2. 湖南省家禽安全生产工程技术研究中心, 长沙 410128;
3. 优质畜禽产品生产省部共建协同创新中心, 长沙 410128
2. Hunan Engineering Research Center of Poultry Production Safety, Changsha 410128, China;
3. Co-Innovation Center of High Quality Livestock and Poultry Product Production by Province and Ministry, Changsha 410128, China
鸡胸肉是肉鸡中体积最大的骨骼肌之一,也是除生长速度之外,最受重视的遗传选育的方向之一[1]。近年来,随着对肉鸡生长速度的过度选育,快大型肉鸡的胸肌组织出现了一种硬质化现象,具体表现为颜色苍白且触感坚硬,施以外部压力时,肉的形变程度小,且伴有明显的脊状凸起。2014年,Sihvo等[2]首次将这种仅出现在快大型肉鸡的胸肌组织上的症状命名为木质化鸡胸肉(wooden breast,WB)。此后,有一定数量的文献报道WB在不同试验环境下的发生率,其中最高的WB发生率由Lake等[3]报道,严重程度在轻微及以上的WB占比79%。每年仅WB这种肌肉疾病就给美国肉鸡饲养行业带来超过2 500万美元的经济损失[4],因此WB足以引起市场和肉鸡饲养行业的重视。本文进一步深入探讨了谢谦等[5]综述的内容,在此基础上增加了2018年至2022年间关于WB的特点、发生机理、缓解方式和检测手段等的最新研究进展,以期为快大型肉鸡养殖业中降低WB发病率以及降低WB带来的经济损失提供参考。
1 WB组织的病理学变化 1.1 肉鸡的外观和体态变化鸟类的胸肌发达程度直接反映鸟类的飞行能力,生活于海拔更高地域的鸟类通常胸肌组织较低海拔栖居的鸟类更加发达[6]。在肉鸡上,胸肌率则是反映肉鸡肉用性状的重要指标。对于罹患WB的肉鸡,首先则表现为趴卧时间较长等异常的生长状态。Norring等[7]通过活体触摸区分了正常肉鸡和WB肉鸡,进而对罹患WB鸡进行长期监视后发现,WB鸡的步态较差,步行次数较少,总趴卧时间长。Iwasaki等[8]通过翅膀抬起试验,即手动提起翅膀根部,观察肉鸡翅膀是否可从背部并拢,发现罹患WB的肉鸡翅膀从背部合拢困难(图 1),且雄性肉鸡翅膀可并拢率较雌性更低。对于罹患WB肉鸡的体态变化报道仍需要更多的数据支撑,以便后续针对肉鸡进行活体观测即可对罹患WB的肉鸡进行初筛和及时淘汰。
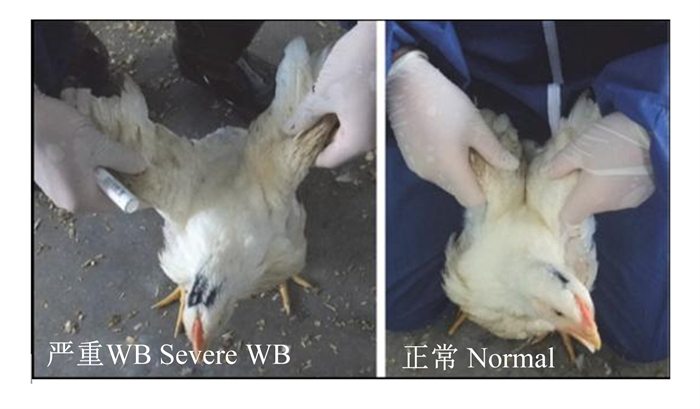
|
图 1 WB肉鸡体态变化 Fig. 1 Body changes of WB broilers[8] |
现阶段对WB严重程度的分级方式通常有2种,即3分法(正常、轻微或中等和严重)和4分法(正常、轻微、中等和严重)[9]。其中,3分法较早由Sihvo等[2]提出,他也是最早报道WB的研究学者之一,但后续一些研究为了进一步区别不同严重程度的WB,进而启用了4分法[10]。2种分类学方法均主要根据胸大肌的硬质化病变面积的大小而区分严重程度,正常鸡胸肉从上部(颅部、大端)到下部(尾部、小端)均柔软,无硬化;轻微WB主要为上部有轻微潜在病灶和弥漫性苍白,但中部和下部柔软;中等WB为上部和中部硬化均硬化,但尾端区域柔韧;严重WB为超过50%的胸大肌均硬质化,施予外部压力下变性程度小。WB的评分方式主要以触感反馈为主,轻微和中等WB病变界限较为模糊,所以不同文献中对于WB评分采取的3分和4分分类方式均获得一定程度的认可。除了质地的硬化,部分WB还可能伴有出现渗血、黏液等[11]。图 2展示了Kuttappan等[12]报道的WB最具代表性的外观变化。
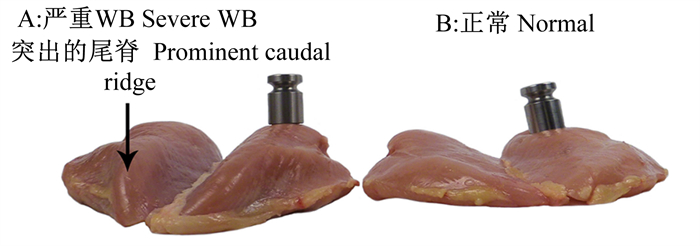
|
图 2 WB和正常鸡胸肉 Fig. 2 WB and normal breast of chicken[12] |
肌肉组织中,平行排列的肌原纤维构成骨骼肌纤维,多个肌纤维形成肌束,肌束排列形成肌肉组织[13],因此骨骼肌中的肌纤维是在光学显微镜下可观察到的最小单位。大量研究是通过伊红-苏木素(HE)、Masson等染色技术对WB的微观组织形态学进行呈现,同时结合多种分子生物学技术,例如免疫荧光染色、荧光原位杂交(FISH)技术和透射电镜等对WB的组织形态学变化进行广泛和深入地报道。Sihvo等[2, 14]较早发现,严重的WB组织中有大量的退行性肌纤维,肌纤维间质区域由嗜酸性物质和结缔组织填充,进而使肌纤维间隙进一步扩大,血管周围可见淋巴细胞浸润,且静脉周围炎性细胞数量与WB评分呈显著正相关。后续Sanden等[15]、Ferreira等[16]通过傅里叶变换红外(FTIR)显微光谱仪,验证了WB的肌纤维间隙中存在大量的胶原纤维。Papah等[17]使用透射电镜研究发现,WB的肌纤维的线粒体结构异化、间质纤维变性,肌原纤维存在分裂变性、不规则变性、移位变性和Z线(Z-line)变性,且肌内膜和肌束膜厚度下降。WB和发生在鸡胸肉中的另一种病变——白条肉(white striping,WS)有一定的相似性,但WS主要为肌纤维间的脂肪沉积异常[12]。总之,WB的主要表现为肌纤维萎缩、空泡化、炎性细胞浸润、血管炎症和密度下降、胶原蛋白浸润以及卫星细胞修复功能失效等。
2 WB对肉品质和营养价值的影响 2.1 肉品质下降WB对肉品质有较大影响,表 1总结了近年来国内外主要研究WB的团队报道的WB的肉品质变化[18-32],可见WB显著提高了蒸煮损失和滴水损失,肌肉保水能力显著下降;不过,WB对pH、肉色、剪切力等指标的影响不同的报道结果未能完全一致。
