2. 广东省水产动物精准营养高效饲料工程研究中心, 湛江 524088;
3. 南方海洋科学与工程广东省实验室(湛江), 湛江 524025;
4. 宜昌华太生物科技有限公司, 宜昌 443500
2. Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang 524088, China;
3. Southern Marine Science and Engineering Guangdong Laboratory(Zhanjiang), Zhanjiang 524025, China;
4. Yichang Huatai Biological Technology Co., Ltd., Yichang 443500, China
鱼粉(FM)具有蛋白质含量高、氨基酸(AA)组成平衡、富含不饱和脂肪酸、抗营养因子含量低等优点,是水产动物尤其是肉食性鱼类的最佳蛋白质来源。鱼粉资源紧缺,导致价格不断攀升,给水产养殖业带来了巨大的成本压力,严重限制了水产饲料工业和养殖业的快速、可持续发展。尽管应用粮源性蛋白质饲料替代鱼粉在养殖动物上取得了较好效果[1],一定程度缓解了因鱼粉的高额成本带来的经济压力。然而,随着人们生活水平的提高,动物性食品的占比逐年升高,人畜争粮日益严重,据推测,未来10年间,粮食单产的提升速度远不及饲用粮需求的增长速度[2]。粮食需求量的刚性增长,以及人们对动物性食品的需求,要求最大程度减少饲料工业对粮源性蛋白质饲料的依赖[3]。充分开发、高效利用非粮蛋白质源替代鱼粉对饲料工业,尤其是水产饲料行业的发展具有重大意义。
随着人民生活水平的提高以及社会经济的快速发展,消费者对营养价值高、口感鲜美的优质水产品需求愈来愈强烈。水产品品质不仅受水产动物自身影响,如品系[4]和规格[5],还受外部因素调控,如投饲策略[6]、养殖水体温度[7]、盐度[8]、溶解氧[9]、密度[10]以及饲料[11]。研究发现,饲料中添加3%的酿酒酵母水解物能够显著提高斜带石斑鱼(Epinephelus coioides)肌肉中粗蛋白质(CP)含量,降低粗脂肪(EE)含量[12]。25%的豆粕替代鱼粉,显著提高了日本鲈鱼(Lateolabrax japonicus)肌肉中水分和C18 ∶ 3n-3含量[13]。9%的蚕豆粉代替大豆粉,显著降低了草鱼(Ctenoparyngodon idellus)肌肉中n-6多不饱和脂肪酸(n-6 PUFA)和n-3多不饱和脂肪酸(n-3 PUFA)含量,单不饱和脂肪酸(MUFA)含量以及肌肉的硬度、咀嚼性和胶黏性也显著提高[14]。鸡肉粉是家禽加工过程中的副产物,是经过蒸煮、压制、干燥和粉碎后产生的动物蛋白质源。研究发现,鸡肉粉替代75%的鱼粉,显著降低了大菱鲆(Psetta maeotica)肌肉的CP含量,提高了水分和粗灰分的含量[15]。随着鸡肉粉替代鱼粉比例的升高,大西洋鲷(Sparus aurata)[16]和珍珠龙胆石斑鱼(E. fuscoguttatus ♀×E. lanceolatus ♂)[17]肌肉中EE含量显著降低。将鸡肉粉酶解可优化其营养价值[18]。酶解鸡肉粉(enzyme-digested poultry by-product meal, EP)CP含量高,富含游离氨基酸和小肽。小肽可被直接吸收利用,能促进蛋白质的合成,加快矿物元素的吸收,改善适口性和诱食性,可提高经济效益[19]。
2020年中国渔业统计年鉴显示,海水养殖鱼类中,石斑鱼产量达18.31万t,位居第二[20]。珍珠龙胆石斑鱼是石斑鱼养殖的主要品种之一,具有抗病力强、肉质鲜美、经济效益高、生长速度快和发展潜力大等特点[21]。鱼粉替代技术及石斑鱼产品品质的改善均影响着石斑鱼产业的进一步发展。因此,本试验以珍珠龙胆石斑鱼为研究对象,探究非粮蛋白源EP替代鱼粉对珍珠龙胆石斑鱼肌肉品质及肌肉生长相关基因表达的影响,为开拓非粮蛋白质源的使用,研制石斑鱼资源节约型配合饲料提供理论基础。
1 材料与方法 1.1 试验饲料鱼粉和酶解鸡肉粉(100%来源于鸡胫骨肌肉,由宜昌某生物技术有限公司提供)的概略营养组成见表 1,酶解鸡肉粉的肽分子质量分布见图 1。配制以0(EP0组,作为对照组)、3%(EP3组)、6%(EP6组)、9%(EP9组)、12%(EP12组)、15%(EP15组)和18%(EP18组)酶解鸡肉粉等量替代鱼粉的7种等氮等脂的试验饲料。饲料原料经粉碎后,通过60目筛,按照表 2准确称量,通过V型立式混合机(JS-14S型,浙江正泰电器股份有限公司)充分搅拌,加入预先称重的豆油、大豆磷脂、鱼油和水,二次搅拌混合,用膨化机制得直径为3.00 mm的颗粒。将颗粒饲料置于25 ℃空调房干燥48 h,直至水分含量约为10%,后储存于-20 ℃保存备用。
|
|
表 1 鱼粉和酶解鸡肉粉的概略营养组成(干物质基础) Table 1 Proximate nutrient composition of the FM and EP (DM basis) |
|
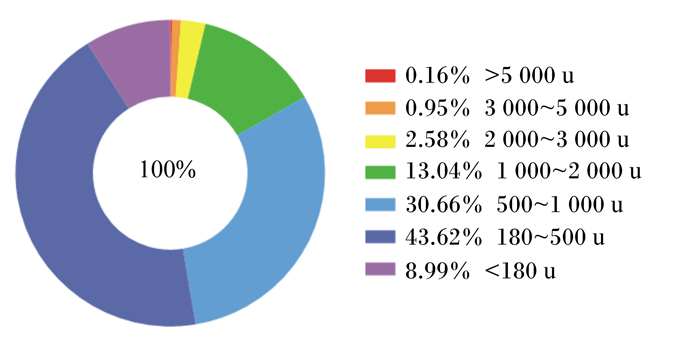
图 1 酶解鸡肉粉的肽分子质量分布 Fig. 1 Peptide molecular weight distribution of EP |
|
|
表 2 试验饲料组成及营养水平(干物质基础) Table 2 Composition and nutrient levels of experimental diets (DM basis) |
