奶牛泌乳过程由多个组织器官协同完成。反刍动物具有瘤胃这一特殊的消化道器官,饲粮中的营养物质首先在瘤胃中被微生物发酵后再到达小肠和后肠道,这一过程中饲粮中的物质发生了巨大的变化,如碳水化合物发酵为挥发性脂肪酸(volatile fatty acid,VFA),蛋白质发酵为小肽和氨态氮,脂类被微生物利用组合产生新的脂肪酸等。瘤胃发酵产物经消化道吸收后,在肝脏代谢作用下,最终在乳腺中合成乳蛋白、乳脂及乳糖等营养物质。提高乳产量和乳品质是奶牛行业一直追求的目标,因此,深入了解饲粮在反刍动物体内消化、吸收和代谢的调控机制,不仅能精准调控乳产量和乳品质,对奶牛的健康养殖也有重要意义。本文系统阐述了胃肠道-肝脏-乳腺等多器官一体化调控乳成分的合成机理,旨在为提高乳品质及奶牛产奶效率提供理论依据。
1 胃肠道-肝脏-乳腺协同调控乳蛋白合成乳蛋白是乳中干物质的重要组成成分,其含量是衡量乳品质优劣的关键指标。乳蛋白在机体内的消化吸收率高达98%,可提供机体所需的各种必需氨基酸,并在抵抗高血压及增强免疫力等生理功能中发挥重要作用[1]。
乳蛋白合成过程如图 1所示。反刍动物瘤胃微生物利用肽、氨基酸和氨等小分子物质合成微生物蛋白(microbial protein,MCP),这些小分子物质来源于瘤胃中的细菌、原虫及真菌等对机体摄入部分蛋白质的降解[2]。饲粮中瘤胃非降解蛋白质与MCP一起到达小肠被分解成小肽和氨基酸,小肽和氨基酸经小肠壁吸收后由门静脉进入肝脏,经血液循环后分配给乳腺组织利用[3]。瘤胃微生物可通过改变MCP而间接影响乳蛋白合成,研究发现乳蛋白含量与脱硫弧菌属(Desulfovibrio)、普雷沃氏菌属(Prevotella)以及丁酸弧菌属(Butyrivibrio)呈正相关关系[4-5],与欧陆森氏菌属(Olsenella)呈负相关关系[6]。同时,瘤胃中的普雷沃氏菌属和溶纤维丁酸弧菌(Butyrivibrio fibrisolvens)等蛋白质降解菌能够加速饲粮中的蛋白质形成游离氨基酸和寡肽等MCP合成前体物质[7]。
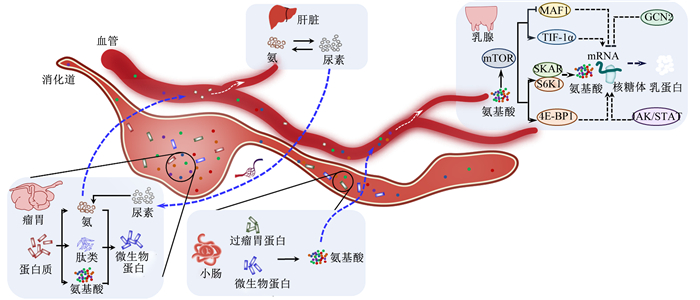
|
mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;MAF1:RNA聚合酶Ⅲ负调控因子negative regulator of RNA polymerase Ⅲ;TIF-1α:转录调节因子-1α transcriptional intermediary factor-1α;SKAR:DNA聚合酶δ相互作用蛋白3 DNA polymerase delta interaction protein 3;S6K1:核糖体S6蛋白激酶1 ribosomal S6 protein kinase 1;4E-BP1:4E结合蛋白1 4E-binding protein 1;GCN2:一般性调控阻遏蛋白激酶2 general control nonderepressible 2;JAK/STAT:Janus激酶/信号传导及转录激活因子Janus kinase/signal transducer and activator of transcription。 图 1 奶畜乳蛋白合成与调控示意图 Fig. 1 Schematic diagram of synthesis and regulation of dairy milk protein |
瘤胃微生物分解饲粮中的非蛋白氮产生氨,一部分用于合成MCP,另一部分被瘤胃壁吸收,经门静脉运送到肝脏重新转化为尿素[8]。氨和尿素的生成过程不断循环,称为瘤胃-肝脏中的尿素氮循环。氮素循环在反刍动物乳蛋白的合成中发挥重要作用,反刍动物通过尿素氮循环利用大量的氮,其在肝脏中合成的尿素有40%~80%进入消化道[8]。乳蛋白产量高的奶牛比乳蛋白产量低的奶牛瘤胃中氨态氮浓度高[9]。
乳腺利用经瘤胃-小肠释放到血液中的游离氨基酸以及肝脏合成的部分小肽、蛋白质等合成乳蛋白。哺乳动物雷帕霉素靶蛋白复合物1(mammalian target of rapamycin complex 1,mTORC1)通路是调控乳腺中乳蛋白合成的重要通路,主要通过RNA聚合酶Ⅲ负调控因子(negative regulator of RNA polymerase Ⅲ,MAF1)、转录调节因子-1α(transcriptional intermediary factor-1α,TIF-1α)、DNA聚合酶δ相互作用蛋白3(DNA polymerase delta interaction protein 3,SKAR)、核糖体S6蛋白激酶1(ribosomal S6 protein kinase 1,S6K1)、4E结合蛋白1(4E-binding protein 1,4E-BP1)等蛋白调控乳蛋白合成[10]。一般性调控阻遏蛋白激酶2(general control nonderepressible 2, GCN2)属于保守的丝氨酸/苏氨酸激酶家族,通过真核生物起始因子2α(eIF2α)调控蛋白合成速率[11]。本研究团队前期以新生奶公犊牛胰腺原代腺泡细胞为模型,研究发现添加苯丙氨酸能增加胰酶mRNA表达及mTORC1下游蛋白(S6K1、4E-BP1)的磷酸化水平,促进胰腺淀粉酶的合成与分泌[12]。在该模型中,添加亮氨酸不仅激活了磷脂酰肌醇-3-羟激酶(phosphatidylinositol-3-hydroxy kinase,PI3K)-蛋白激酶B(protein kinase B, Akt)-mTORC1轴,同时也抑制了GCN2信号通路,以促进蛋白质合成和抑制蛋白质降解的方式促进淀粉酶的合成[13-14]。此外,Janus激酶/信号传导及转录激活因子(Janus kinase/signal transducer and activator of transcription, JAK/STAT)信号通路能够调控奶牛乳蛋白基因的转录和翻译[15-16](图 1)。
2 胃肠道-肝脏-乳腺协同调控乳脂合成乳脂合成是一个复杂的生物学过程,该过程受脂质合成与转运相关因子及相关信号通路的调控(图 2)。甘油三酯(triacylglycerol, TAG)是乳脂的主要成分,占乳脂总量的95%~98%。乳脂中除TAG外,还含有大量活性物质,如共轭亚油酸、鞘磷脂等,其中共轭亚油酸具有抗动脉硬化和免疫调节特性,神经鞘磷脂及其代谢产物能够抑制肿瘤[17-18]。
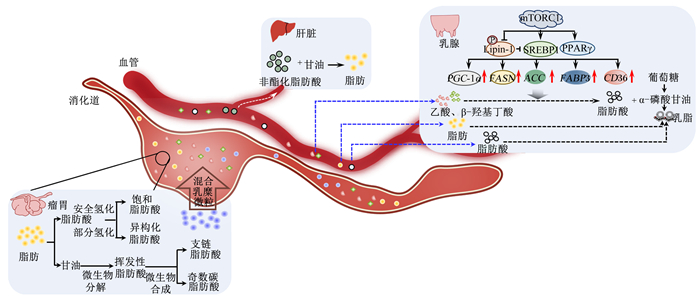
|