|
|
表 1 WB对胸肌肉品质的影响 Table 1 Effects of WB on meat quality of breast muscle |
WB对鸡胸肉的多种组成成分有较大影响。多数研究报道,WB较正常鸡胸肉的含水量和脂肪含量显著提高,蛋白质含量降低,胶原蛋白、钙和钠含量提高,粗灰分含量降低[27, 33]。在脂肪酸组成方面,Petracci等[34]报道,WB中二十碳五烯酸和二十二碳五烯酸的含量显著下降,饱和脂肪酸、单不饱和脂肪酸和多不饱和脂肪酸的含量与正常鸡胸肉无显著差异,羰基化合物和脂质过氧化物含量显著高于正常鸡胸肉。在氨基酸组成方面,与正常鸡胸肉相比,WB的必需氨基酸中赖氨酸、亮氨酸、缬氨酸和异亮氨酸含量下降;非必需氨基酸中半胱氨酸含量提高,谷氨酸、丙氨酸和天冬氨酸含量下降,其他氨基酸的含量无显著差异[35]。
3 WB形成的影响因素 3.1 遗传背景快大型肉鸡均处于相同的饲养管理条件下,却并非所有的肉鸡均罹患肌肉疾病,所以遗传背景差异可能影响了WB的进展。Sihvo等[2]报道并定义了WB这种肌病后,即有研究探讨了是否因生长速度过快导致了WB。Santos等[36]比较了14个不同品系的快大型肉鸡的WB发生率,在同一品系内,根据肉鸡生长速度(快速、中速和慢速)分类统计发病率,发现长速快则发病率高,二者呈显著正相关;Livingston等[37]比较了3种商品代快大型肉鸡和雅典加拿大随机繁殖(ACRB)鸡的WB发病率发现,品种与WB评分有关,而孵化温度、性别与WB评分无关;Zhang等[38]比较了5个品种的快大型肉鸡,发现肉鸡出栏时的体重不是WB发生率的决定因素,而平均胸肌质量与WB的发生率相关性较大;Chen等[39]也报道了42日龄胸肌率高的肉鸡WB发病率显著升高。但与之相反的,Bailey等[40]统计了154 781只鸡的性状,发现WB等肌病与体重和胸肌产量的遗传相关性范围为-0.06~0.41,非遗传效应对WB表型方差的贡献大于71.5%,说明环境因素对WB表型的影响较遗传因素更大。Lake等[3]通过全基因组测序和全基因组关联分析(GWAS)发现与WB有关的28个数量性状位点(QTL),但WB的遗传力估计为0.5左右,属于较低遗传力。综上所述,遗传背景对WB发生率可能存在影响,但环境和饲养管理因素可能作用更大,下文将从多角度进行探讨。
3.2 饲养管理现代肉鸡饲养业的高密度养殖模式,对于生长速度快、抗病力强的品种需求旺盛,对于饲养管理水平也提出了较高的要求。在孵化温度方面,Oviedo-Rondón等[41]报道了较为完整的温度-肌病理论体系,认为孵化温度升高和孵化箱中的含氧量下降会导致血管收缩频率提高,从而调节下丘脑的激素分泌水平,而肌肉组织的供氧量不足导致大量的糖酵解进行供能,进而引起脂质代谢混乱和肌纤维降解,使出生后的肌纤维更易受损。Tejeda等[42]试验结果也证实,种蛋在孵化箱中孵化位置的不同影响了肉鸡胴体和胸肉产量及WB发生率。
4 WB形成原因和发生机理 4.1 局部缺氧胸肌组织的局部缺氧诱导可能引起WB的理论基础在于:对肉鸡生长速率和鸡胸肉比率的过度选育,胸肌组织中的肌纤维生长和肥大速度过快,而毛细血管密度下降,进而导致肌纤维的长期供血不足;肌纤维代谢废物无法及时排出,进一步导致渗透压失衡、氧化应激等多种下游反应;肌纤维因细胞间的多种来源的代谢废物累积而受到刺激、受损,随后巨噬细胞活化、募集吞噬肌纤维碎片,大量的炎性细胞浸润加重了局部组织的代谢压力,进一步导致缺氧[34, 43]。此外,长期缺氧导致血管内皮生长因子的表达下降,血管内皮细胞生理性清除因缺氧而受损的线粒体的机制受损,可能加剧了WB[44]。Lake等[45]、Papah等[17]针对胸肌组织的组织形态学研究也证实,WB组织中存在毛细血管密度低于正常鸡胸肉以及静脉炎等现象,且罹患WB肉鸡的血液中钾含量较高,氧分压较低,上静脉血氧饱和度较低。
虽然局部缺氧理论在动物生理方面逻辑清晰,但仍有一些试验结果无法解释。如Sihvo等[46]、Cônsolo等[47]研究报道,肉鸡生长前期(2周龄时)即可检测到WB,生长早期肌纤维肥大速率较慢,这与胸肌高速生长引起供血不足的理论相悖。因此,局部缺氧可能对于WB的形成处于中下游位置,不能将WB与正常鸡胸肉的差异现象都归结于缺氧导致的,所以局部缺氧理论仍需要进一步的研究和完善。
4.2 多种物质代谢紊乱可能共同加剧WBWB的代谢紊乱主要涉及糖酵解、脂质过氧化和蛋白质的磷酸化等。Abasht等[48]通过质谱检测发现WB中有糖代谢紊乱的迹象,主要表现为糖酵解中间产物葡萄糖-6-磷酸和果糖-6-磷酸含量下降。而后Lake等[49]发现,糖酵解基因6-磷酸果糖激酶-2/果糖-2, 6-二磷酸酶3(PFKFB3)在WB中显著下调,PFKFB3编码的酶催化果糖-6-磷酸转化为2, 6-二磷酸果糖,可能表明WB中分流了葡萄糖到磷酸戊糖途径。结合Lake等[45]之前报道的,WB肉鸡的血糖水平与正常肉鸡无显著差异;Kawasaki等[50]报道,部分WB肉鸡肝脏有组织形态学病变;肝脏中糖原合成可能受损[49]。WB的胸肌组织糖的代谢方向可能和正常鸡胸肉组织不同,葡萄糖的酵解减少,更多地进入了其他通道,如葡萄糖醛酸和磷酸戊糖等[51]。哺乳动物的葡萄糖转运进入骨骼肌主要依靠葡萄糖转运蛋白(GLUT)家族[52],而鸡天生具有高血糖和胰岛素抵抗能力[53-55],且鸡没有GLUT4,其他常见GLUT(GLUT1、GLUT2、GLUT3和GLUT8)的表达水平也非常低[56],以上说明鸡的葡萄糖转运模式与哺乳动物不同,骨骼肌吸收血糖与胰岛素水平相关性小,因此葡萄糖的进入骨骼肌的转运模式是否是影响WB的主要原因仍需进一步验证。
