1 试验动物选取284头同一牧场2胎次的健康泌乳前期荷斯坦奶牛,并根据牛场原有记录剔除乳房炎病牛,同时记录奶牛的泌乳日龄(24~77 d)、产奶量[(46.93±7.09) kg]。所有试验奶牛散栏式饲养,饲喂同一全混合日粮(TMR),饲粮组成及营养水平见表 1,饲粮的15N丰度为0.55‰。所有奶牛自由采食和饮水,每日饲喂3次,挤奶3次。
|
|
表 1 饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the diet (DM basis) |
分别在05:00、14:00、17:00利用转盘式挤奶台进行机械挤奶。记录奶牛产奶量的同时采集奶样,共采集3次奶样,并按照早中晚比例4 ∶ 3 ∶ 3混合均匀,取样50 mL添加防腐剂(北京嘉源科技有限公司提供,主要成分为溴硝丙二醇),4 ℃保存送至当地检测中心利用近红外光谱仪进行乳蛋白、尿素氮含量检测。
1.2.2 瘤胃液采集与瘤胃发酵参数测定晨饲后2 h内使用瘤胃采集器经口腔采集瘤胃液,将抽取的前100 mL瘤胃液舍弃,采集到的瘤胃液分装于15 mL离心管中,置于液氮中冷冻保存。待检前,将冻存瘤胃液解冻,取2 mL瘤胃液,4 ℃、13 000×g离心5 min,取600 mL瘤胃上清液,加入50 μL 25%偏磷酸,12 000×g离心20 min,上清液置于-20 ℃保存。氨态氮含量采用苯酚-次氯酸钠比色法测定[8]。挥发性脂肪酸含量使用气相色谱仪测定(安捷伦7890A)[9]。
1.2.3 血样采集与血浆生化指标测定晨饲后2 h内使用肝素抗凝管采集尾静脉血10 mL,4 000×g离心5 min,血浆分装置于-20 ℃保存。采用全自动生化仪(AU 5800,贝克曼)检测血浆尿酸、肌酐、β-羟丁酸、游离脂肪酸、葡萄糖、尿素氮、乙酰乙酸含量。游离氨基酸含量使用总氨基酸测定试剂盒(南京建成生物工程研究所)检测。采用双抗体夹心法在450 nm处测定血浆尿囊素含量(上海科淘生物科技中心)。嘌呤衍生物含量是尿酸含量与尿囊素含量之和。参照贺媛媛[10]的检测方法,用Isoprime 100元素分析-同位素比率质谱仪(英国Isoprime公司)分别测定饲粮和血浆的15N丰度。Δ15N是血浆15N丰度与饲粮15N丰度之差,它可以克服个体层面的差异,尤其适用于大群体奶牛,有效地评估氮利用率[11]。
1.3 数据统计分析使用Excel 2010对数据进行初步整理,计算各指标的平均值、标准差、中位数、最大值、最小值以及变异系数。利用GraphPad Prism 7.0进行单因子方差分析和线性回归分析,同时进行皮尔逊相关性分析,P < 0.05表示显著相关,P < 0.01表示极显著相关。
2 结果 2.1 奶牛的血浆嘌呤衍生物含量、产奶性能、瘤胃发酵参数和血浆生化指标表 1列出了284头荷斯坦奶牛的产奶性能、瘤胃发酵参数和血浆生化指标。由表可知,奶牛血浆中尿酸含量的平均值为38.23 μmol/L,最大值为80 μmol/L。尿囊素含量的平均值为0.30 μmol/L,最大值为0.46 μmol/L。嘌呤衍生物含量的平均值为38.53 μmol/L,最大值为38.53 μmol/L。尿酸、尿囊素和嘌呤衍生物的变异系数分别为35.91%、24.36%、35.59%。
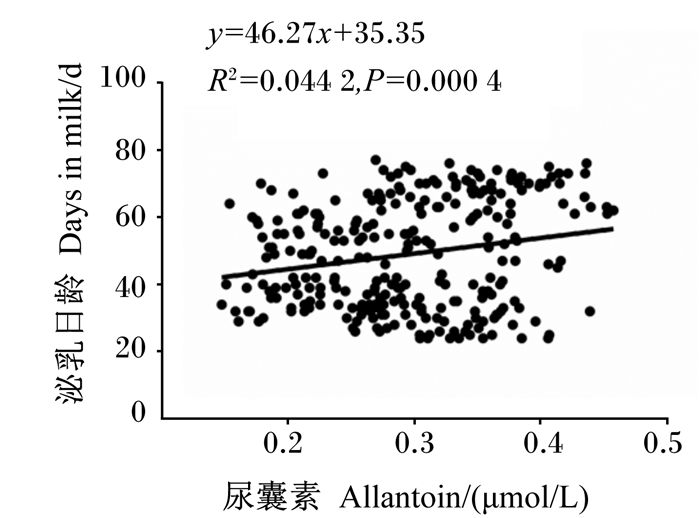
2.2 血浆嘌呤衍生物含量与奶牛产奶性能的相关性由表 2可知,血浆尿酸含量与泌乳日龄、乳蛋白产量均呈显著正相关(P < 0.05),与产奶量呈极显著正相关(P < 0.01),而与乳尿素氮、乳蛋白含量无显著相关性(P>0.05)。血浆尿囊素含量与泌乳日龄、乳蛋白含量呈极显著正相关(P < 0.01,图 1),与乳尿素氮含量、产奶量、乳蛋白产量无显著相关性(P>0.05)。血浆嘌呤衍生物含量与泌乳日龄、乳蛋白产量呈显著正相关(P < 0.05),与产奶量呈极显著正相关(P < 0.01),而与乳尿素氮、乳蛋白含量无显著相关性(P>0.05)。上述相关性多属于弱相关,在统计分析上呈显著性。
|
|
表 2 奶牛的血浆嘌呤衍生物含量、产奶性能、瘤胃发酵参数和血浆生化指标 Table 2 Plasma purine derivative contents, milk performance, rumen fermentation parameters and plasma biochemical indices of dairy cows |
|
图 1 泌乳日龄与血浆尿囊素含量散点图 Fig. 1 Scatter plot of lactation days with plasma allantoin content |