珍珠龙胆石斑鱼幼鱼购自广东省湛江市东南码头石斑鱼苗厂,鱼苗购回后在广东省湛江海洋高新科技园室外水泥池暂养2周,期间投喂石斑鱼商业饲料(广东粤海饲料集团,CP含量为50%)。养殖试验开始前,禁食24 h后将630尾初均重为(7.50±0.02) g的珍珠龙胆石斑鱼随机放养于21个玻璃钢桶(300 L),每桶30尾鱼,每种试验饲料投喂3桶。每天表观饱食投喂2次(08:00和16:00),共计56 d。并根据天气变化及吃料情况调整投喂量。每天每个玻璃钢桶中更换约70%的水。养殖期间,养殖水体温度为30.80~31.80 ℃,盐度为29~32,溶解氧含量高于6.15 mg/L,pH为6.80~7.20。
1.3 样品收集与分析 1.3.1 肌肉及饲料营养成分含量测定饲养试验结束后,每桶随机收集3尾石斑鱼的背肌,储存于-20 ℃,以检测肌肉营养成分含量。使用AOAC(1997)[22]方法分析饲料和肌肉的营养成分含量。105 ℃恒重干燥法测定水分含量;索氏抽提法(抽提剂为石油醚)测定粗脂肪EE含量;凯氏定氮法(KjeltecTM 8400定氮仪,瑞典)测定CP含量;550 ℃灼烧法(马弗炉)测定粗灰分含量。饲料中氨基酸组成按照GB/T 18246—2000[23]进行测定。肌肉中氨基酸组成按照GB 5009.124—2016[24]进行测定,脂肪酸组成按照GB 5009.168—2016[25]进行测定。EP的肽分子量分布采用《海洋鱼低聚肽粉》(GB/T 22729—2008)[26]进行检测。
1.3.2 肌肉系水力及质构特性测定随机从每桶选择3尾鱼,取3.00~4.00 g背肌,准确称量,悬挂于充满空气的保鲜袋,于4 ℃冰箱悬吊24 h,再次称重背肌重量,以计算滴水损失[27];另取3.00~4.00 g背肌,用保鲜膜包裹密封,于100 ℃水浴锅蒸煮5 min,冷却至室温,称重以计算蒸煮损失[28]。随机从每桶取6尾鱼,剥离背肌,剪切为3.00 cm(长)×3.00 cm(宽)×1.00 cm(高)的规格,通过质构仪(TMS-PRO,Food Technology Corporation,美国)对进行质构特性分析。
1.3.3 肌肉生长相关基因表达测定随机从每桶选择3尾鱼,解剖并取得背肌,迅速将背肌放置于含有RNA-later(Ambion,美国)的EP管于-80 ℃保存,用于肌肉生长相关基因表达的分析。使用Trizol试剂(北京转基因生物技术有限公司)提取背肌总RNA,1%琼脂糖凝胶电泳验证总RNA的完整性。使用DNase试剂(TaKaRa,日本)处理RNA提取物以去除可能污染的DNA,并使用分光光度计(ND-1000,Nano-Drop Technologies,Wilmington,美国)评估RNA的提取质量。使用Prime ScriptTM RT试剂盒(TaKaRa,日本)进行反转录试验,以获得cDNA。根据SYBR ® Premix Ex TaqTM Ⅱ试剂盒说明书进行实时荧光定量PCR(qRT-PCR)。qRT-PCR的反应体系为10 μL,其中包括5 μL SYBR ® Green Real-Time PCR Master Mix,3.2 μL无菌双蒸水,正向和反向引物各0.4 μL和1 μL cDNA。在荧光定量热循环仪(Bio-Rad CFX96,Bio-Rad Labs,美国)中,以95 ℃,30s;95 ℃,5 s,60 ℃,20 s,40个循环;65 ℃,15 s的条件进行qRT-PCR。内参基因为β-肌动蛋白(β-actin),qRT-PCR引物序列见表 3。根据Mu等[29]的2-ΔΔCt法来计算目的基因的相对表达量。
|
|
表 3 qRT-PCR引物序列 Table 3 Primer sequences for qRT-PCR |
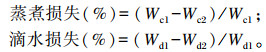
|
式中:Wc1是背肌蒸煮前重量(g);Wc2是背肌蒸煮后重量(g);Wd1是背肌悬挂静置前重量(g);Wd2是背肌悬挂静置后重量(g)。
1.5 统计分析所有数据均通过SPSS 22.0软件进行单因素方差分析(one-way ANOVA),用Tukey法进行多重比较。P < 0.05表示差异显著。结果以平均值±标准误表示。
2 结果 2.1 酶解鸡肉粉对珍珠龙胆石斑鱼肌肉营养成分含量的影响由表 4可知,酶解鸡肉粉替代不同比例鱼粉对珍珠龙胆石斑鱼背肌中水分、CP、EE和粗灰分含量没有显著影响(P>0.05)。
|
|
表 4 饲喂不同饲料石斑鱼的肌肉营养成分含量(湿重基础) Table 4 Muscle nutritional component contents of groupers fed different diets (wet weight basis) |
由表 5可知,酶解鸡肉粉替代不同比例鱼粉对珍珠龙胆石斑鱼肌肉的内聚性和弹性未产生显著影响(P>0.05)。EP6组的蒸煮损失显著低于EP0、EP3、EP9和EP18组(P < 0.05)。EP12、EP15和EP18组的滴水损失显著低于EP3组(P < 0.05)。EP6组的硬度与EP18组无显著差异(P>0.05),但显著高于其他各组(P < 0.05)。EP6组的胶黏性显著高于EP0、EP12和EP15组(P < 0.05)。EP6组的咀嚼性与EP0和EP18组无显著差异(P>0.05),但显著高于其他各组(P < 0.05)。
|
|
表 5 饲喂不同饲料石斑鱼的肌肉系水力和质构特性 Table 5 Muscle water holding capacity and texture properties of groupers fed different diets |