正常鸡胸肉的脂肪含量较低,但在WB中均可观察到肌束膜间有明显的肌间脂肪细胞填充,这提示WB的脂肪化代谢方向可能与正常鸡胸肉有差异。Maharjan等[57]报道,罹患WB肉鸡的肝脏和腹脂中的甘油三酯转运速率显著提高,且转录因子和肌肉细胞分化相关基因[锌指蛋白234(ZNF234)、B细胞异位基因2(BTG2)]和肌肉生长相关基因胰岛素样生长因子1(IGF1)显著上调,肝脏合成的甘油三酯可能大部分都通过肝脏转运到了肉鸡胸肌进行脂解作用和脂肪酸氧化代谢,以满足肉鸡胸肌细胞能量需求。Papah等[58]通过RNA原位杂交,发现WB中的脂蛋白脂肪酶(LPL)的mRNA信号大量富集在静脉内皮细胞中,正常鸡胸肉中脂滴包被蛋白1(PLIN1)基因基本不表达,而WB中脂肪细胞高表达PLIN1,在一定程度上揭示了WB中脂肪代谢的动力学差异。
WB中蛋白质修饰可能也存在阻碍,WB中涉及泛素-蛋白酶体途径的几个基因显著下调,包括泛素特异性肽酶2(USP2)、valosin蛋白(VCP)、泛素融合降解1样蛋白(UFD1L)和蛋白酶体亚基β4(PSMB4),以上结果表明,WB的泛素-蛋白酶体活性可能降低,进而导致错误折叠或功能失调的蛋白质堆积[59]。Zhang等[60]通过对WB组织的蛋白质组学研究发现,蛋白质泛素化途径的热休克蛋白β1(HSPB1)和泛素羧基末端水解酶1(UCHL1)表达量显著上调,HSPB1上调表明WB中可能存在肌动蛋白细胞骨架坍塌或肌原纤维结构退化,这与Velleman等[61]、Sihvo等[46]的透射电镜观测到的WB肌节结构的M线和Z线的混乱的结果一致。
4.3 多种细胞的级联反应正常胸肌组织不仅包含构成肌纤维的肌细胞、运送氧气的红细胞,还包含血管内皮细胞、卫星细胞和炎性细胞等细胞类型,这些细胞类型可能都参与了WB的形成。Abasht等[62]研究发现,在炎症等病理疾病的血管内皮细胞中高表达的内皮细胞特异性分子1(ESM1)基因,且随WB严重程度呈线性上调,电镜下的正常的胸肌组织的内皮细胞层超微结构较薄,细胞器不明显,而WB的内皮细胞层较厚,细胞器明显。Ferreira等[16]研究表明,WB中成肌分化抗原阳性(MYOD+)的卫星细胞数量显著下降,配对盒基因家族转录因子7(PAX7)阳性(PAX7+)的卫星细胞数量增多;Emami等[63]报道,WB组织中配对盒基因家族转录因子3(PAX3)、PAX7和生肌因子5(MYF5)表达量显著低于正常鸡胸肉。此外,在WB的肌肉切片中可观察到大量单核炎性细胞浸润,骨骼肌中的巨噬细胞响应卫星细胞来源的谷氨酰胺,进而分化后对于清除肌纤维碎片起着重要作用[64],但目前研究大多将WB中的巨噬细胞功能归结于促炎因子引发肌纤维局部炎症,进而促进WB中的肌纤维进一步裂解。综上所述,WB中多种类型细胞间的互作效应,仍未充分展示,需要进一步研究。
4.4 肠道菌群代谢物的作用肠道菌群与多种肌肉疾病有着密不可分的关系,如肌少症、重症肌无力、杜氏肌病和神经性肌萎缩等[65]。在罹患WB的肉鸡上,Maharjan等[66]报道了乳杆菌属在罹患WB的肉鸡的肠道中丰度显著提高,Ligilactobacillus acidipiscis的丰度显著降低;Zhang等[67]研究发现,牛硒单胞菌和大叶拟杆菌是罹患WB的肉鸡肠道中的2个优势菌种,根据肠道菌群结构预测WB肉鸡肠道菌群的功能发现,糖酵解和尿素循环通路下调。结合前人报道,可以推测WB的肉鸡肠道菌群结构与正常肉鸡存在差异,肠道菌群可能参与了肉鸡WB的形成过程。
5 WB调控和缓解方式 5.1 能量、蛋白质和氨基酸水平饲粮氨基酸比例、蛋白质水平等可直接影响WB发生率,因此调节饲粮营养成分可作为缓解WB的潜在方式之一。Vieira等[68]、Iwasaki等[8]和Meloche等[69]分别通过降低饲粮能量水平和动态饲喂降能饲粮的方式,均在一定程度上降低了WB的发生率,具体降能方式有:降至代谢能需要量的90%,降低生长后期ME至2 930 kcal/kg等。但以上报道的降低WB发生率行之有效的降能方式,均降低了肉鸡的生长性能。Maharjan等[70]通过回归分析得出,3.17~3.48 g/Mcal可消化赖氨酸的饲粮可在保证鸡胸肉最快增速的同时最大程度降低WB发生率。
赖氨酸与精氨酸的比率对肌肉生长和肉品质有重要影响[71]。Meloche等[72]将肉鸡12~18日龄饲粮的赖氨酸水平降低至0.88%,WB的发病率降低了17.8%;Zampiga等[73]将肉鸡饲粮分为4个阶段,分别在1~12日龄和13~24日龄提高20%和30%的赖氨酸与精氨酸的比率,结果表明提高30%的赖氨酸与精氨酸的比率降低了WB发病率;Cruz等[74]研究表明,增加可消化赖氨酸水平改善了生长性能和胴体性状,并提高了WS和WB病变的发生和严重程度。以上这些研究表明,氨基酸平衡对于胸肌组织的发育和代谢平衡有着重要作用,饲粮的氨基酸配比向理想蛋白质靠拢可能是未来缓解WB的重要研究方向之一。
5.2 饲养管理措施限饲是降低WB发生率的有效手段之一,但同时会对肉鸡的生长性能带来消极影响。Livingston等[37]从肉鸡8日龄开始采取限制肉鸡09:00—17:00采食时间的方式进行限饲;Simões等[75]在肉鸡8~49日龄饲喂正常采食肉鸡50%~90%的饲粮;Trocino等[29]在肉鸡13~21日龄饲喂正常采食量80%的饲粮;Radaelli等[76]采用13~21日龄饲喂80%采食量的限饲策略,以上报道的限饲策略均不同程度地降低了WB发生率。
