玉米赤霉烯酮(zearalenon, ZEN)是镰刀菌产生的有毒害作用的霉菌毒素,家畜可通过被污染的谷物接触到ZEN,影响生长、繁殖、免疫等功能,其中猪是对ZEN最敏感的动物[1]。因此,寻找一种有效抵抗真菌毒素的添加剂有重要意义。低聚壳聚糖硒作为有机硒多糖,结合了硒和低聚壳聚糖两者的功能,可增强机体抗氧化能力、提高繁殖性能、促进免疫器官发育[2-4]。从食物摄入的毒素首先攻击的是胃肠道,已有研究表明,ZEN可诱导猪肠上皮细胞产生线粒体损伤和氧化应激[5-6]。内质网可维持细胞稳态,是蛋白质的合成、加工的重要场所[7]。Ben等[8]发现番红花和槲皮素可通过减少内质网应激保护人结肠癌细胞和人胚胎肾细胞免受ZEN诱导的细胞凋亡。目前并未见有关于低聚壳聚糖硒通过内质网应激途径缓解ZEN对猪肠道损伤的研究。因此,本试验采用猪肠上皮细胞(IPEC-J2细胞)为材料,研究低聚壳聚糖硒在ZEN诱导的细胞氧化损伤和内质网应激中的作用机制,以期为低聚壳聚糖硒保护ZEN对肠道的损伤提供参考依据。
1 材料与方法 1.1 试验材料IPEC-J2细胞(天津师范大学动物营养与饲料实验室惠赠),低聚壳聚糖硒(天津农学院基础兽医实验室制,硒(Se)含量为22 g/kg,合成方法参照徐春兰等[9]),低聚壳聚糖(Comybion公司,相对分子质量2 000),亚硒酸钠(成都艾科达化学试剂有限公司,AR,98%),玉米赤霉烯酮(上海源叶生物科技有限公司),线粒体膜电位(JC-1)检测试剂盒、活性氧(ROS)检测试剂盒(南京凯基科技有限公司),细胞计数检测试剂盒(CCK8)、β-肌动蛋白(β-actin)兔单克隆抗体、山羊抗兔二抗(碧云天生物技术公司),乳酸脱氢酶(LDH)检测试剂盒(南京建成生物工程研究所),葡萄糖调节蛋白78(glucose regulated protine 78, GRP78)、磷酸化蛋白激酶R样内质网激酶/蛋白激酶R样内质网激酶(phosphorylated protein kinase receptor like endoplasmic reticulum kinase/protein kinase receptor like endoplasmic reticulum kinase, P-PERK/PERK)、转录激活因子4(activating transcription factor 4, ATF4)、C/EBP同源蛋白(C/EBP-homologous protein, CHOP)、转录因子E2相关因子(nuclear factor E2-related factor 2, Nrf2)、血红素加氧酶-1(heme oxygenase-1, HO-1)抗体(Proteintech公司),聚氰基丙烯酸正丁酯(BCA)蛋白检测试剂盒(索莱宝科技有限公司)。
1.2 细胞存活率的测定用培养基调节细胞含量至1×105个/mL,接种于96孔板中,分为5组:对照组(C组)、ZEN组(30 μg/mL)、ZEN+0.5 Se组(30 μg/mL ZEN+0.5 μmol/L低聚壳聚糖硒,以Se计)、ZEN+1.5 Se组(30 μg/mL ZEN+1.5 μmol/L低聚壳聚糖硒,以Se计)、ZEN+3.0 Se组(30 μg/mL ZEN+3.0 μmol/L低聚壳聚糖硒,以Se计),每组重复6次。细胞长至60%~70%时,ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组更换为含对应浓度低聚壳聚糖硒的培养液,其他组更换为正常培养液。12 h后向含ZEN组加入ZEN,继续培养24 h。接着加入CCK8培养45 min,按说明书操作,在450 nm波长测定每孔的吸光度(OD)值。计算细胞存活率,计算公式如下:

|
细胞分组及处理同1.2,收集细胞培养基,按照LDH试剂盒说明操作,测定各个样品中LDH活性,在450 nm波长测定每孔的OD值。培养基中LDH活性计算公式如下:

|
用培养基调节细胞含量至4×105个/mL,接种于6孔板中,分组及处理同1.2。将按上述处理培养好的6孔板细胞弃上清,用磷酸盐缓冲液(PBS)洗3遍,加入稀释好的二氯二氢荧光素-乙酰乙酸酯(DCFH-DA)1 mL于每孔中,37 ℃孵育20 min,用不含血清的培养基洗3遍,于荧光倒置显微镜下观察。
1.5 线粒体膜电位的测定细胞分组及处理同1.2,使用线粒体探针对细胞进行染色。按照说明书加入JC-1工作液,于细胞培养箱中37 ℃孵育20 min,孵育结束后,吸除上清,用PBS洗2次,加入培养液,于荧光倒置显微镜下观察。