由表 6可知,酶解鸡肉粉替代不同比例鱼粉对珍珠龙胆石斑鱼肌肉中亮氨酸(Leu)、异亮氨酸(Ile)、蛋氨酸(Met)、赖氨酸(Lys)、精氨酸(Arg)、苯丙氨酸(Phe)、苏氨酸(Thr)、酪氨酸(Tyr)、天冬氨酸(Asp)、谷氨酸(Glu)、甘氨酸(Gly)、丙氨酸(Ala)、丝氨酸(Ser)以及总氨基酸、总必需氨基酸、总鲜味氨基酸和总芳香族氨基酸含量均未产生显著影响(P>0.05)。EP0组缬氨酸(Val)含量显著高于EP12组(P < 0.05)。EP0和EP9组组氨酸(His)含量显著高于EP18组(P < 0.05)。EP0组脯氨酸(Pro)含量显著高于EP18组(P < 0.05)。
|
|
表 6 饲喂不同饲料石斑鱼的肌肉氨基酸组成(干物质基础) Table 6 Muscle amino acid composition of groupers fed different diets (DM basis) |
由表 7可知,随着酶解鸡肉粉替代鱼粉比例的增加,珍珠龙胆石斑鱼肌肉C15 ∶ 0、C17 ∶ 0、C20 ∶ 0和C16 ∶ 1n-7的含量逐渐上升,均在EP18组达到最大值,肌肉C22 ∶ 1n-9、C22 ∶ 6n-3(DHA)、∑MUFA、∑n-3 PUFA、总n-3高不饱和脂肪酸(∑n-3 HUFA)的含量及n-3/n-6 PUFA比值呈现先升高后降低的变化趋势。EP9组C22 ∶ 1n-9含量显著高于EP0、EP3、EP6和EP18组(P < 0.05)。EP0组∑MUFA含量显著低于EP12和EP15组(P < 0.05)。EP3组n-3/n-6 PUFA比值及DHA、∑n-3 HUFA和∑n-3 PUFA含量显著高于EP12、EP15和EP18组(P < 0.05)。EP0、EP3和EP6组C24 ∶ 1n-9含量显著高于EP15和EP18组(P < 0.05)。肌肉中其余脂肪酸以及总饱和脂肪酸(∑SFA)、∑n-6 PUFA含量及EPA(C20 ∶ 5n-3)/DHA比值未受到酶解鸡肉粉替代鱼粉比例的影响(P>0.05)。
|
|
表 7 饲喂不同饲料石斑鱼的肌肉脂肪酸组成(干物质基础) Table 7 Muscle fatty acid composition of groupers fed different diets (DM basis) |
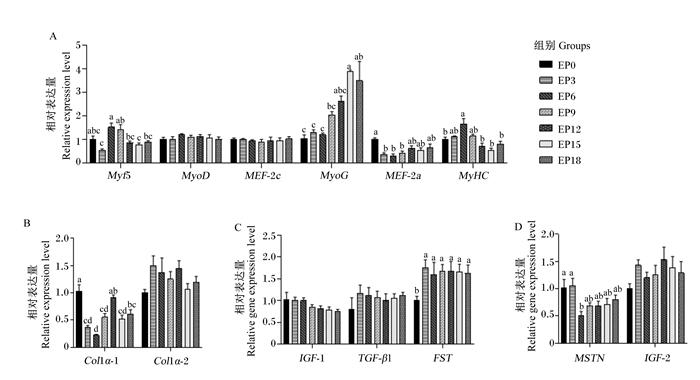
由图 2可知,珍珠龙胆石斑鱼肌肉中肌源性分化因子(MyoD)和肌细胞增强因子2c(MEF-2c)、Ⅰ型胶原蛋白编码基因α-2(Col1α-2)、胰岛素样生长因子-1(IGF-1)和转化生长因子-β1(TGF-β1)、胰岛素样生长因子-2(IGF-2)的相对表达量受饲料影响不显著(P>0.05)。EP6组肌细胞因子5(Myf5)和肌球蛋白重链(MyHC)的相对表达量显著高于EP12、EP15和EP18组(P < 0.05)。EP15组肌细胞生成素(MyoG)的相对表达量显著高于EP0、EP3、EP6和EP9组(P < 0.05)。EP0组肌细胞增强因子-2a(MEF-2a)的相对表达量显著高于EP3、EP6和EP9组(P < 0.05)。EP0组Ⅰ型胶原蛋白编码基因α-1(Col1α-1)的相对表达量与EP12组无显著差异(P>0.05),但显著高于其他各组(P < 0.05)。EP0组卵泡抑素(FST)的相对表达量显著低于其他各组(P < 0.05)。EP0和EP3组肌肉生长抑制素(MSTN)的相对表达量显著高于EP6组(P < 0.05)。
|
数据柱形标注不同字母表示差异显著(P < 0.05),相同字母或无字母标注表示差异不显著(P>0.05)。 Value columns with different letters mean significant difference (P < 0.05), while with the same letters or no letters mean no significant difference (P>0.05). 图 2 饲喂不同饲料石斑鱼的肌肉生肌调节因子(A)、编码胶原蛋白(B)、肌肉生长正调控因子(C)和肌肉生长负调控因子(D)相对表达量 Fig. 2 Relative expression levels of myogenic regulatory factors (A), encoding collagens (B), positive regulators of muscle growth (C) and negative regulators of muscle growth (D) in muscle of groupers fed different diets. |
肌肉作为被食用的主要部分,其营养成分含量则是影响品质的重要因素[30]。在本试验中,随着酶解鸡肉粉替代鱼粉比例的增加,珍珠龙胆石斑鱼肌肉中水分、CP、EE和粗灰分含量无显著变化。在评价鱼类肌肉营养价值的指标中,蛋白质尤为重要。氨基酸作为蛋白质的基本单元,其组成和含量的变化影响着肌肉的营养价值[31-32]。研究表明,珍珠龙胆石斑鱼背肌中含量最丰富的氨基酸是Glu,其次是Asp、Lys和Leu[33-34],本试验中也发现了相同的结果。风味是口腔的触觉、味觉和嗅觉的复合感觉[35],肌肉中必需氨基酸、鲜味氨基酸和芳香族氨基酸的含量与风味密切相关[36]。Glu和Asp主要呈现的是鲜味,Gly和Ala呈现的是甜味,此外,Ser和Pro也与甜味的出现有关[37]。在本试验中,随着酶解鸡肉粉替代鱼粉比例的增加,必需氨基酸Val和His,总鲜味氨基酸及总芳香族氨基酸的含量均呈现下降趋势,意味着酶解鸡肉粉替代鱼粉后,珍珠龙胆石斑鱼肌肉的风味可能会受到影响。