由表 3可知,血浆尿酸和嘌呤衍生物含量与瘤胃丁酸含量呈显著正相关(P < 0.01),与瘤胃异戊酸含量呈极显著负相关(P < 0.01);血浆尿囊素含量与瘤胃异戊酸含量呈显著负相关(P < 0.05),而与瘤胃丁酸含量无显著相关性(P>0.05)。除此之外,血浆尿酸、尿囊素、嘌呤衍生物含量与瘤胃乙酸、丙酸、异丁酸、氨态氮含量及乙酸/丙酸均无显著相关性(P>0.05)。上述相关性多属于弱相关,在统计分析上呈显著性。
|
|
表 3 血浆嘌呤衍生物含量与奶牛产奶性能的相关性 Table 3 Correlations of plasma purine derivative contents with milk performance of dairy cows |
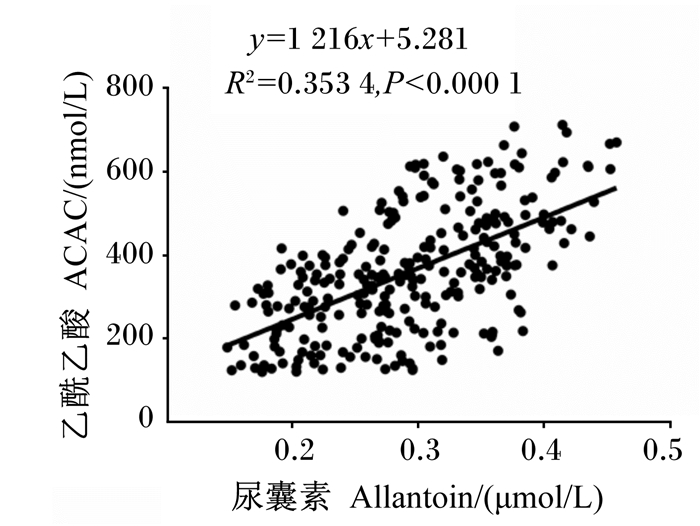
由表 4可知,血浆尿酸含量与Δ15N、血浆肌酐含量呈极显著负相关(P < 0.01),与血浆游离脂肪酸、葡萄糖含量呈极显著或显著正相关(P < 0.01或P < 0.05),但与血浆β-羟丁酸、尿素氮、乙酰乙酸、游离氨基酸含量无显著相关性(P>0.05)。血浆尿囊素含量与血浆肌酐、乙酰乙酸、尿素氮含量呈极显著或显著正相关(P < 0.01或P < 0.05,图 2),与血浆游离脂肪酸、葡萄糖含量呈极显著或显著负相关(P < 0.01或P < 0.05),而与Δ15N及血浆β-羟丁酸、游离氨基酸含量无显著相关性(P>0.05)。血浆嘌呤衍生物含量与Δ15N、血浆肌酐含量呈极显著负相关(P < 0.01),与血浆游离脂肪酸、葡萄糖含量呈极显著正相关(P < 0.01),但与血浆尿素氮、乙酰乙酸、游离氨基酸含量无显著相关性(P>0.05)。上述相关性多属于弱相关,在统计分析上呈显著性。
|
|
表 4 血浆嘌呤衍生物含量与瘤胃发酵参数的相关性 Table 4 Correlations of plasma purine derivatives contents with rumen fermentation parameters |
|
|
表 5 血浆嘌呤衍生物含量与血浆生化指标的相关性 Table 5 Correlations of plasma purine derivatives contents with plasma biochemical indices |
|
图 2 血浆乙酰乙酸含量与血浆尿囊素含量散点图 Fig. 2 Scatter plot of plasma acetoacetic acid content with plasma allantoin content |
在相同营养条件下,奶牛对饲粮蛋白质的消化和吸收不同,从而导致血浆嘌呤衍生物含量不同。血浆嘌呤衍生物排出途径主要是经肾脏随尿液排出,其次是通过乳腺或唾液排出,并且血浆嘌呤衍生物含量与尿嘌呤衍生物排泄量呈高度正相关[12]。在本试验中,血浆尿酸、尿囊素含量的平均值分别为38.23和0.30 μmol/L,且血浆尿酸含量占嘌呤衍生物含量的99%。Gonzalez-Ronquillo等[4]研究表明,泌乳前期和泌乳后期的奶牛尿嘌呤衍生物含量和乳嘌呤衍生物排泄量不同。Giesecke等[13]研究发现,奶牛产奶量与血浆尿囊素含量(r=0.78)、乳尿囊素排泄量(r=0.95)呈显著正相关。本试验结果也表明了泌乳日龄、产奶量和乳蛋白产量与血浆嘌呤衍生物含量呈正相关。因此,泌乳日龄和产奶量影响机体对嘌呤碱基的吸收和代谢。
3.2 血浆嘌呤衍生物含量与瘤胃发酵参数的相关性瘤胃氨态氮和挥发性脂肪酸含量是评价瘤胃发酵特征的主要指标。氨态氮含量反映了瘤胃内蛋白质平衡状况,其含量受瘤胃微生物利用速度和瘤胃壁吸收速度的影响[2]。挥发性脂肪酸主要来源于瘤胃微生物对碳水化合物的消化,是动物机体主要的能量来源[14]。研究表明,血浆嘌呤衍生物含量与瘤胃微生物蛋白产量呈显著正相关,而瘤胃微生物蛋白产量与能量载体物质和含氮物质密切相关[15]。本研究结果表明,瘤胃氨态氮、乙酸、丙酸和异丁酸含量与血浆嘌呤衍生物含量无显著相关性,而瘤胃丁酸含量与血浆嘌呤衍生物含量呈显著正相关,瘤胃戊酸、异戊酸含量与血浆嘌呤衍生物含量呈显著负相关性,这说明了血浆嘌呤衍生物含量与碳水化合物代谢相关,但相关性较弱。瘤胃丁酸含量与血浆嘌呤衍生物含量表现的强相关,可能是由于丁酸可以促进微生物的生长活动,从而使微生物更好地利用氮源去合成微生物蛋白[16]。异戊酸属于支链挥发性脂肪酸,可以为氨基酸的合成提供碳骨架,但因异戊酸和戊酸含量总占比不足10%,目前相关研究较少,其相互作用关系还需进一步深入研究。