mTORC1:哺乳动物雷帕霉素靶蛋白复合物1 mammalian target of rapamycin complex 1;Lipin-1:磷脂酸磷酸水解酶-1 phosphatidic acid phosphohydrolase-1;SREBP1:固醇调节元件结合蛋白1 sterol regulatory element-binding protein 1;PPARγ:过氧化物酶体增殖物激活受体γ peroxisome proliferators-activated receptor γ;PGC-1α:过氧化物酶体增殖物激活受体γ辅激活因子-1α peroxisome proliferators-activated receptor γ coactivator-1α;FASN:脂肪酸合成酶fatty acid synthetase;ACC:乙酰辅酶A羧化酶acetyl-CoA carboxylase;FABP3:脂肪酸结合蛋白3 fatty acid binding protein 3;CD36:白细胞分化抗原36 leukocyte differentiation antigen 36。 图 2 奶畜乳脂合成与调控示意图 Fig. 2 Schematic diagram of synthesis and regulation of dairy milk fat |
脂肪酸是合成TAG的主要原料,反刍动物主要通过“从头合成”和“血液吸收”2条途径合成脂肪酸。在乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC)及脂肪酸合成酶(fatty acid synthetase, FASN)作用下,乳腺上皮细胞利用瘤胃产生的乙酸、β-羟基丁酸等前体物质从头合成短链和中链脂肪酸(<16碳)[19]。与干奶期相比,泌乳期奶牛乳腺组织中ACC和FASN的mRNA表达量显著上调[20]。固醇调节元件结合蛋白(sterol regulatory element-binding protein, SREBP)1是乳脂合成的核心转录因子,其促进FASN、过氧化物酶体增殖物激活受体γ辅激活因子-1α(peroxisome proliferators-activated receptor γ coactivator-1α, PGC-1α)及ACC等基因的表达,进而对脂肪酸和胆固醇的合成进行正调节[21]。除SREBP1外,过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptor γ, PPARγ)也是调控脂肪合成的关键受体。研究表明,PPARγ在泌乳牛乳腺中的表达量上调,能通过ACC、FASN及SREBP等蛋白调控脂肪酸的代谢,还可调控白细胞分化抗原36(leukocyte differentiation antigen 36, CD36)、脂肪酸结合蛋白(fatty acid binding protein, FABP)3等脂肪酸转运相关蛋白的表达[22]。mTORC1也是调控乳脂合成的关键因子。mTORC1的抑制剂雷帕霉素能够抑制PPARγ的活性[23];mTORC1能通过磷脂酸磷酸水解酶1(phosphatidic acid phosphohydrolase 1, Lipin-1)负调控SREBP1的表达及活性,进而影响脂肪酸的合成[21]。
奶牛泌乳期间,肝脏通过能量迁移作用将乳腺外组织的营养物质优先流向乳腺,为乳脂合成提供更多的前体物质,如非酯化脂肪酸(non-esterified fatty acid, NEFA)[24]。围产期奶牛肝脏中未被氧化的NEFA被重新酯化成TAG,进一步形成极低密度脂蛋白(very low-density lipoprotein, VLDL)后被转运出肝脏入血[25]。乳腺上皮细胞可以识别并摄取血液中的VLDL及乳糜微粒携带的长链脂肪酸(long-chain fatty acid, LCFA)[26],其主要来源于饲粮TAG在瘤胃微生物作用下水解形成的脂肪酸。绝大部分胞外LCFA需要在膜转运蛋白的参与下进入乳腺中才能被利用合成乳脂。CD36是介导反刍动物乳腺摄取LCFA的主要转运蛋白,其能够识别并富集相应的LCFA,将LCFA定位在细胞外膜的脂筏结构上并通过极性C端进入胞内[27]。LCFA在细胞内的转运还需要脂肪酸转运蛋白的协助,FABP是奶牛乳腺细胞内主要的脂肪酸转运蛋白。奶牛乳腺组织中主要表达的是FABP3、FABP4和FABP5,其中FABP3能将长链脂酰辅酶A转运到特定的细胞器中[28]。此外,丙酸、戊酸和异丁酸等被瘤胃微生物利用合成奇数碳原子脂肪酸及支链脂肪酸,沉积于反刍动物乳脂中[29]。饲粮物理有效中性洗涤纤维(physically effective neutral detergent fiber,peNDF)含量可通过改变瘤胃发酵而影响乳脂肪酸组成,这种变化在饲喂高精料饲粮情况下更为显著[30-31]。基于饲粮peNDF和瘤胃降解淀粉(rumen degraded starch,RDS)含量,本团队总结出碳水化合物平衡指数(carbohydrate balance index,CBI=peNDF/RDS)可通过影响养分利用和消化道健康而改变泌乳反刍动物产奶量和乳成分[32]。
动物的采食触发胰腺释放胆囊收缩素,并将胆汁酸释放到胃肠道。胆汁酸作为肠-肝轴的重要媒介,在脂肪乳化、脂肪酸吸收等方面发挥重要的作用[33-34]。本研究团队发现,饲喂高RDS的饲粮导致奶山羊瘤胃异常发酵,影响肠道健康,干扰胆汁酸的肠肝循环、肠道脂肪酸吸收和乳腺乳脂合成,降低乳脂率[35]。在不改变主要养分含量的情况下,适当增加奶山羊饲粮中过瘤胃淀粉的含量能提高产奶量;但过高RDS饲粮使瘤胃氢化细菌丰度降低、脂肪酸和胆固醇合成相关基因的表达量下降,从而降低乳脂含量[36]。
3 胃肠道-肝脏-乳腺协同调控乳糖合成如图 3所示,奶牛通过内源合成和小肠吸收2种方式获取葡萄糖。
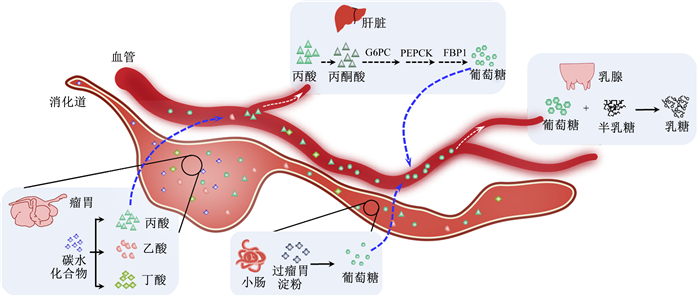
|
G6PC:葡萄糖-6-磷酸酶催化亚基glucose-6-phosphatase catalytic subunit;PEPCK:磷酸烯醇丙酮酸羧化激酶phosphoenolpyruvate carboxy kinase;FBP1:果糖-1, 6-二磷酸酶1 fructose-1, 6-bisphosphatase 1。 图 3 奶畜乳糖合成与调控示意图 Fig. 3 Schematic diagram of synthesis and regulation of dairy milk lactose |