1.6 氧化应激相关蛋白表达细胞分组及处理同1.2,用细胞裂解液裂解细胞,置冰上30 min,将细胞刮下,离心取上清,用BCA试剂盒测定蛋白浓度;加入5×Buffer变性煮10 min得目的蛋白。Western blot测定Nrf2、HO-1蛋白在IPEC-J2细胞中的表达量。
1.7 内质网应激通路相关蛋白表达细胞分组及处理同1.2,Western blot测定GRP78、ATF4、P-PERK/PERK、CHOP蛋白在IPEC-J2细胞中的表达量。
1.8 数据统计测定值以平均值±标准差表示,采用SPSS 18.0统计分析软件对试验数据进行单因素方差分析(one way-ANOVA),若存在差异显著性,再进行多重比较(LSD法)。P < 0.05为差异显著,P < 0.01为差异极显著。
2 结果 2.1 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞存活率的影响由图 1可知,与对照组相比,添加ZEN可极显著降低IPEC-J2细胞存活率(P < 0.01);ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组细胞存活率极显著高于ZEN组(P < 0.01);ZEN+3.0 Se组细胞存活率与对照组无显著差异(P>0.05)。
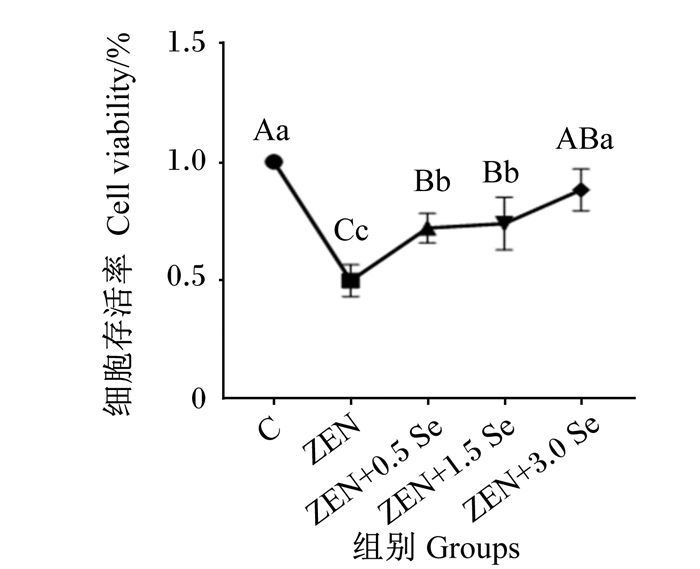
|
数据点标注不同大写字母表示极显著差异(P < 0.01),不同小写字母表示差异显著(P < 0.05)。 Data points with different capital letters mean extremely significant difference (P < 0.01), and with different small letters mean significant difference (P < 0.05). 图 1 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞存活率的影响 Fig. 1 Effects of oligomeric chitosan selenium on viability of IPEC-J2 cells induced by ZEN |
由表 1可知,ZEN组LDH活性相比对照组显著升高(P < 0.05);ZEN+3.0 Se组LDH活性与ZEN组相比显著降低(P < 0.05);ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组LDH活性与对照组无显著差异(P>0.05)。
|
|
表 1 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞LDH活性的影响 Table 1 Effects of oligomeric chitosan selenium on LDH activity of IPEC-J2 cells induced by ZEN |
通过荧光强度来反映ROS含量,由图 2可知,ZEN组的ROS含量极显著高于对照组(P < 0.01);ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组的ROS含量与ZEN组相比均极显著降低(P < 0.01)。
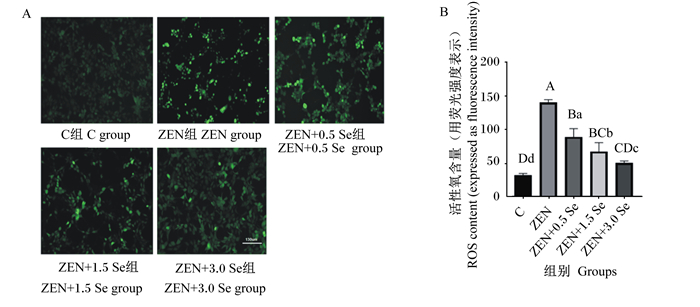
|
A:荧光显微镜观察低聚壳聚糖硒抗ZEN对IPEC-J2细胞活性氧含量的影响;B:低聚壳聚糖硒抗ZEN对IPEC-J2细胞活性氧含量的影响。 数据柱标注不同大写字母表示极显著差异(P < 0.01),不同小写字母表示差异显著(P < 0.05)。图 3同。 A: effects of oligomeric chitosan selenium anti-ZEN on reactive oxygen content in IPEC-J2 cells observed by fluorescence microscopy; B: effects of oligomeric chitosan selenium anti-ZEN on reactive oxygen content in IPEC-J2 cells. Value bars with different capital letter superscripts mean extremely significant difference (P < 0.01), and with different small letter superscripts mean significant difference (P < 0.05). The same as Fig. 3. 