3.3 血浆嘌呤衍生物含量与血浆生化指标的相关性氮同位素分馏使自然丰度下的15N差异富集,其中Δ15N可以表征不同饮食和饲养条件下奶牛氮利用率的变化[17]。据报道,Δ15N与氮利用率呈显著负相关,与瘤胃微生物蛋白合成效率呈负相关[11, 18]。本试验发现,Δ15N与血浆嘌呤衍生物含量呈显著负相关,说明嘌呤衍生物与机体的氮代谢有关。肌酐是动物肌肉中磷酸肌酸的代谢产物,反映机体内蛋白质代谢水平[19-22]。血液中肌酐含量越高说明机体内肌肉降解程度越高[23]。本研究中,血浆肌酐含量与血浆嘌呤衍生物含量呈显著负相关,说明机体内氨基酸被充分利用,减少肌肉降解,有利于微生物蛋白的合成。游离脂肪酸和葡萄糖含量间接反映了奶牛的能量代谢状况[24-25]。游离脂肪酸经过β氧化产生酮体,主要包括β-羟丁酸和乙酰乙酸,它们与动物机体内脂肪的动员程度密切相关[26]。本研究表明,血浆游离脂肪酸、葡萄糖含量与血浆嘌呤衍生物含量呈显著正相关,血浆β-羟丁酸和乙酰乙酸含量与血浆嘌呤衍生物含量无显著相关性,但血浆乙酰乙酸含量与血浆尿囊素含量呈显著正相关,进一步说明机体的嘌呤代谢与能量代谢息息相关。血浆游离氨基酸主要被乳腺利用合成乳蛋白[24-29]。尿素氮是衡量机体内蛋白质代谢及氨基酸平衡的重要指标[30-31]。但本试验结果表明,血浆游离氨基酸和尿素氮含量与血浆嘌呤衍生物含量均无显著相关性,与上述结果不一致,提示了体内试验较为复杂,血浆生化指标受多个因素的干扰。因此,在血浆利用嘌呤衍生物含量估算瘤胃微生物蛋白合成量时,应尽量避免上述影响因素,以提高计算的准确性。
4 结论本试验通过血浆嘌呤衍生物含量与泌乳前期奶牛的产奶性能、瘤胃发酵参数以及血浆生化指标的相关性分析,表明了影响奶牛血浆嘌呤衍生物的生理因素包括:泌乳日龄、乳蛋白产量、产奶量,瘤胃异戊酸、戊酸、丁酸含量,血浆Δ15N和肌酐、游离脂肪酸、葡萄糖含量。
| [1] |
PUCHALA R, KULASEK G W. Estimation of microbial protein flow from the rumen of sheep using microbial nucleic acid and urinary excretion of purine derivatives[J]. Canadian Journal of Animal Science, 1992, 72(4): 821-830. DOI:10.4141/cjas92-094 |
| [2] |
MCALLAN A B. The fate of nucleic acids in ruminants[J]. Proceedings of the Nutrition Society, 1982, 41(3): 309-317. DOI:10.1079/PNS19820046 |
| [3] |
GONZALEZ-RONQUILLO M, BALCELLS J, GUADA J A, et al. Purine derivative excretion in dairy cows: endogenous excretion and the effect of exogenous nucleic acid supply[J]. Journal of Dairy Science, 2003, 86(4): 1282-1291. DOI:10.3168/jds.S0022-0302(03)73712-6 |
| [4] |
DÓREA J R R, DANÉS M A C, ZANTON G I, et al. Urinary purine derivatives as a tool to estimate dry matter intake in cattle: a Meta-analysis[J]. Journal of Dairy Science, 2017, 100(11): 8977-8994. DOI:10.3168/jds.2017-12908 |
| [5] |
KOZLOSKI G V, STEFANELLO C M, OLIVEIRA L, et al. Technical note: evaluation of urinary purine derivatives in comparison with duodenal purines for estimating rumen microbial protein supply in sheep[J]. Journal of Animal Science, 2017, 95(2): 884-891. |
| [6] |
GONDA H L, EMANUELSON M, MURPHY M. The effect of roughage to concentrate ration in the diet on nitrogen and purine metabolism in dairy cows[J]. Animal Feed Science and Technology, 1996, 64(1): 27-42. DOI:10.1016/S0377-8401(96)01049-8 |
| [7] |