现阶段肉鸡饲养行业的高密度养殖,肉鸡活动空间较小,快大型肉鸡也因体重过大导致长期趴卧,缺少运动可能是导致WB发生的潜在因素。对此,Pedersen等[77]更改了鸡舍布局,使肉鸡在饮水和料槽之间有1.6~5.0 m的距离,结果表明,提高肉鸡的运动量对降低肌病的发生并未带来实质效果,但对肉鸡腿部肌肉性状有一定改善效果。如上文所述,鸡胸肉是鸟类飞翔行为主要调动的肌肉群,提高肉鸡行走距离对于缓解WB效果有限。
5.3 维生素和微量元素调节饲粮维生素和微量元素水平可否降低WB发生率存在一定争议。Wang等[78-79]在肉鸡饲粮中添加不同水平的维生素E,降低了轻微WB的比率,但对严重WB的比率无显著影响;Livingston等[80]在肉鸡生长前期中期和后期添加更高水平的微量矿物元素钾至1.01%,结果表明,提高饲粮中有效钾和有效磷水平显著降低了WB评分;Fatemi等[81]在蛋内注射2.4 μg维生素D3,结合生长期饲粮钙磷水平降低20%,结果表明,钙磷限饲显著降低了肉鸡WB发生率;Sirri等[82]在肉鸡饲粮中用有机锌、锰和铜替代无机微量矿物元素均提高了体重、平均日增重和饲料利用率,但未能影响WB和WS发生率。饲粮中的维生素和微量元素水平调节WB也是未来精准营养的研究方向,对于提高肉鸡经济效益有重要价值。
5.4 饲料添加剂除了调节饲粮中必需的营养成分外,一些饲料添加剂也可在一定程度上降低WB发生率和严重程度。例如胍基乙酸,胍基乙酸是脊椎动物合成肌酸的唯一前体[83],但植物来源的饲料中胍基乙酸的含量较低,肉鸡饲粮中添加0.06%的胍基乙酸即可显著降低WB评分[84];Greene等[85]报道,饲粮添加植酸酶提高了氧稳态相关基因血红蛋白β家族基因HBBR、HBM、HBZ和HEPH的mRNA相对表达量,降低了WB发病率;Kuttappan等[86]报道,抗氧化剂乙氧基喹啉、甲硫氨酸羟基类似物螯合物可显著降低WB发生率和严重程度;Wang等[87]报道,饲粮添加α-硫辛酸可降低3周龄时WB的严重程度;Erinle等[88]在肉鸡饲粮中添加2.5%的葡萄渣,改善了肉品质,对WB的发生率有降低的趋势。以上为目前报道的能有效缓解WB的饲料添加剂,其他添加剂诸如蓝莓果渣、枯草芽孢杆菌和磷脂酸等收效甚微[89-91]。
6 食品加工处理改善WB的口感有研究应用食品加工方式改良WB的风味和口感。Oliveira等[92]和Madruga等[93]通过将WB加工成香肠和肉肠,结果发现,WB制成的肉肠的外观、香气、味道、质地和整体接受度均与正常鸡胸肉制成的无显著差异。Santos等[94]将WB制成汉堡肉饼,统计食用人员的评分,结果显示,WB制成的肉品在纹理、风味、芳香、外貌和颜色上均与正常鸡胸肉制作无显著差异。Xing等[95]研究表明,对WB施以超声波振幅粉碎制备成肉糊,改善了WB肉的胶原化特性,并可继续用于生产凝胶型肉制品。
通过深加工处理将WB制成鸡肉类加工食品,虽然在一定程度上弥补了肉鸡饲养业的损失,让消费市场消化了这些鸡胸肉,但这些方式仍有一定的风险,因为WB客观而言发生了炎性细胞浸润、肌纤维裂解等现象,这些因素并不会因为食品加工而消除。同时,对于WB的食品深加工是否具有食品安全隐患,仍需进一步探求,且大量WB涌入食品加工市场,可能会影响消费者对食品加工行业的信任,给行业带来冲击。因此,从本质上揭示WB的形成机理,通过育种、营养调控手段降低WB发生率可能更有意义。
7 WB的检测手段WB的活体触摸判断主观性强且难以判断,以个人经验判断也容易出现误判,近年来有一些研究探究了WB的客观检测和评价方式。Siddique等[9]报道,因WB的组成成分与正常鸡胸肉不同,所以鸡胸肉不同部位的电阻与正常鸡胸肉不同;通过多段电阻仪,以机器学习造模和神经网络训练电阻谱与WB严重程度的相关性,检测准确率可达81.48%;De Carvalho等[96]通过红外光谱扫描可鉴别正常鸡胸肉和病变鸡胸肉,但该法的缺陷在于不能有效区分WB和WS肉。此外还有图像评判法,根据测量屠宰后肉鸡的体尺,结合颅骨、龙骨和胸肌小端的角度判定[97];红外热成像法,以红外扫描肉鸡的鸡胸肉,辅助以活体胸肌厚度检查等等[98]。在国内,孙啸等[99]报道了一种硬度检测探头的设备,可通过建立神经网络模型,对于WB的严重程度进行识别,成功率达90%以上。Kong等[100]建立了线性回归方程,WB肉鸡血清肌酶水平和鸡胸肉硬度相关系数(r)=0.608,后续可结合更多生物标志物建立预测模型。总之,目前由于检测技术的限制,客观上可接近100%识别准确率的检测方式还未见报道,仍需要研究人员进一步开发新的检测技术和方法。
8 小结随着生活水平的提高,人们对于畜禽产品的品质要求也逐渐提高,所以在满足消费者对鸡肉数量的追求的同时提高质量,是家禽生产行业的不懈追求。揭示WB的形成机制,对于后续遗传育种和设计营养调控,以及在保证肉鸡生长速度的同时,降低WB发生率有着重要意义。WB的形成是遗传和环境共同作用产生的结果,通过调节饲粮营养水平和添加抗氧化剂是缓解WB的有效方式之一。此外,发展WB检测技术准确区分正常鸡胸肉和WB并加以解决,亦可降低家禽生产行业的经济损失。
| [1] |