图 2 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞ROS含量的影响 Fig. 2 Effects of oligomeric chitosan selenium on reactive oxygen content in IPEC-J2 cells induced by ZEN |
通过荧光强度来反映线粒体膜电位的变化,由图 3可知,ZEN组的线粒体膜电位极显著低于对照组(P < 0.01);ZEN+1.5 Se和ZEN+3.0 Se组的线粒体膜电位与ZEN组相比极显著升高(P < 0.01)。
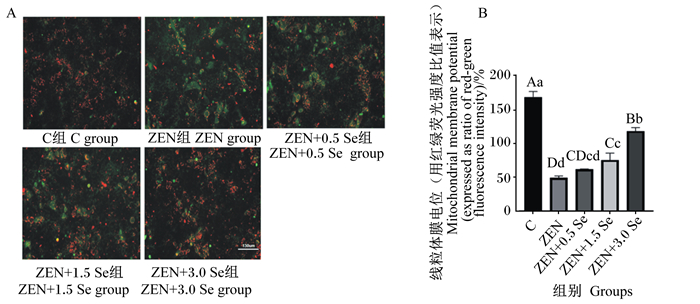
|
A:荧光显微镜观察低聚壳聚糖硒抗ZEN对IPEC-J2细胞线粒体膜电位的影响;B:低聚壳聚糖硒抗ZEN对IPEC-J2细胞线粒体膜电位的影响。 A: effects of oligomeric chitosan selenium anti-ZEN on mitochondrial membrane potential of IPEC-J2 cells observed by fluorescence microscopy; B: effects of oligomeric chitosan selenium anti-ZEN on mitochondrial membrane potential in IPEC-J2 cells. 图 3 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞线粒体膜电位的影响 Fig. 3 Effects of oligomeric chitosan selenium on mitochondrial membrane potential of IPEC-J2 cells induced by ZEN |
由图 4和表 2可知,ZEN组Nrf2蛋白表达量显著低于对照组(P < 0.05),ZEN+0.5 Se、ZEN+1.5 Se和ZEN+3.0 Se组Nrf2蛋白表达量显著或极显著高于对照组(P < 0.01或P < 0.05);且ZEN+0.5 Se、ZEN+1.5 Se和ZEN+3.0 Se组Nrf2蛋白表达量极显著高于ZEN组(P < 0.01)。ZEN和ZEN+0.5 Se组HO-1蛋白表达量显著低于对照组(P < 0.05),ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组与ZEN组无显著差异(P>0.05),但ZEN+1.5 Se、ZEN+3.0 Se组与对照组也无显著差异(P>0.05)。
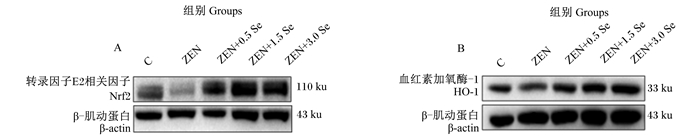
|
A:Nrf2蛋白条带图Nrf2 protein band map;B:HO-1蛋白条带图HO-1 protein band map。 图 4 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞氧化应激相关蛋白表达的条带图 Fig. 4 Band map of oligomeric chitosan selenium on expression of oxidative stress-related proteins in IPEC-J2 cells induced by ZEN |
|
|
表 2 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞氧化应激相关蛋白表达的影响 Table 2 Effects of oligomeric chitosan selenium on expression of oxidative stress-related proteins in IPEC-J2 cells induced by ZEN |
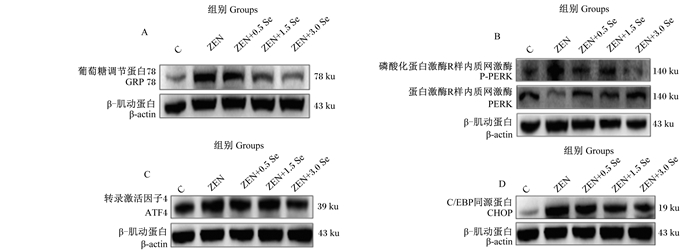