各组试验饲料的EE含量保持不变,酶解鸡肉粉中小肽的高效利用可以节约蛋白质,充分发挥饲料中脂类的能量效应,促进脂肪的分解,使得珍珠龙胆石斑鱼肌肉脂肪酸组成发生变化[38]。有研究发现,不饱和脂肪酸(UFA),尤其是PUFA,能够改善肌肉的风味[39],肌肉的整体可接受程度及肉香味与C16 ∶ 1n-7含量呈正相关关系[40]。在本试验中,随着酶解鸡肉粉替代鱼粉比例的增加,肌肉C16 ∶ 1n-7含量呈现上升趋势,MUFA、∑n-3 PUFA和∑n-3 HUFA含量呈现先升高后降低的变化趋势,∑n-6 PUFA含量呈现先降低后升高的变化趋势。3%和6%酶解鸡肉粉等量替代鱼粉时,肌肉中∑n-3 PUFA和∑n-3 HUFA含量达到最大值。从上述结果可以得出,鱼粉被酶解鸡肉粉替代后,珍珠龙胆石斑鱼肌肉的风味会被改善,然而,肌肉中UFA含量增多,会存在被氧化的风险,产生醇类或者醛类的复合物,使肌肉气味变臭,影响肌肉品质[41]。此外,较高水平的n-3 PUFA具有抗炎作用[42],高水平的n-6 PUFA可以引起血管收缩、血小板聚集和炎症反应[43]。人类食物中n-3/n-6 PUFA比值的推荐值为0.10~0.25[44],比值越低,人类患高脂血症、肥胖症和乳腺癌的风险越高[45-46]。在本试验中,珍珠龙胆石斑鱼肌肉的n-3/n-6 PUHA比值为0.51~0.60。随着酶解鸡肉粉替代鱼粉比例的增加,n-3/n-6 PUHA比值逐渐变小,逐步向食物最佳n-3/n-6 PUHA比值靠近。
系水力是衡量肌肉品质的一个重要指标,能够反映肌肉中可溶性物质及液态物质的流失情况,对肌肉的营养成分的保留及风味的保持具有重要意义[47]。硬度、内聚性、弹性、胶黏性和咀嚼性是肌肉的主要质构特性[48],不仅影响水产品的外观,还影响口感。在本试验中,当6%酶解鸡肉粉等量替代鱼粉时,珍珠龙胆石斑鱼肌肉的蒸煮损失显著降低,硬度、胶黏性和咀嚼性均显著提高,提示EP6组珍珠龙胆石斑鱼肌肉的风味及其营养物质得到较好的保留,质地较硬,更具有嚼劲。
生肌调节因子(MRFs)及其相关调节基因是影响肌肉品质的内因[49],对肌原细胞分化、肌纤维发育以及肌肉组织的形成和生长具有重要的调节作用[50-51]。MRFs家族的表达具有时序性,在体细胞形成过程中,首先表达的是Myf5,其次是MyoD和MEF-2c,然后是MEF-2a和MyoG,最后是MyHC[52]。其中,Myf5和MyoD主要作为生肌决定因子[53],MyoG在肌细胞分化中起着重要作用[54]。在本试验中,6%酶解鸡肉粉等量替代鱼粉后,珍珠龙胆石斑鱼肌肉中Myf5和MyHC的相对表达量显著提高,改善了肌肉细胞的分化。IGF-1是一种重要的生长因子,在鱼类肌肉中具有多种受体,能促进细胞活化、增殖和分化,是MRFs的正调节因子[55]。相反,IGF-2是MRFs的负调节因子[56]。在本试验中,IGF-1和IGF-2的表达受饲料的影响不显著。鱼类肌肉中胶原蛋白的含量丰富,主要的胶原蛋白类型为Ⅰ型,由2条α-1链(Col1α-1)和1条α-2链(Col1α-2)编码[57]。胶原蛋白的合成和交联主要受TGF-β1调控,TGF-β1介导Ⅰ型胶原蛋白的表达[58]。在本试验中,TGF-β1和Col1α-2的表达受饲料的影响不显著,酶解鸡肉粉替代鱼粉后,下调了Col1α-1的表达,对胶原蛋白的形成产生负面影响。MSTN编码肌肉生长抑制素蛋白,是肌肉生长的负调节因子[51]。FST可以抑制MSTN对肌肉生长的负调节作用,促进肌肉的生长发育[59]。饲料中鱼粉被6%酶解鸡肉粉等量替代后,珍珠龙胆石斑鱼肌肉中MSTN的相对表达量显著降低,FST的相对表达量显著提高,表明6%酶解鸡肉粉等量替代鱼粉有利于珍珠龙胆石斑鱼肌肉的分化和生长,能够提高肌肉的生长。
4 结论① 6%的酶解鸡肉粉等量替代鱼粉能够显著改善珍珠龙胆石斑鱼肌肉的系水力和质构特性。
② 6%的酶解鸡肉粉等量替代鱼粉能够显著提高珍珠龙胆石斑鱼肌肉中MRFs家族及肌肉生长正调控因子的表达,抑制肌肉生长负调控因子的表达。
| [1] |
GÓMEZ-REQUENI P, MINGARRO M, CALDUCH-GINER J A, et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata)[J]. Aquaculture, 2004, 232. |
| [2] |
单国星. 人畜争粮矛盾下饲草料基地建设对农牧民养殖效益的影响——以新疆新源县喀拉布拉镇为例[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2020. SHAN G X. The impact of forage base construction on farmers and herdsmen's breeding benefits under the contradiction between people and animals for grain-taking Kerala town, Xinyuan County, Xinjiang as an example[D]. Master's Thesis. Yangling: Northwest A & F University, 2020. (in Chinese) |
| [3] |
YTRESTØYL T, AAS T S, ÅSGÅRD T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway[J]. Aquaculture, 2015, 448: 365-374. DOI:10.1016/j.aquaculture.2015.06.023 |
| [4] |
JOHNSTON I A, LI X J, VIEIRA V A, et al. Muscle and flesh quality traits in wild and farmed Atlantic salmon[J]. Aquaculture, 2006, 256(1/2/3/4): 323-336. |