DALLE ZOTTE A, RICCI R, CULLERE M, et al. Research note: effect of chicken genotype and white striping-wooden breast condition on breast meat proximate composition and amino acid profile[J]. Poultry Science, 2020, 99(3): 1797-1803. DOI:10.1016/j.psj.2019.10.066 |
| [2] |
SIHVO H K, IMMONEN K, PUOLANNE E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers[J]. Veterinary Pathology, 2014, 51(3): 619-623. DOI:10.1177/0300985813497488 |
| [3] |
LAKE J A, DEKKERS J C M, ABASHT B. Genetic basis and identification of candidate genes for wooden breast and white striping in commercial broiler chickens[J]. Scientific Reports, 2021, 11(1): 6785. DOI:10.1038/s41598-021-86176-4 |
| [4] |
ZANETTI M A, TEDESCO D C, SCHNEIDER T, et al. Economic losses associated with wooden breast and white striping in broilers[J]. Semina: Ciências Agrárias, 2018, 39(2): 887-892. DOI:10.5433/1679-0359.2018v39n2p887 |
| [5] |
谢谦, 蒋小碟, 宋泽和, 等. 木质化鸡胸肉的形成及营养调控[J]. 动物营养学报, 2019, 31(12): 5431-5437. XIE Q, JIANG X D, SONG Z H, et al. Formation of wooden breast and nutritional regulation[J]. Chinese Journal of Animal Nutrition, 2019, 31(12): 5431-5437 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.12.006 |
| [6] |
XIONG Y, FAN L Q, HAO Y, et al. Physiological and genetic convergence supports hypoxia resistance in high-altitude songbirds[J]. PLoS Genetics, 2020, 16(12): e1009270. DOI:10.1371/journal.pgen.1009270 |
| [7] |
NORRING M, VALROS A, VALAJA J, et al. Wooden breast myopathy links with poorer gait in broiler chickens[J]. Animal, 2019, 13(8): 1690-1695. DOI:10.1017/S1751731118003270 |
| [8] |
IWASAKI T, WATANABE T, HASEGAWA Y, et al. Nutrition during the early rearing period affects the incidence of wooden breasts in broilers[J]. The Journal of Poultry Science, 2021, 58(3): 177-185. DOI:10.2141/jpsa.0200034 |
| [9] |
SIDDIQUE A, SHIRZAEI S, SMITH A E, et al. Acceptability of artificial intelligence in poultry processing and classification efficiencies of different classification models in the categorisation of breast fillet myopathies[J]. Frontiers in Physiology, 2021, 12: 712649. DOI:10.3389/fphys.2021.712649 |
| [10] |
TIJARE V V, YANG F L, KUTTAPPAN V A, et al. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies[J]. Poultry Science, 2016, 95(9): 2167-2173. DOI:10.3382/ps/pew129 |
| [11] |
XING T, ZHAO X, XU X L, et al. Physiochemical properties, protein and metabolite profiles of muscle exudate of chicken meat affected by wooden breast myopathy[J]. Food Chemistry, 2020, 316: 126271. DOI:10.1016/j.foodchem.2020.126271 |