由图 5和表 3可知,ZEN组GRP78、P-PERK/PERK、ATF4、CHOP蛋白表达量均显著或极显著高于对照组(P < 0.05或P < 0.01);ZEN+3.0 Se组GRP78蛋白表达量显著低于ZEN组(P < 0.05);ZEN+0.5 Se、ZEN+1.5 Se和ZEN+3.0 Se组P-PERK/PERK蛋白表达量显著或极显著低于ZEN组(P < 0.05或P < 0.01);ZEN+1.5 Se和ZEN+3.0 Se组ATF4蛋白表达量显著或极显著低于ZEN组(P < 0.05或P < 0.01);ZEN+3.0 Se组CHOP蛋白表达量显著低于ZEN组(P < 0.05)。
|
A:GRP78蛋白条带图GRP78 protein band map;B:P-PERK/PERK蛋白条带图P-PERK/PERK protein band map;C:ATF4蛋白条带图ATF4 protein band map;D:CHOP蛋白条带图CHOP protein band map。 图 5 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞内质网应激相关蛋白表达的条带图 Fig. 5 Band map of oligomeric chitosan selenium on expression of endoplasmic reticulum stress-related proteins in IPEC-J2 cells induced by ZEN |
|
|
表 3 低聚壳聚糖硒对ZEN诱导条件下IPEC-J2细胞内质网应激相关蛋白表达的影响 Table 3 Effects of oligomeric chitosan selenium on expression of endoplasmic reticulum stress-related proteins in IPEC-J2 cells induced by ZEN |
本试验研究表明,IPEC-J2细胞受ZEN损伤后,细胞活力降低,培养上清中的LDH活性升高,而添加低聚壳聚糖硒可增加细胞活力,降低LDH的活性。LDH是一种存在于胞质中的酶,当细胞受到外界刺激引起细胞损伤或凋亡时,LDH就会释放到外界,并存在于细胞培养液中。这表明低聚壳聚糖硒可抵抗ZEN引起的IPEC-J2细胞损伤。
细胞受到刺激时,线粒体膜通透性改变,线粒体膜电位就会降低,与前人研究结果[10]一致,ZEN降低线粒体膜电位,而添加低聚壳聚糖硒后线粒体膜电位会增加,提高了红色荧光的比例,恢复了细胞的状态。
正常的细胞中ROS以较低水平来维持正常的细胞生理活动。氧化应激是ZEN毒性作用的一个重要途径,长期保持高含量的ROS会引起DNA、蛋白质和脂质受损,当超过机体的抗氧化能力后,就会引起细胞损伤和凋亡[11]。据报道,ZEN可引起多种细胞氧化损伤[12-14]。抗氧化剂预处理可降低ZEN引起的ROS的产生[10, 15]。ROS作为一种强氧化应激信号,不仅可以直接损伤细胞,也可以激活一系列损伤、凋亡相关的传导途径。本研究表明,ZEN会使ROS含量大量增加,导致IPEC-J2细胞氧化损伤;添加低聚壳聚糖硒后,ROS含量降低。这说明低聚壳聚糖硒对ROS的产生有一定的抑制作用,可启动细胞内的抗氧化机制。
Nrf2是具有细胞保护功能的抗氧化剂的主要调节剂,可保护细胞不会受到自由基的影响,抵御外来氧化刺激,抑制细胞凋亡,促进细胞存活[16],它是激活HO-1重要的因素,HO-1也是典型的氧化应激标记物[17],是一种经典的抗氧化酶,有良好的抵抗氧化应激的作用[18]。多项研究表明,Nrf2信号通路在抗氧化系统中发挥重要作用。Xiao等[19]研究发现,通过激活Nrf2途径,可改善肠上皮细胞的氧化损伤。酵母硒可激活肾脏中的Nrf2信号通路,改善Nrf2和Kelch样环氧氯丙烷相关蛋白(Keap)的含量,减轻赭曲霉毒素诱导的氧化损伤[20]。与之一致,本试验中,ZEN使Nrf2蛋白表达量显著下降,低聚壳聚糖硒的添加显著增加了Nrf2蛋白表达量。但是对于HO-1,添加ZEN可使其蛋白表达量显著降低,且ZEN+0.5 Se组也显著低于对照组,ZEN+0.5 Se、ZEN+1.5 Se、ZEN+3.0 Se组与ZEN组无显著差异,但ZEN+1.5 Se、ZEN+3.0 Se组与对照组也无显著差异。从而说明本试验剂量下的低聚壳聚糖硒可显著增加Nrf2蛋白表达量,虽然未达到明显增加HO-1蛋白表达量的作用,但是有改善的趋势,可能是低聚壳聚糖硒剂量还不够,至使Nrf2未能完全激活HO-1。
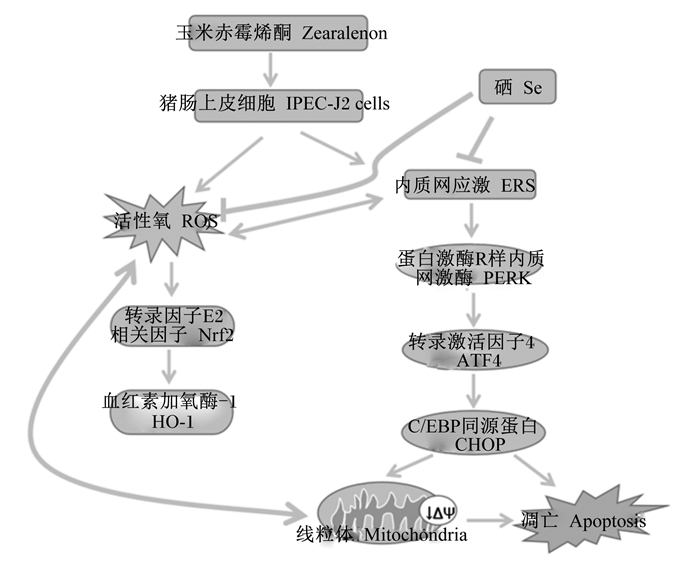