将瘤胃微生物发酵产生的VFA等多种非糖物质经肝脏糖异生过程转变成葡萄糖或糖原,是保障反刍动物乳腺葡萄糖供应充足的主要途径[37-39]。各种糖异生前体物质在糖异生中的贡献度分别是:丙酸(60%~74%)、乳酸(16%~26%)、丙氨酸(3%~5%)、戊酸和异丁酸(5%~6%)、丙三醇(0.5%~3%)及其他生糖氨基酸(8%~11%)[40-41]。丙酸被吸收进入门静脉,其中90%被肝脏摄取并转化形成丙酮酸,丙酮酸在葡萄糖-6-磷酸酶催化亚基(glucose-6-phosphatase catalytic subunit,G6PC)、磷酸烯醇丙酮酸羧化激酶(phosphoenolpyruvate carboxy kinase,PEPCK)和果糖-1, 6-二磷酸酶1(fructose-1, 6-bisphosphatase 1,FBP1)这3个限速酶的作用下生成葡萄糖[42](图 3)。研究发现,山羊产前补充莫能菌素能增加瘤胃丙酸的产生,进而诱导肝脏PEPCK的表达[43];同时,丙酸能调控奶牛小肠上皮细胞PEPCK、FBP1和PGC-1α等糖异生基因的表达,进而调控血浆葡萄糖稳态[44]。在产后奶牛饲粮中添加发酵氨化浓缩乳清能增加瘤胃内丙酸的含量,提高血液胰岛素和葡萄糖水平[45]。同时,使用一定量甘油代替饲粮中的玉米,可以上调奶牛肝脏PEPCK的表达,促进肝脏糖异生作用[46]。本团队研究发现,围产期奶牛补饲生物素和烟酰胺,显著增加奶牛肝脏中丙酮酸羧化酶的mRNA丰度,促进肝脏糖异生[47]。此外,我们通过研究还发现,mTORC1通过PGC-1α调控反刍动物的肝脏糖异生相关酶的表达[48]。
3.2 小肠吸收在多数情况下,小肠多种消化酶分解过瘤胃淀粉形成葡萄糖,并通过小肠上皮细胞吸收入血[39, 49-50]。除此之外,氨基酸也是反刍动物体内重要的糖异生底物来源,部分生糖氨基酸在肝脏中经脱氨基作用转化为葡萄糖[51]。
在乳腺中,葡萄糖经变构后生成尿苷二磷酸(uridine diphosphate,UDP)-半乳糖,与葡萄糖结合形成乳糖[52-53]。葡萄糖代谢紊乱可能导致严重的奶牛营养代谢疾病,影响奶牛健康和产奶性能,例如围产后期,由于生糖前体缺乏,导致奶牛发生酮病[54]。
4 其他乳成分的合成与功能牛奶是矿物质的重要来源,富含钙、磷、钠、钾、氯、碘、镁及少量的铁元素,这些矿物质的高生物利用度增加了牛奶独特的营养价值。目前对于牛奶中矿物元素调控研究最深入的是钙元素。乳腺上皮细胞的钙敏感受体(calcium-sensing receptor,CaSR)能感应细胞内钙浓度,并通过钙释放激活钙通道蛋白1(calcium release-activated calcium modulator 1,ORAI1)增加钙的流入。牛奶中的钙约有60%是通过乳腺细胞质膜钙ATP酶2(membrane calcium ATPase2,PMCA2)转运出细胞,约有40%以与乳蛋白结合的方式分泌。因此,对乳蛋白的调控一定程度上也会影响乳钙含量[55]。鉴于钙代谢与机体1, 25-二羟维生素D3和25-羟维生素D3的紧密关系,研究发现妊娠后期奶牛补充25-羟维生素D3可提高血液1, 25-二羟维生素D3浓度,进而改变牛奶中25-羟维生素D3浓度[56]。本研究团队也发现,1, 25-二羟维生素D3可以剂量和能量依赖方式调节乳腺上皮细胞钙转运[57]。
牛奶还是维生素的宝贵来源,能够为机体提供充足的维生素D、维生素A和维生素B12。因奶牛乳腺细胞无法自主合成维生素和矿物质,乳成分中大部分脂溶性维生素(脂溶性维生素A、维生素D3和维生素E)和矿物质主要来源于饲粮(图 4)。虽然脂溶性维生素K3和多数水溶性B族维生素均可在瘤胃合成[58],但近年来也有研究发现高产奶牛仍需在饲粮中额外补充这些维生素[59]。近期的肠道微生物基因组学发现,尽管众多微生物具有维生素B和维生素K2合成基因,但只有少数微生物基因组具有完整的维生素B12从头合成途径,且饲粮纤维水平对瘤胃维生素B12至关重要[60]。这意味着在高精料饲喂情况下,需要额外补充维生素B12才能保证牛奶中有充足的维生素B12。另外,也有研究发现,饲粮改变引起的肠道微生物群落变化可通过胆汁酸代谢影响机体对维生素A的吸收,进而可能改变牛奶中维生素A含量[61]。
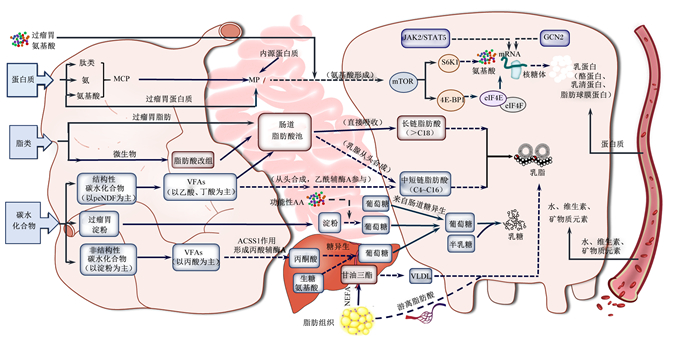
|
MCP:微生物蛋白microbial protein;MP:代谢蛋白质metabolizable protein;peNDF:物理有效中性洗涤纤维physically effective neutral detergent fiber;VFAs:挥发性脂肪酸volatile fatty acids;AA:氨基酸amino acid;ACSS1:酰基辅酶A合成酶短链家族成员1 acyl-CoA synthetase short-chain family member 1;NEFA:非酯化脂肪酸non-esterified fatty acid;JAK2/STAT5:Janus激酶2/信号传导及转录激活因子5 Janus kinase 2/signal transducer and activator of transcription 5;GCN2:一般性调控阻遏蛋白激酶2 general control nonderepressible 2;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;S6K1:核糖体S6蛋白激酶1 ribosomal S6 protein kinase 1;4E-BP1:4E结合蛋白1 4E-binding protein 1;eIF4:真核生物起始因子4 eukaryotic initiation factor 4;VLDL:极低密度脂蛋白very low-density lipoprotein。 图 4 奶畜乳成分合成与调控示意图 Fig. 4 Schematic diagram of synthesis and regulation of dairy milk components |
不同于单胃动物,反刍动物能够高效消化利用粗饲料,这得益于瘤胃这一独特消化道器官的存在。有证据表明,瘤胃微生物区系与乳成分和产奶效率密切相关[6, 62-65]。目前,全球奶牛养殖业面临的重要挑战之一是如何提高饲料利用率。奶牛产奶效率是一个复杂的生物性状,主要受自身遗传、消化道微生物组、营养素的消化吸收以及饲养管理等多因素的调控[66]。然而,消化道微生物组-宿主-营养素交互的具体调节机制和调控技术尚不十分清楚。因此,精准揭示消化道微生物组-宿主-营养素互作对产奶效率的整合调控机理与技术,可能是提高奶牛产奶效率,实现低碳养殖,提升我国牧场竞争力,推动牧场持续盈利的核心技术手段。
此外,乳成分与机体激素水平、基因表达和瘤胃微生物之间存在复杂的互作关系,国内外都在加紧这方面的研究,并已取得了一些进展,但研究结果仍局限于传统相关分析。因此,利用现代生物信息学分析,从激素、瘤胃微生物与发酵产物、基因网络和信号传导等多个水平深入、系统研究乳成分合成的调控机理,是制定精准营养干预策略,提高奶牛生产性能的重要理论基础。