| [12] |
KUTTAPPAN V A, HARGIS B M, OWENS C M. White striping and woody breast myopathies in the modern poultry industry: a review[J]. Poultry Science, 2016, 95(11): 2724-2733. DOI:10.3382/ps/pew216 |
| [13] |
BRADLEY C P, EMAMY N, ERTL T, et al. Enabling detailed, biophysics-based skeletal muscle models on HPC systems[J]. Frontiers in Physiology, 2018, 9: 816. DOI:10.3389/fphys.2018.00816 |
| [14] |
SIHVO H K, LINDÉN J, AIRAS N, et al. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers[J]. Veterinary Pathology, 2017, 54(1): 119-128. DOI:10.1177/0300985816658099 |
| [15] |
SANDEN K W, BÖCKER U, OFSTAD R, et al. Characterization of collagen structure in normal, wooden breast and spaghetti meat chicken fillets by FTIR microspectroscopy and histology[J]. Foods, 2021, 10(3): 548. DOI:10.3390/foods10030548 |
| [16] |
FERREIRA T Z, KINDLEIN L, FLEES J J, et al. Characterization of pectoralis major muscle satellite cell population heterogeneity, macrophage density, and collagen infiltration in broiler chickens affected by wooden breast[J]. Frontiers in Physiology, 2020, 11: 529. DOI:10.3389/fphys.2020.00529 |
| [17] |
PAPAH M B, BRANNICK E M, SCHMIDT C J, et al. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler chickens[J]. Avian Pathology, 2017, 46(6): 623-643. DOI:10.1080/03079457.2017.1339346 |
| [18] |
DE ALMEIDA ASSUNÇÃO A S, GARCIA R G, KOMIYAMA C M, et al. Wooden breast myopathy on broiler breast fillets affects quality and consumer preference[J]. Tropical Animal Health and Production, 2020, 52(6): 3555-3565. DOI:10.1007/s11250-020-02392-6 |
| [19] |
CAI K, SHAO W, CHEN X, et al. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat[J]. Poultry Science, 2018, 97(1): 337-346. DOI:10.3382/ps/pex284 |
| [20] |
OLIVEIRA R F D, MELLO J L M D, FERRARI F B, et al. Physical, chemical and histological characterization of pectoralis major muscle of broilers affected by wooden breast myopathy[J]. Animals, 2021, 11(3): 596. DOI:10.3390/ani11030596 |
| [21] |
SUN X, GIAMPIETRO-GANECO A, MUELLER A, et al. Meat quality traits and Blunt Meullenet-Owens Razor Shear characteristics of broiler breast fillets affected by woody breast condition and post-cooking meat temperature[J]. Poultry Science, 2021, 100(8): 101212. DOI:10.1016/j.psj.2021.101212 |
| [22] |