研究发现内质网应激(endoplasmic reticulum stress, ERS)可由ROS产生触发[21],氧化应激通过增加错误折叠的蛋白质破坏氧化还原平衡从而引起内质网应激[22],但也可能是由于ERS产生从而引起活性氧蓄积。当细胞受到刺激时CHOP表达会上升,参与内质网应激凋亡途径[23]。其中PERK可以抑制蛋白质继续翻译,缓解氧化应激,内质网中存在错误折叠的蛋白会诱导PERK激活,促使下游ATF4转录,通过激活凋亡通路PERK/ATF4/CHOP,损伤细胞[24]。GRP78是内质网应激重要的标志性指标,在蛋白质合成和加工过程中起关键作用[25]。ZEN诱导内质网应激信号通路诱导小鼠睾丸细胞凋亡[26]。由呕吐毒素(T-2)引起的ER应激通过内切核糖核酸酶肌醇需要酶1-氨基末端激酶(IRE1-JNK)和PERK-ATF4-CHOP信号转导途径触发山羊子宫内膜上皮细胞凋亡[27]。Se通过抑制ERS从而保护肾脏免受ZEN氧化损伤和凋亡的影响[28]。同样,Xiao等[15]表明,Se改善了ZEN诱导的ROS的产生,并逆转了内质网应激相关基因和GRP78、ATF4的蛋白表达水平,可以缓解ZEN对鸡脾淋巴细胞的损伤。本试验中,ZEN组GRP78、ATF4、P-PERK/PERK、CHOP蛋白表达量相比于对照组均显著增加,并且分别在添加不同浓度低聚壳聚糖硒后表达显著降低,这表明低聚壳聚糖硒通过作用于IPEC-J2细胞的内质网应激通路,表现出对ZEN损伤的保护作用。因此本试验研究发现,低聚壳聚糖硒可能是通过缓解氧化损伤和内质网应激减轻ZEN对IPEC-J2细胞的损伤作用(图 6),当硒作用后,ROS和ERS可被抑制,将不会激活下游CHOP蛋白并且不会改变线粒体膜电位,从而保护IPEC-J2细胞免受ZEN损伤。
|
图 6 低聚壳聚糖硒对ZEN诱导的IPEC-J2细胞氧化应激和内质网应激的机制图 Fig. 6 Mechanism of oligomeric chitosan selenium on oxidative stress and endoplasmic reticulum stress induced by ZEN in IPEC-J2 cells |
低聚壳聚糖硒可缓解ZEN引起的IPEC-J2细胞氧化损伤和内质网应激。
| [1] |
KNUTSEN H, ALEXANDER J, BARREGÅRD L, et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed[J]. European Food Safety Authority, 2017, 15(7): e04851. |
| [2] |
胡成. 酵母硒对保育猪生长性能及腹泻防控效果试验[J]. 养猪, 2021(1): 25-27. HU C. Effect of yeast selenium on growth performance and diarrhea prevention and control of nursery pig[J]. Swine Production, 2021(1): 25-27 (in Chinese). DOI:10.3969/j.issn.1002-1957.2021.01.009 |
| [3] |
刘莹莹, 张星, 任慧波, 等. 低聚壳聚糖对母猪繁殖性能、泌乳性能和血浆生化指标的影响[J]. 动物营养学报, 2017, 29(12): 4525-4533. LIU Y Y, ZHANG X, REN H B, et al. Effects of oligo-chitosan on reproduction performance, lactation performance and plasma biochemical parameters of sows[J]. Chinese Journal of Animal Nutrition, 2017, 29(12): 4525-4533 (in Chinese). DOI:10.3969/j.issn.1006-267x.2017.12.034 |
| [4] |
张干, 张瑞强, 令狐克川, 等. 低聚壳聚糖对中华绒螯蟹生长性能、体成分、非特异性免疫及抗氧化能力的影响[J]. 水产学报, 2020, 44(8): 1340-1348. ZHANG G, ZHANG R Q, LING HU K C, et al. Effects of oligo-chitosan supplementation on growth performance, body composition, non-specific immunity, and antioxidant capacity of Eriocheir sinensis[J]. Journal of Fisheries of China, 2020, 44(8): 1340-1348 (in Chinese). |