| [1] |
FONTECHA J, CALVO M V, JUAREZ M, et al. Milk and dairy product consumption and cardiovascular diseases: an overview of systematic reviews and meta-analyses[J]. Advances in Nutrition, 2019, 10(Suppl 2): S164-S189. |
| [2] |
MATTHEWS C, CRISPIE F, LEWIS E, et al. The rumen microbiome: a crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency[J]. Gut Microbes, 2019, 10(2): 115-132. DOI:10.1080/19490976.2018.1505176 |
| [3] |
张兴夫, 杜瑞平, 高民. 奶牛对日粮中蛋白质的摄取利用特点及影响因素[J]. 中国畜牧杂志, 2014, 50(18): 42-47. ZHANG X F, DU R P, GAO M. Response of intake and utilization to dietary protein and influencing factors in lactating dairy cows[J]. Chinese Journal of Animal Science, 2014, 50(18): 42-47 (in Chinese). DOI:10.3969/j.issn.0258-7033.2014.18.009 |
| [4] |
李子健. 不同生理阶段奶牛瘤胃细菌菌群数量与多样性的比较研究[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2018. LI Z J. Comparative study on rumen bacteria quantity and diversity of dairy cows in different physiological stages[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2018. (in Chinese) |
| [5] |
FAN Q S, WANAPAT M, HOU F J. Chemical composition of milk and rumen microbiome diversity of yak, impacting by herbage grown at different phenological periods on the Qinghai-Tibet plateau[J]. Animals: an Open Access Journal From MDPI, 2020, 10(6): 1030. |
| [6] |
JAMI E, WHITE B A, MIZRAHI I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency[J]. PLoS One, 2014, 9(1): e85423. DOI:10.1371/journal.pone.0085423 |
| [7] |
吴建民, 王雍, 周协琛, 等. 基于宏基因组学解析瘤胃微生物调节荷斯坦奶牛乳蛋白含量的研究[J]. 动物营养学报, 2020, 32(8): 3843-3855. WU J M, WANG Y, ZHOU X C, et al. Rumen microorganism regulating milk protein content in Holstein dairy cows based on metagenomic analysis[J]. Chinese Journal Of Animal Nutrition, 2020, 32(8): 3843-3855 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.08.043 |
| [8] |
HRISTOV A N, BANNINK A, CROMPTON L A, et al. Invited review: nitrogen in ruminant nutrition: a review of measurement techniques[J]. Journal of Dairy Science, 2019, 102(7): 5811-5852. DOI:10.3168/jds.2018-15829 |
| [9] |
FAN Q S, WANAPAT M, HOU F J. Rumen bacteria influence milk protein yield of yak grazing on the Qinghai-Tibet plateau[J]. Animal Bioscience, 2021, 34(9): 1466-1478. DOI:10.5713/ab.20.0601 |
| [10] |
ROSARIO F J, POWELL T L, GUPTA M B, et al. mTORC1 transcriptional regulation of ribosome subunits, protein synthesis, and molecular transport in primary human trophoblast cells[J]. Frontiers in Cell and Developmental Biology, 2020, 8: 583801. DOI:10.3389/fcell.2020.583801 |
| [11] |
CHIKASHIGE Y J, KATO H, THORNTON M, et al. Gcn2 eIF2α kinase mediates combinatorial translational regulation through nucleotide motifs and uORFs in target mRNAs[J]. Nucleic Acids Research, 2020, 48(16): 8977-8992. DOI:10.1093/nar/gkaa608 |
| [12] |