FUJIHARA T, SHEM M N, MATSUI T. Urinary excretion of purine derivatives and plasma allantoin level in sheep and goats during fasting[J]. Animal Science Journal, 2007, 78(2): 129-134. DOI:10.1111/j.1740-0929.2007.00416.x |
| [8] |
BRODERICK G A, KANG J H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media[J]. Journal of Dairy Science, 1980, 63(1): 64-75. DOI:10.3168/jds.S0022-0302(80)82888-8 |
| [9] |
LIANG Y S, LI G Z, LI X Y, et al. Growth performance, rumen fermentation, bacteria composition, and gene expressions involved in intracellular pH regulation of rumen epithelium in finishing Hu lambs differing in residual feed intake phenotype[J]. Journal of Animal Science, 2017, 95(4): 1727-1738. |
| [10] |
贺媛媛. 药食同源产品(玛咖、葛根)产地鉴别技术研究[D]. 硕士学位论文. 北京: 中国农业科学院, 2020. HE Y Y. Research on the authentication technology of geographical origin of medicine and food homogeneous products (Maca & Gegen)[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences thesis, 2020. (in Chinese) |
| [11] |
CANTALAPIEDRA-HIJAR G, DEWHURST R J, CHENG L, et al. Nitrogen isotopic fractionation as a biomarker for nitrogen use efficiency in ruminants: a meta-analysis[J]. Animal, 2018, 12(9): 1827-1837. DOI:10.1017/S1751731117003391 |
| [12] |
ANJANAPRUTHIPONG J K, 史清河, 张颖. 尿中嘌呤衍生物是估测瘤胃微生物合成量的一种指示物[J]. 国外畜牧科技, 1999(5): 7-10. ANJANAPRUTHIPONG J K, SHI Q H, ZHANG Y. Urine purine derivatives are an indicator for estimating rumen microbial synthesis[J]. Technology of Animal Husbandry, 1999(5): 7-10 (in Chinese). |
| [13] |
GIESECKE D, EHRENTREICH L, STANGASSINGER M, et al. Mammary and renal excretion of purine metabolites in relation to energy intake and milk yield in dairy cows[J]. Journal of Dairy Science, 1994, 77(8): 2376-23781. DOI:10.3168/jds.S0022-0302(94)77180-0 |
| [14] |
VAN HOUTERT M F. The production and metabolism of volatile fatty acids by ruminants fed roughages: a review[J]. Animal Feed Science and Technology, 1993, 43(3/4): 189-225. |
| [15] |
WESTON R H, HOGAN J P. The digestion of pasture plants by sheep 1.Ruminal production of volatile fatty acids by sheep offered diets of ryegrass and forage oats[J]. Australian Journal of Agricultural Research, 1968, 19(3): 419-432. DOI:10.1071/AR9680419 |