| [5] |
FAN W T, SHEN T T, DING Q Q, et al. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells[J]. Journal of Biochemical and Molecular Toxicology, 2017, 31(10): e21944. DOI:10.1002/jbt.21944 |
| [6] |
SHEN T T, MIAO Y F, DING C C, et al. Activation of the p38/MAPK pathway regulates autophagy in response to the CYPOR-dependent oxidative stress induced by zearalenone in porcine intestinal epithelial cells[J]. Food and Chemical Toxicology, 2019, 131: 110527. DOI:10.1016/j.fct.2019.05.035 |
| [7] |
郝媛媛, 刘妍, 李翠茹, 等. 通心络对高脂饮食诱导内质网应激致小鼠血管内皮细胞凋亡的保护作用[J]. 世界科学技术-中医药现代化, 2020, 22(12): 4232-4238. HAO Y Y, LIU Y, LI C R, et al. Protective effect of Tongxinluo on vascular endothelial cell apoptosis induced by high-fat diet-induced endoplasmic reticulum stress in mice[J]. World Science and Technology-Modernization of Traditional Chinese Medicine, 2020, 22(12): 4232-4238 (in Chinese). |
| [8] |
BEN SALEM I, PROLA A, BOUSSABBEH M, et al. Crocin and quercetin protect HCT116 and HEK293 cells from zearalenone-induced apoptosis by reducing endoplasmic reticulum stress[J]. Cell Stress & Chaperones, 2015, 20(6): 927-938. |
| [9] |
徐春兰, 钦传光, 牛卫宁, 等. 硒化壳聚糖的制备及其体外抗氧化活性研究[J]. 化学与生物工程, 2009, 26(9): 45-48. XU C L, QIN C G, NIU W N, et al. Preparation of seleno-chitosan and study on its antioxidant activity in vitro[J]. Chemistry & Bioengineering, 2009, 26(9): 45-48 (in Chinese). |
| [10] |
WANG J J, LI M M, ZHANG W, et al. Protective effect of N-acetylcysteine against oxidative stress induced by zearalenone via mitochondrial apoptosis pathway in SIEC02 cells[J]. Toxins, 2018, 10(10): 407. DOI:10.3390/toxins10100407 |
| [11] |
FERRER E, JUAN-GARCÍA A, FONT G, et al. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells[J]. Toxicology in Vitro, 2009, 23(8): 1504-1509. DOI:10.1016/j.tiv.2009.07.009 |