GUO L, TIAN H B, SHEN J, et al. Phenylalanine regulates initiation of digestive enzyme mRNA translation in pancreatic acinar cells and tissue segments in dairy calves[J]. Bioscience Reports, 2018, 38(1): BSR20171189. DOI:10.1042/BSR20171189 |
| [13] |
GUO L, YAO J H, ZHENG C, et al. Leucine regulates α-amylase and trypsin synthesis in dairy calf pancreatic tissue in vitro via the mammalian target of rapamycin signalling pathway[J]. Animal, 2019, 13(9): 1899-1906. DOI:10.1017/S1751731118003683 |
| [14] |
GUO L, LIANG Z Q, ZHENG C, et al. Leucine affects α-amylase synthesis through PI3K/Akt-mTOR signaling pathways in pancreatic acinar cells of dairy calves[J]. Journal of Agricultural and Food Chemistry, 2018, 66(20): 5149-5156. DOI:10.1021/acs.jafc.8b01111 |
| [15] |
KIU H, NICHOLSON S E. Biology and significance of the JAK/STAT signalling pathways[J]. Growth Factors, 2012, 30(2): 88-106. DOI:10.3109/08977194.2012.660936 |
| [16] |
KHAN M Z, KHAN A, XIAO J X, et al. Role of the JAK-STAT pathway in bovine mastitis and milk production[J]. Animals: an Open Access Journal From MDPI, 2020, 10(11): 2107. |
| [17] |
GERSTNER C, SAÍN J, LAVANDERA J, et al. Functional milk fat enriched in conjugated linoleic acid prevented liver lipid accumulation induced by a high-fat diet in male rats[J]. Food & Function, 2021, 12(11): 5051-5065. |
| [18] |
D'ANGELO G, MOORTHI S, LUBERTO C. Role and function of sphingomyelin biosynthesis in the development of cancer[J]. Advances in Cancer Research, 2018, 140: 61-96. |
| [19] |
MU T, HU H H, MA Y F, et al. Regulation of key genes for milk fat synthesis in ruminants[J]. Frontiers in Nutrition, 2021, 8: 765147. DOI:10.3389/fnut.2021.765147 |
| [20] |
BIONAZ M, LOOR J J. Gene networks driving bovine milk fat synthesis during the lactation cycle[J]. BMC Genomics, 2008, 9: 366. DOI:10.1186/1471-2164-9-366 |
| [21] |
WANG G Y, CHEN L, QIN S L, et al. Mechanistic target of rapamycin complex 1: from a nutrient sensor to a key regulator of metabolism and health[J/OL]. Advances in Nutrition, 2022: nmac055. (2022-05-13)[2022-07-22]. https://pubmed.ncbi.nlm.nih.gov/35561748/.
|
| [22] |
刘莉莉. 脂肪酸与PPARγ基因互作对奶牛乳腺上皮细胞乳脂肪合成的调节作用及机理[D]. 博士学位论文. 哈尔滨: 东北农业大学, 2014. LIU L L. The Effect of interaction between fatty acid and PPARγ gene on regulation and mechanism of milk fat synthesis in dairy cow mammary epithelial cells[D]. Ph. D. Thesis. Harbin: Northeast Agricultural University, 2014. (in Chinese) |
| [23] |
KIM J E, CHEN J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis[J]. Diabetes, 2004, 53(11): 2748-2756. DOI:10.2337/diabetes.53.11.2748 |
| [24] |
李心慰. 乙酸、非酯化脂肪酸、生长激素和催乳素调控奶牛肝细胞脂代谢的信号机制[D]. 博士学位论文. 长春: 吉林大学, 2013. LI X W. The signaling mechanism of acetic acid, non-esterified fatty acids, growth hormone and prolactin on the regulation of lipid metabolism in the hepatocytes of dairy cows[D]. Ph. D. Thesis. Changchun: Jilin University, 2013. (in Chinese) |
| [25] |