| [5] |
SHEARER K D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids[J]. Aquaculture, 1994, 119(1): 63-88. DOI:10.1016/0044-8486(94)90444-8 |
| [6] |
IMSLAND A K D, ROTH B, CHRISTENSEN L B, et al. Effects of short-term starvation periods on flesh quality in Arctic charr (Salvelinus alpinus) in different seasons[J]. Aquaculture Research, 2020, 51(10): 4022-4029. DOI:10.1111/are.14745 |
| [7] |
LÓPEZ A O, ABDEL I, PERIAGO M J, et al. Temperature influence on the white muscle growth dynamics of the sea bass Dicentrarchus labrax, L.flesh quality implications at commercial size[J]. Aquaculture, 2008, 277(1/2): 39-51. |
| [8] |
程亚美, 赵金良, 宋凌元, 等. 盐度、碱度对尼罗罗非鱼生长和肌肉品质的影响[J]. 水产科学, 2020, 39(3): 341-349. CHENG Y M, ZHAO J L, SONG L Y, et al. Effects of salinity and alkalinity on growth performance and muscle quality of Nile tilapia Oreochromis niloticus[J]. Fisheries Science, 2020, 39(3): 341-349 (in Chinese). |
| [9] |
ZUANAZZI J S G, DE LARA J A F, GOES E S D R, et al. Anoxia stress and effect on flesh quality and gene expression of tilapia[J]. Food Science and Technology, 2019, 39(1): 195-202. DOI:10.1590/fst.00518 |
| [10] |
SILVA V A, TRUSHENSKI J, SCHWARZ M H, et al. Effects of rearing density on growth, physiological responses, and flesh quality in juvenile cobia (Rachycentron canadum)[J]. Journal of the World Aquaculture Society, 2020, 51(6): 1301-1312. DOI:10.1111/jwas.12721 |
| [11] |
ZHAO Y, LI J Y, YIN L, et al. Effects of dietary glutamate supplementation on flesh quality, antioxidant defense and gene expression related to lipid metabolism and myogenic regulation in Jian carp (Cyprinus carpio var. Jian)[J]. Aquaculture, 2019, 502: 212-222. DOI:10.1016/j.aquaculture.2018.12.050 |
| [12] |
YANG X Y, HE Y F, CHI S Y, et al. Supplementation with Saccharomyces cerevisiae hydrolysate in a complex plant protein, low-fishmeal diet improves intestinal morphology, immune function and Vibrio harveyi disease resistance in Epinephelus coioides[J]. Aquaculture, 2020, 529: 735655. DOI:10.1016/j.aquaculture.2020.735655 |
| [13] |
LIANG X F, HU L, DONG Y C, et al. Substitution of fish meal by fermented soybean meal affects the growth performance and flesh quality of Japanese seabass (Lateolabrax japonicus)[J]. Animal Feed Science and Technology, 2017, 229: 1-12. DOI:10.1016/j.anifeedsci.2017.03.006 |
| [14] |