| [12] |
CAO H W, ZHI Y, XU H B, et al. Zearalenone causes embryotoxicity and induces oxidative stress and apoptosis in differentiated human embryonic stem cells[J]. Toxicology in Vitro, 2019, 54: 243-250. DOI:10.1016/j.tiv.2018.09.020 |
| [13] |
VENKATARAMANA M, NAYAKA S C, ANAND T, et al. Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine[J]. Food and Chemical Toxicology, 2014, 65: 335-342. DOI:10.1016/j.fct.2013.12.042 |
| [14] |
付玉蓉. 虎杖苷缓解玉米赤霉烯酮对奶牛乳腺上皮细胞损伤效果的研究[D]. 硕士学位论文. 长春: 吉林大学, 2020. FU Y R. Effect of polydation on bovine mammary epithelial cells injury induced by zearalenone[D]. Master's Thesis. Changchun: Jilin University, 2020. (in Chinese) |
| [15] |
XIAO Y X, XU S W, ZHAO S C, et al. Protective effects of selenium against zearalenone-induced apoptosis in chicken spleen lymphocyte via an endoplasmic reticulum stress signaling pathway[J]. Cell Stress & Chaperones, 2019, 24(1): 77-89. |
| [16] |
KOBAYASHI A, OHTA T, YAMAMOTO M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes[J]. Methods in Enzymology, 2004, 378: 273-286. |
| [17] |
LI C M, WANG Y R, LI L, et al. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production[J]. Cell Stress & Chaperones, 2019, 24(2): 453-460. DOI:10.1007/s12192-019-00982-4/email/correspondent/c1/new |
| [18] |
乔丽杰, 王延让, 张明. Nrf2/HO-1通路在氧化损伤保护机制中研究进展[J]. 中国职业医学, 2013, 40(1): 82-84. QIAO L J, WANG Y R, ZHANG M. Research progress of Nrf2/HO-1 pathway in mechanism of oxidative damage and protection[J]. China Occupational Medicine, 2013, 40(1): 82-84 (in Chinese). |
| [19] |
XIAO X, SONG D G, CHENG Y Z, et al. Biogenic nanoselenium particles activate Nrf2-ARE pathway by phosphorylating p38, ERK1/2, and AKT on IPEC-J2 cells[J]. Journal of Cellular Physiology, 2019, 234(7): 11227-11234. DOI:10.1002/jcp.27773 |
| [20] |
LI K, CAO Z J, GUO Y, et al. Selenium yeast alleviates ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 4048706. |