VAN DORLAND H A, SADRI H, MOREL I, et al. Coordinated gene expression in adipose tissue and liver differs between cows with high or low NEFA concentrations in early lactation[J]. Journal of Animal Physiology and Animal Nutrition, 2012, 96(1): 137-147. DOI:10.1111/j.1439-0396.2011.01130.x |
| [26] |
DOEGE H, STAHL A. Protein-mediated fatty acid uptake: novel insights from in vivo models[J]. Physiology (Bethesda, Md.), 2006, 21: 259-268. |
| [27] |
GLATZ J F C, LUIKEN J J F P. From fat to FAT (CD36/SR-B2): understanding the regulation of cellular fatty acid uptake[J]. Biochimie, 2017, 136: 21-26. DOI:10.1016/j.biochi.2016.12.007 |
| [28] |
BIONAZ M, LOOR J J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation[J]. The Journal of Nutrition, 2008, 138(6): 1019-1024. DOI:10.1093/jn/138.6.1019 |
| [29] |
ABDOUL-AZIZ S K A, ZHANG Y D, WANG J Q. Milk odd and branched chain fatty acids in dairy cows: a review on dietary factors and its consequences on human health[J]. Animals: an Open Access Journal From MDPI, 2021, 11(11): 3210. |
| [30] |
CAO Y C, WANG D D, WANG L M, et al. Physically effective neutral detergent fiber improves chewing activity, rumen fermentation, plasma metabolites, and milk production in lactating dairy cows fed a high-concentrate diet[J]. Journal of Dairy Science, 2021, 104(5): 5631-5642. DOI:10.3168/jds.2020-19012 |
| [31] |
王砀砀, 赵会会, 肖凯丽, 等. 全混合日粮物理有效中性洗涤纤维水平对泌乳中期奶牛瘤胃液和乳中脂肪酸组成的影响[J]. 动物营养学报, 2018, 30(7): 2841-2849. WANG D D, ZHAO H H, XIAO K L, et al. Effects of physically effective neutral detergent fiber level in total mixed ration on fatty acid composition in rumen fluid and milk of dairy cows during mid-lactation period[J]. Chinese Journal of Animal Nutrition, 2018, 30(7): 2841-2849 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.07.046 |
| [32] |
姚军虎, 申静. 瘤胃可降解淀粉: 决定反刍动物消化道健康与养分利用的关键日粮因子[J]. 饲料工业, 2020, 41(8): 1-7. YAO J H, SHEN J. Rumen degradable starch regulate the gut health and nutrient utilization in ruminants[J]. Feed Industry, 2020, 41(8): 1-7 (in Chinese). |
| [33] |
WAHLSTRÖM A, SAYIN S I, MARSCHALL H U, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism[J]. Cell Metabolism, 2016, 24(1): 41-50. DOI:10.1016/j.cmet.2016.05.005 |
| [34] |
DAWSON P A, HUBBERT M L, RAO A. Getting the mOST from OST: role of organic solute transporter, OSTα-OSTβ, in bile acid and steroid metabolism[J]. Biochimica et Biophysica Acta, 2010, 1801(9): 994-1004. DOI:10.1016/j.bbalip.2010.06.002 |
| [35] |
申静. 日粮瘤胃可降解淀粉调控奶山羊瘤胃与肝脏代谢的机制[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2020. SHEN J. Effects of dietary rumen degradable starch on rumen and liver metabolism in dairy goats[D]. Ph. D. Thesis. Yangling: Northwest A&F University, 2020. (in Chinese) |
| [36] |
ZHENG L X, WU S R, SHEN J, et al. High rumen degradable starch decreased goat milk fat via trans-10, cis-12 conjugated linoleic acid-mediated downregulation of lipogenesis genes, particularly, INSIG1[J]. Journal of Animal Science and Biotechnology, 2020, 11: 30. DOI:10.1186/s40104-020-00436-3 |