OLIVEIRA R F D, MELLO J L M D, FERRARI F B, et al. Effect of aging on the quality of breast meat from broilers affected by wooden breast myopathy[J]. Animals, 2021, 11(7): 1960. DOI:10.3390/ani11071960 |
| [23] |
BALDI G, SOGLIA F, LAGHI L, et al. Comparison of quality traits among breast meat affected by current muscle abnormalities[J]. Food Research International, 2019, 115: 369-376. DOI:10.1016/j.foodres.2018.11.020 |
| [24] |
XING T, ZHAO X, ZHANG L, et al. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China[J]. Poultry Science, 2020, 99(1): 620-628. DOI:10.3382/ps/pez560 |
| [25] |
MUDALAL S, LORENZI M, SOGLIA F, et al. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat[J]. Animal, 2015, 9(4): 728-734. DOI:10.1017/S175173111400295X |
| [26] |
MALILA Y, U-CHUPAJ J, SRIMARUT Y, et al. Monitoring of white striping and wooden breast cases and impacts on quality of breast meat collected from commercial broilers (Gallus gallus)[J]. Asian-Australasian Journal of Animal Sciences, 2018, 31(11): 1807-1817. DOI:10.5713/ajas.18.0355 |
| [27] |
SOGLIA F, MUDALAL S, BABINI E, et al. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality[J]. Poultry Science, 2016, 95(3): 651-659. DOI:10.3382/ps/pev353 |
| [28] |
GRATTA F, FASOLATO L, BIROLO M, et al. Effect of breast myopathies on quality and microbial shelf life of broiler meat[J]. Poultry Science, 2019, 98(6): 2641-2651. DOI:10.3382/ps/pez001 |
| [29] |
TROCINO A, PICCIRILLO A, BIROLO M, et al. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens[J]. Poultry Science, 2015, 94(12): 2996-3004. DOI:10.3382/ps/pev296 |
| [30] |
BOWKER B, ZHUANG H. Detection of razor shear force differences in broiler breast meat due to the woody breast condition depends on measurement technique and meat state1[J]. Poultry Science, 2019, 98(11): 6170-6176. DOI:10.3382/ps/pez334 |
| [31] |
KUTTAPPAN V A, OWENS C M, COON C, et al. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters[J]. Poultry Science, 2017, 96(8): 3005-3009. DOI:10.3382/ps/pex072 |
| [32] |
DALLE ZOTTE A, TASONIERO G, PUOLANNE E, et al. Effect of "wooden breast" appearance on poultry meat quality, histological traits, and lesions characterization[J]. Czech Journal of Animal Science, 2017, 62: 51-57. DOI:10.17221/54/2016-CJAS |
| [33] |