| [21] |
BOUSSABBEH M, BEN SALEM I, PROLA A, et al. Patulin induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway[J]. Toxicological Sciences, 2015, 144(2): 328-337. DOI:10.1093/toxsci/kfu319 |
| [22] |
PLAISANCE V, BRAJKOVIC S, TENENBAUM M, et al. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL[J]. PloS One, 2016, 11(9): e0163046. DOI:10.1371/journal.pone.0163046 |
| [23] |
JIANG Q, LIU G, CHEN J S, et al. Crosstalk between nuclear glucose-regulated protein 78 and tumor protein 53 contributes to the lipopolysaccharide aggravated apoptosis of endoplasmic reticulum stress-responsive porcine intestinal epithelial cells[J]. Cellular Physiology and Biochemistry, 2018, 48(6): 2441-2455. DOI:10.1159/000492682 |
| [24] |
HU H, TIAN M X, DING C. CHOP调控内质网应激介导细胞凋亡的机制[J]. 中国预防兽医学报, 2019, 41(2): 219. HU H, TIAN M X, DING C. Mechanisms of CHOP regulating endoplasmic reticulum stress-mediated apoptosis[J]. Chinese Journal of Preventive Veterinary Medicine, 2019, 41(2): 219 (in Chinese). |
| [25] |
RON D, WALTER P. Signal integration in the endoplasmic reticulum unfolded protein response[J]. Nature Reviews Molecular Cell Biology, 2007, 8(7): 519-529. DOI:10.1038/nrm2199 |
| [26] |
冯楠楠, 王冰洁, 郑王龙, 等. PERK在玉米赤霉烯酮诱导TM4细胞凋亡中的作用[J]. 中国兽医学报, 2018, 38(7): 1416-1423. FENG N N, WANG B J, ZHENG W L, et al. The role of PERK in ZEA induced TM4 apoptosis[J]. Chinese Journal of Veterinary Science, 2018, 38(7): 1416-1423 (in Chinese). |
| [27] |
YI Y L, ZHAO F, WANG N, et al. Endoplasmic reticulum stress is involved in the T-2 toxin-induced apoptosis in goat endometrium epithelial cells[J]. Journal of Applied Toxicology, 2018, 38(12): 1492-1501. DOI:10.1002/jat.3655 |
| [28] |
ZHANG Y, HU B, WANG M Y, et al. Selenium protects against zearalenone-induced oxidative stress and apoptosis in the mouse kidney by inhibiting endoplasmic reticulum stress[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 6059058. |