LI X X, CHEN S J, SUN J J, et al. Partial substitution of soybean meal with faba bean meal in grass carp (Ctenopharyngodon idella) diets, and the effects on muscle fatty acid composition, flesh quality, and expression of myogenic regulatory factors[J]. Journal of the World Aquaculture Society, 2020, 51(5): 1145-1160. DOI:10.1111/jwas.12671 |
| [15] |
YIGIT M, ERDEM M, KOSHIO S, et al. Substituting fish meal with poultry by-product meal in diets for black sea turbot Psetta maeotica[J]. Aquaculture Nutrition, 2006, 12(5): 340-347. DOI:10.1111/j.1365-2095.2006.00409.x |
| [16] |
KARAPANAGIOTIDIS I T, PSOFAKIS P, MENTE E, et al. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata)[J]. Aquaculture Nutrition, 2019, 25(1): 3-14. DOI:10.1111/anu.12824 |
| [17] |
ZHOU Z Y, YAO W, YE B, et al. Effects of replacing fishmeal protein with poultry by-product meal protein and soybean meal protein on growth, feed intake, feed utilization, gut and liver histology of hybrid grouper (Epinephelus fuscoguttatus ♀×Epinephelus lanceolatus ♂) juveniles[J]. Aquaculture, 2020, 516: 734503. DOI:10.1016/j.aquaculture.2019.734503 |
| [18] |
BARBOUR G W, NEMASETONI R, LILBURN M S, et al. The effect of enzyme predigestion on the quality of poultry by-product meal from whole turkey mortality[J]. Poultry Science, 1995, 74(7): 1180-1190. DOI:10.3382/ps.0741180 |
| [19] |
MARTÍNEZ-ALVAREZ O, CHAMORRO S, BRENES A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review[J]. Food Research International, 2015, 73: 204-212. DOI:10.1016/j.foodres.2015.04.005 |
| [20] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 中国渔业统计年鉴2020[M]. 北京: 中国农业出版社, 2020: 22-26. Fishery Administration Bureau of Ministry of Agriculture and Rural Areas, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2020[M]. Beijing: China Agriculture Press, 2020: 22-26 (in Chinese). |
| [21] |
唐怀庆, 张健东, 陈刚, 等. 养殖密度、投喂频率和投喂水平对珍珠龙胆石斑鱼特定生长率、饲料转化率和胃蛋白酶活力的协同影响[J]. 广东海洋大学学报, 2018, 38(1): 22-31. TANG H Q, ZHANG J D, CHEN G, et al. Combined effects of breeding density, feeding frequency and feeding level on specific growth rate, feed conversion rate and pepsin activity of juvenile hybrid groupers (Epinephelus fuscoguttatus ♀×E. lanceolatus ♂)[J]. Journal of Guangdong Ocean University, 2018, 38(1): 22-314 (in Chinese). DOI:10.3969/j.issn.1673-9159.2018.01.004 |
| [22] |
AO AC. Official methods of analysis of AOAC International.Volume Ⅰ, agricultural chemicals, contaminants, drugs[[M]. Gaithersburg: AOAC International, 2010.