| [37] |
ASCHENBACH J R, KRISTENSEN N B, DONKIN S S, et al. Gluconeogenesis in dairy cows: the secret of making sweet milk from sour dough[J]. IUBMB Life, 2010, 62(12): 869-877. DOI:10.1002/iub.400 |
| [38] |
SHENNAN D B, PEAKER M. Transport of milk constituents by the mammary gland[J]. Physiological Reviews, 2000, 80(3): 925-951. DOI:10.1152/physrev.2000.80.3.925 |
| [39] |
REYNOLDS C K, HUNTINGTON G B, TYRRELL H F, et al. Net portal-drained visceral and hepatic metabolism of glucose, L-lactate, and nitrogenous compounds in lactating Holstein cows[J]. Journal of Dairy Science, 1988, 71(7): 1803-1812. DOI:10.3168/jds.S0022-0302(88)79749-0 |
| [40] |
LARSEN M, KRISTENSEN N B. Effect of abomasal glucose infusion on splanchnic amino acid metabolism in periparturient dairy cows[J]. Journal of Dairy Science, 2009, 92(7): 3306-3318. DOI:10.3168/jds.2008-1889 |
| [41] |
REYNOLDS C K, AIKMAN P C, LUPOLI B, et al. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation[J]. Journal of Dairy Science, 2003, 86(4): 1201-1217. DOI:10.3168/jds.S0022-0302(03)73704-7 |
| [42] |
WESTERMEIER F, HOLYOAK T, ASENJO J L, et al. Gluconeogenic enzymes in β-cells: pharmacological targets for improving insulin secretion[J]. Trends in Endocrinology & Metabolism, 2019, 30(8): 520-531. |
| [43] |
KARCHER E L, PICKETT M M, VARGA G A, et al. Effect of dietary carbohydrate and monensin on expression of gluconeogenic enzymes in liver of transition dairy cows[J]. Journal of Animal Science, 2007, 85(3): 690-699. DOI:10.2527/jas.2006-369 |
| [44] |
ZHAN K, YANG T Y, CHEN Y Y, et al. Propionate enhances the expression of key genes involved in the gluconeogenic pathway in bovine intestinal epithelial cells[J]. Journal of Dairy Science, 2020, 103(6): 5514-5524. DOI:10.3168/jds.2019-17309 |
| [45] |
CAPUTO OLIVEIRA R, ERB S J, PRALLE R S, et al. Postpartum supplementation with fermented ammoniated condensed whey altered nutrient partitioning to support hepatic metabolism[J]. Journal of Dairy Science, 2020, 103(8): 7055-7067. DOI:10.3168/jds.2019-17790 |
| [46] |
WHITE H M, CARVALHO E R, KOSER S L, et al. Short communication: regulation of hepatic gluconeogenic enzymes by dietary glycerol in transition dairy cows[J]. Journal of Dairy Science, 2016, 99(1): 812-817. DOI:10.3168/jds.2015-9953 |
| [47] |
WEI X S, YIN Q Y, ZHAO H H, et al. Metabolomics for the effect of biotin and nicotinamide on transition dairy cows[J]. Journal of Agricultural and Food Chemistry, 2018, 66(22): 5723-5732. DOI:10.1021/acs.jafc.8b00421 |
| [48] |
WANG G Y, ZHANG J, WU S R, et al. The mechanistic target of rapamycin complex 1 pathway involved in hepatic gluconeogenesis through peroxisome-proliferator-activated receptor γ coactivator-1α[J/OL]. Animal Nutrition. (2022-08-08)[2022-08-10]. https://www.sciencedirect.com/science/article/pii/S2405654522000968.