| [16] |
KRISTENSEN N B, HARMON D L. Effect of increasing ruminal butyrate absorption on splanchnic metabolism of volatile fatty acids absorbed from the washed reticulorumen of steers[J]. Journal of Animal Science, 2004, 82(12): 3549-3559. DOI:10.2527/2004.82123549x |
| [17] |
CANTALAPIEDRA-HIJAR G, FOUILLET H, HUNEAU J F, et al. Relationship between efficiency of nitrogen utilization and isotopic nitrogen fractionation in dairy cows: contribution of digestion v. metabolism?[J]. Animal, 2016, 10(2): 221-229. DOI:10.1017/S1751731115002025 |
| [18] |
CHENG L, EDWARDS G R, DEWHURST R J, et al. The effect of dietary water soluble carbohydrate to nitrogen ratio on nitrogen partitioning and isotopic fractionation of lactating goats offered a high-nitrogen diet[J]. Animal, 2016, 10(5): 779-785. DOI:10.1017/S1751731115002335 |
| [19] |
VALADARES R F D, BRODERICK G A, FILHO S C V, et al. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives[J]. Journal of Dairy Science, 1999, 82(12): 2686-2696. DOI:10.3168/jds.S0022-0302(99)75525-6 |
| [20] |
WANG H, LONG R, ZHOU W, et al. A comparative study on urinary purine derivative excretion of yak (Bos grunniens), cattle (Bos taurus), and crossbred (Bos taurus×Bos grunniens) in the Qinghai-Tibetan plateau, China[J]. Journal of Animal Science, 2009, 87(7): 2355-2362. DOI:10.2527/jas.2008-1544 |
| [21] |
VAN NIEKERK B D H, BENSADOUN A, PALADINES O L, et al. A study of some of the conditions affecting the rate of excretion and stability of creatinine in sheep urine[J]. Journal of Nutrition, 1963, 79(3): 373-380. DOI:10.1093/jn/79.3.373 |
| [22] |
FAICHNEY G J, WELCH R J, BROWN G H. Prediction of the excretion of allantoin and total purine derivatives by sheep from the 'creatinine coefficient'[J]. The Journal of Agricultural Science, 1995, 125(3): 425-428. DOI:10.1017/S0021859600084938 |
| [23] |