|
| [23] |
国家质量技术监督局. 饲料中氨基酸的测定: GB/T 18246-2000[S]. 北京: 中国标准出版社, 2000. The State Bureau of Quality and Technical Supervision. Determination of amino acids in feeds: GB/T 18246-2000[S]. Beijing: Standards Press of China, 2000. (in Chinese) |
| [24] |
国家卫生和计划生育委员会, 国家食品药品监督管理总局. 食品安全国家标准食品中氨基酸的测定: GB 5009.124-2016[S]. 北京: 中国标准出版社, 2016. National Health and Family Planning Commission of the People's Republic of China, China Food and Drug Administration. National food safety standards-Determination of amino acids in food: GB 5009.124-2016[S]. Beijing: Standards Press of China, 2016. (in Chinese) |
| [25] |
国家卫生和计划生育委员会, 国家食品药品监督管理总局. 食品安全国家标准食品中脂肪酸的测定: GB 5009.168-2016[S]. 北京: 中国标准出版社, 2016. National Health and Family Planning Commission of the People's Republic of China, China Food and Drug Administration. National food safety standards-Determination of fatty acids in food: GB 5009.168-2016[S]. Beijing: Standards Press of China, 2016. (in Chinese) |
| [26] |
中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员. 海洋鱼低聚肽粉: GB/T 22729-2008[S]. 北京: 中国标准出版社, 2008. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. Oligopeptides powder of marine fish: GB/T 22729-2008[S]. Beijing: Standards Press of China, 2008. (in Chinese) |
| [27] |
BIDNER B S, ELLIS M, WITTE D P, et al. Influence of dietary lysine level, pre-slaughter fasting, and rendement napole genotype on fresh pork quality[J]. Meat Science, 2004, 68(1): 53-60. DOI:10.1016/j.meatsci.2003.10.018 |
| [28] |
BERTRAM H C, ANDERSEN H J, KARLSSON A H, et al. Prediction of technological quality (cooking loss and napole yield) of pork based on fresh meat characteristics[J]. Meat Science, 2003, 65(2): 707-712. DOI:10.1016/S0309-1740(02)00272-3 |
| [29] |
MU H, SHEN H H, LIU J H, et al. High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea[J]. Fish & Shellfish Immunology, 2018, 77: 465-473. |
| [30] |
VIDELER J J. An opinion paper: emphasis on white muscle development and growth to improve farmed fish flesh quality[J]. Fish Physiology and Biochemistry, 2011, 37(2): 337-343. DOI:10.1007/s10695-011-9501-4 |
| [31] |
黄洋, 黄海立, 杜涛, 等. 野生多鳞鱚(Sillago sihama)肌肉营养成分分析及品质评价[J]. 广东海洋大学学报, 2015, 35(6): 9-14. HUANG Y, HUANG H L, DU T, et al. Analysis and evaluation of main nutritive composition in the muscle of wild Sillago sihama[J]. Journal of Guangdong Ocean University, 2015, 35(6): 9-14 (in Chinese). DOI:10.3969/j.issn.1673-9159.2015.06.002 |
| [32] |
SANGIAO-ALVARELLOS S, LAIZ-CARRIÓN R, GUZMÁN J M, et al. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs[J]. American Journal of Physiology: Regulatory Integrative and Comparative Physiology, 2003, 285(4): R897-R907. DOI:10.1152/ajpregu.00161.2003 |
| [33] |
于宏, 万刚涛, 程民杰, 等. 龙虎斑鱼肌肉营养成分分析[J]. 广东海洋大学学报, 2014, 34(6): 83-87. YU H, WAN G T, CHENG M J, et al. Analysis of the nutritive components of muscle from pearl gentian grouper (Epinephelus fuscoguttatus ♀×E. lanceolatus ♂)[J]. Journal of Guangdong Ocean University, 2014, 34(6): 83-87 (in Chinese). DOI:10.3969/j.issn.1673-9159.2014.06.014 |
| [34] |
王继英, 张德瑞, 马晶晶, 等. 珍珠龙胆石斑鱼肌肉营养成分分析与品质评价[J]. 海洋湖沼通, 2015(4): 61-69. WANG J Y, ZHANG D R, MA J J, et al. Nutritional components analysis and nutritive value evaluation of ♀Epinephelus fuscoguttatus×E. lanceolatus ♂ muscles[J]. Transactions of Oceanology and Limnology, 2015(4): 61-69 (in Chinese). |
| [35] |
胡宇超, 王园, 孟子琪, 等. 发酵麸皮多糖对肉羊肉品质、肌肉氨基酸组成及肌肉抗氧化酶和肌纤维类型相关基因表达的影响[J]. 动物营养学报, 2020, 32(2): 932-940. HU Y C, WANG Y, MENG Z Q, et al. Effects of fermented wheat bran polysaccharides on meat quality, muscle amino acid composition and expression of antioxidant enzymes and muscle fiber type-related genes in muscle of mutton sheep[J]. Chinese Journal of Animal Nutrition, 2020, 32(2): 932-940 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.02.049 |
| [36] |
金亚东, 杨万宗, 吴爽, 等. 饲粮精料比例对羊屠宰性能和肉品质的影响及其作用机理[J]. 动物营养学报, 2019, 31(10): 4450-4457. JIN Y D, YANG W Z, WU S, et al. Effects of dietary concentrate ratios on slaughter performance and meat quality in sheep and its mechanisms[J]. Chinese Journal of Animal Nutrition, 2019, 31(10): 4450-4457 (in Chinese). |
| [37] |
PARK J N, WATANABE T, ENDOH K I, et al. Taste-active components in a Vietnamese fish sauce[J]. Fisheries Science, 2002, 68(4): 913-920. DOI:10.1046/j.1444-2906.2002.00510.x |
| [38] |
DREVON C A. Fatty acids and expression of adipokines[J]. Biochimica et Biophysica Acta (BBA): Molecular Basis of Disease, 2005, 1740(2): 287-292. DOI:10.1016/j.bbadis.2004.11.019 |