|
| [49] |
BAIRD G D, LOMAX M A, SYMONDS H W, et al. Net hepatic and splanchnic metabolism of lactate, pyruvate and propionate in dairy cows in vivo in relation to lactation and nutrient supply[J]. The Biochemical Journal, 1980, 186(1): 47-57. DOI:10.1042/bj1860047 |
| [50] |
YOUNG J W. Gluconeogenesis in cattle: significance and methodology[J]. Journal of Dairy Science, 1977, 60(1): 1-15. DOI:10.3168/jds.S0022-0302(77)83821-6 |
| [51] |
李延涛. 泌乳奶山羊主要组织器官氨基酸代谢规律研究[D]. 博士学位论文. 泰安: 山东农业大学, 2021. LI Y T. Research on the metabolism regulation of amino acids in the major organs and tissues of lactating dairy goats[D]. Ph. D. Thesis. Tai'an: Shandong Agricultural University, 2021. (in Chinese) |
| [52] |
KUHN N J, CARRICK D T, WILDE C J. Lactose synthesis: the possibilities of regulation[J]. Journal of Dairy Science, 1980, 63(2): 328-336. DOI:10.3168/jds.S0022-0302(80)82934-1 |
| [53] |
姚军虎. 反刍动物碳水化合物高效利用的综合调控[J]. 饲料工业, 2013, 34(17): 1-12. YAO J H. Comprehensive regulation of efficient carbohydrate utilization in ruminants[J]. Feed Industry, 2013, 34(17): 1-12 (in Chinese). |
| [54] |
何生虎, 晁向阳, 王明成. 奶牛酮病的发病机理研究现状及进展[J]. 草食家畜, 2004(3): 15-17. HE S H, CHAO X Y, WANG M C. Pathology mechanism research current and progress of dairy cow ketosis[J]. Grass-Feeding Livestock, 2004(3): 15-17 (in Chinese). DOI:10.3969/j.issn.1003-6377.2004.03.005 |
| [55] |
HERNANDEZ L L. TRIENNIAL LACTATION SYMPOSIUM/BOLFA: serotonin and the regulation of calcium transport in dairy cows[J]. Journal of Animal Science, 2017, 95(12): 5711-5719. DOI:10.2527/jas2017.1673 |
| [56] |
WEISS W P, AZEM E, STEINBERG W, et al. Effect of feeding 25-hydroxyvitamin D3 with a negative cation-anion difference diet on calcium and vitamin D status of periparturient cows and their calves[J]. Journal of Dairy Science, 2015, 98(8): 5588-5600. DOI:10.3168/jds.2014-9188 |
| [57] |
SUN F F, CAO Y C, YU C, et al. 1, 25-dihydroxyvitamin D3 modulates calcium transport in goat mammary epithelial cells in a dose- and energy-dependent manner[J]. Journal of Animal Science and Biotechnology, 2016, 7: 41. DOI:10.1186/s40104-016-0101-0 |
| [58] |
KON S K, PORTER J W. The intestinal synthesis of vitamins in the ruminant[J]. Vitamins and Hormones, 1954, 12: 53-68. |
| [59] |
SACADURA F C, ROBINSON P H, EVANS E, et al. Effects of a ruminally protected B-vitamin supplement on milk yield and composition of lactating dairy cows[J]. Animal Feed Science and Technology, 2008, 144(1/2): 111-124. |
| [60] |
JIANG Q, LIN L M, XIE F, et al. Metagenomic insights into the microbe-mediated B and K2 vitamin biosynthesis in the gastrointestinal microbiome of ruminants[J]. Microbiome, 2022, 10(1): 109. DOI:10.1186/s40168-022-01298-9 |
| [61] |
ZHANG T, SUN P, GENG Q, et al. Disrupted spermatogenesis in a metabolic syndrome model: the role of vitamin A metabolism in the gut-testis axis[J]. Gut, 2022, 71(1): 78-87. DOI:10.1136/gutjnl-2020-323347 |
| [62] |
CUNHA C S, VELOSO C M, MARCONDES M I, et al. Assessing the impact of rumen microbial communities on methane emissions and production traits in Holstein cows in a tropical climate[J]. Systematic and Applied Microbiology, 2017, 40(8): 492-499. DOI:10.1016/j.syapm.2017.07.008 |
| [63] |
LI F Y, LI C X, CHEN Y H, et al. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle[J]. Microbiome, 2019, 7(1): 92. DOI:10.1186/s40168-019-0699-1 |
| [64] |
SHABAT S K B, SASSON G, DORON-FAIGENBOIM A, et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants[J]. The ISME Journal, 2016, 10(12): 2958-2972. DOI:10.1038/ismej.2016.62 |
| [65] |
XUE M Y, SUN H Z, WU X H, et al. Assessment of rumen bacteria in dairy cows with varied milk protein yield[J]. Journal of Dairy Science, 2019, 102(6): 5031-5041. DOI:10.3168/jds.2018-15974 |
| [66] |
吴家劲, 朱森林, 周密, 等. 奶牛瘤胃微生物研究进展和趋势[J]. 生物技术通报, 2020, 36(2): 27-38. WU J J, ZHU S L, ZHOU M, et al. Research progress and trends on rumen microbiota in dairy cows[J]. Biotechnology Bulletin, 2020, 36(2): 27-38 (in Chinese). DOI:10.13560/j.cnki.biotech.bull.1985.2019-1180 |