郭仕辉, 余永涛, 赵清梅, 等. 围产期奶牛主要血液生化指标的监测[J]. 畜牧与兽医, 2020, 52(11): 128-133. GUO S H, YU Y T, ZHAO Q M, et al. Monitoring of major blood biochemical indexes in perinatal cows[J]. Chinese Journal of Animal Science and Veterinary, 2020, 52(11): 128-133 (in Chinese). |
| [24] |
WANG J F, FU S P, LI S N, et al. Short-chain fatty acids inhibit growth hormone and prolactin gene transcription via cAMP/PKA/CREB signaling pathway in dairy cow anterior pituitary cells[J]. International Journal of Molecular Sciences, 2013, 14(11): 21474-21488. DOI:10.3390/ijms141121474 |
| [25] |
STAPLES C R, THATCHER W W, CLARK J H. Relationship between ovarian activity and energy status during the early postpartum period of high producing dairy cows[J]. Journal of Dairy Science, 1990, 73(4): 938-947. DOI:10.3168/jds.S0022-0302(90)78750-4 |
| [26] |
ZHANG H, WANG Z, LIU G, et al. Effect of dietary fat supplementation on milk components and blood parameters of early-lactating cows under heat stress[J]. Slovak Journal of Animal Science, 2011, 44(2): 52-58. |
| [27] |
REYNOLDS C K, HARMON D L, CECAVA M J. Absorption and delivery of nutrients for milk protein synthesis by portal-drained viscera[J]. Journal of Dairy Science, 1994, 77(9): 2787-2808. DOI:10.3168/jds.S0022-0302(94)77220-9 |
| [28] |
NICHOLS J R, SCHINGOETHE D J, MAIGA HA, et al. Evaluationof corn distillers grains and ruminally protected lysine and methionine for lactating dairy cows[J]. Journal of Dairy Science, 1998, 81(2): 482-491. DOI:10.3168/jds.S0022-0302(98)75600-0 |
| [29] |
MERCHEN N R, TITGEMEYER E C. Manipulation of amino acid supply to the growing ruminant[J]. Journal of Animal Science, 1992, 70(10): 3238-3247. DOI:10.2527/1992.70103238x |
| [30] |
STANLEY C C, WILLIAMS C C, JENNY B F, et al. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves[J]. Journal of Dairy Science, 2002, 85(9): 2335-2343. DOI:10.3168/jds.S0022-0302(02)74313-0 |
| [31] |
KANJANAPRUTHIPONG J, THABOOT B. Effects of neutral detergent fiber from rice straw on blood metabolites and productivity of dairy cows in the tropics center[J]. Asian-Australasian Journal of Animal Sciences, 2006, 19(3): 356-362. DOI:10.5713/ajas.2006.356 |