| [39] |
WOOD J D, ENSER M, FISHER A V, et al. Fat deposition, fatty acid composition and meat quality: a review[J]. Meat Science, 2008, 78(4): 343-358. DOI:10.1016/j.meatsci.2007.07.019 |
| [40] |
LUCHTMAN D W, SONG C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies[J]. Neuropharmacology, 2013, 64: 550-565. DOI:10.1016/j.neuropharm.2012.07.019 |
| [41] |
SECCI G, PARISI G. From farm to fork: lipid oxidation in fish products.A review[J]. Italian Journal of Animal Science, 2016, 15(1): 124-136. DOI:10.1080/1828051X.2015.1128687 |
| [42] |
MCMURRAY D N, JOLLY C A, CHAPKIN R S. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model[J]. The Journal of Infectious Diseases, 2000, 182(Suppl.1): S103-S107. |
| [43] |
黄伟卿, 阮少江, 周逢芳, 等. 饲料中添加太子参提取物对大黄鱼肌肉营养的影响[J]. 饲料研究, 2020, 43(9): 50-55. HUANG W Q, RUAN S J, ZHOU F F, et al. Effect of Pseudostellaria heterohpylla extracts on nutrient quality in muscle of large yellow croaker[J]. Feed Research, 2020, 43(9): 50-55 (in Chinese). |
| [44] |
COLLETT E D, DAVIDSON L A, FAN Y Y, et al. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes[J]. American Journal of Physiology: Cell Physiology, 2001, 280(5): C1066-C1075. DOI:10.1152/ajpcell.2001.280.5.C1066 |
| [45] |
AILHAUD G, MASSIERA F, WEILL P, et al. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity[J]. Progress in Lipid Research, 2006, 45(3): 203-236. DOI:10.1016/j.plipres.2006.01.003 |
| [46] |
JEROMSON S, GALLAGHER I J, GALLOWAY S D R, et al. Omega-3 fatty acids and skeletal muscle health[J]. Marine Drugs, 2015, 13(11): 6977-7004. DOI:10.3390/md13116977 |
| [47] |
BRINKER A, FRIEDRICH C. Fish meal replacement by plant protein substitution and guar gum addition in trout feed.Part Ⅱ: effects on faeces stability and rheology[J]. Biorheology, 2012, 49(1): 27-48. DOI:10.3233/BIR-2012-0605 |
| [48] |
CHEN W J, WANG Y, HAN D, et al. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio)[J]. Aquaculture Nutrition, 2019, 25(5): 1145-1155. DOI:10.1111/anu.12930 |
| [49] |
CHENG J H, SUN D W, HAN Z, et al. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review[J]. Comprehensive Reviews in Food Science and Food Safety, 2014, 13(1): 52-61. DOI:10.1111/1541-4337.12043 |
| [50] |
LIN Y Q, ZHOU J S, LI R W, et al. Cloning and expression patterns of MRFs and effect of replacing dietary fish oil with vegetable oils on MRFs expression in grass carp (Ctenopharyngodon idellus)[J]. Turkish Journal of Fisheries and Aquatic Sciences, 2015, 15: 255-264. |
| [51] |
ZHENG G D, SUN C F, PU J W, et al. Two myostatin genes exhibit divergent and conserved functions in grass carp (Ctenopharyngodon idellus)[J]. General and Comparative Endocrinology, 2015, 214: 68-76. DOI:10.1016/j.ygcen.2015.03.008 |
| [52] |
WATABE S. Myogenic regulatory factors and muscle differentiation during ontogeny in fish[J]. Journal of Fish Biology, 1999, 55(sA): 1-18. DOI:10.1111/j.1095-8649.1999.tb01042.x |
| [53] |
RUDNICKI M A, JAENISCH R. The MyoD family of transcription factors and skeletal myogenesis[J]. BioEssays, 1995, 17(3): 203-209. DOI:10.1002/bies.950170306 |
| [54] |
KASSAR-DUCHOSSOY L, GAYRAUD-MOREL B, GOMōS D, et al. Mrf4 determines skeletal muscle identity in Myf5:myod double-mutant mice[J]. Nature, 2004, 431(7007): 466-471. DOI:10.1038/nature02876 |
| [55] |
CASTILLO J, AMMENDRUP-JOHNSEN I, CODINA M, et al. IGF-I and insulin receptor signal transduction in trout muscle cells[J]. American Journal of Physiology: Regulatory Integrative and Comparative Physiology, 2006, 290(6): R1683-R1690. DOI:10.1152/ajpregu.00294.2005 |
| [56] |
SNIJDERS T, NEDERVEEN J P, MCKAY B R, et al. Satellite cells in human skeletal muscle plasticity[J]. Frontiers in Physiology, 2015, 6: 283. |
| [57] |
GELSE K, PÖSCHL E, AIGNER T. Collagens-structure, function, and biosynthesis[J]. Advanced Drug Delivery Reviews, 2003, 55(12): 1531-1546. DOI:10.1016/j.addr.2003.08.002 |
| [58] |
CHEN Y W, NAGARAJU K, BAKAY M, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy[J]. Neurology, 2005, 65(6): 826-834. DOI:10.1212/01.wnl.0000173836.09176.c4 |
| [59] |
REBHAN Y, FUNKENSTEIN B. Inhibition of fish myostatin activity by recombinant fish follistatin and myostatin prodomain: potential implications for enhancing muscle growth in farmed fish[J]. Aquaculture, 2008, 284(1/2/3/4): 231-238. |