RIGDON M, STELZLENI A M, MCKEE R W, et al. Texture and quality of chicken sausage formulated with woody breast meat[J]. Poultry Science, 2021, 100(3): 100915. DOI:10.1016/j.psj.2020.12.014 |
| [34] |
PETRACCI M, SOGLIA F, MADRUGA M, et al. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities[J]. Comprehensive Reviews in Food Science and Food Safety, 2019, 18(2): 565-583. DOI:10.1111/1541-4337.12431 |
| [35] |
THANATSANG K V, MALILA Y, ARAYAMETHAKORN S, et al. Nutritional properties and oxidative indices of broiler breast meat affected by wooden breast abnormality[J]. Animals, 2020, 10(12): 2272. DOI:10.3390/ani10122272 |
| [36] |
SANTOS M N, ROTHSCHILD D, WIDOWSKI T M, et al. In pursuit of a better broiler: carcass traits and muscle myopathies in conventional and slower-growing strains of broiler chickens[J]. Poultry Science, 2021, 100(9): 101309. DOI:10.1016/j.psj.2021.101309 |
| [37] |
LIVINGSTON M L, LANDON C, BARNES H J, et al. White striping and wooden breast myopathies of broiler breast muscle is affected by time-limited feeding, genetic background, and egg storage[J]. Poultry Science, 2019, 98(1): 217-226. DOI:10.3382/ps/pey333 |
| [38] |
ZHANG X, TO K V, JARVIS T R, et al. Broiler genetics influences proteome profiles of normal and woody breast muscle[J]. Poultry Science, 2021, 100(4): 100994. DOI:10.1016/j.psj.2021.01.017 |
| [39] |
CHEN L R, SUYEMOTO M M, SARSOUR A H, et al. Temporal characterization of wooden breast myopathy ("woody breast") severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain[J]. Avian Pathology, 2019, 48(4): 319-328. DOI:10.1080/03079457.2019.1598541 |
| [40] |
BAILEY R A, SOUZA E, AVENDANO S. Characterising the influence of genetics on breast muscle myopathies in broiler chickens[J]. Frontiers in Physiology, 2020, 11: 1041. DOI:10.3389/fphys.2020.01041 |
| [41] |
OVIEDO-RONDÓN E O, VELLEMAN S G, WINELAND M J. The role of incubation conditions in the onset of avian myopathies[J]. Frontiers in Physiology, 2020, 11: 545045. DOI:10.3389/fphys.2020.545045 |
| [42] |
TEJEDA O J, MELOCHE K J, STARKEY J D. Effect of incubator tray location on broiler chicken growth performance, carcass part yields, and the meat quality defects wooden breast and white striping[J]. Poultry Science, 2021, 100(2): 654-662. DOI:10.1016/j.psj.2020.10.035 |
| [43] |
孔富丽, 刘冉冉, 赵桂苹, 等. 肉鸡木质肉和白条纹肉研究进展[J]. 畜牧兽医学报, 2020, 51(10): 2329-2340. KONG F L, LIU R R, ZHAO G P, et al. Research progress on wooden breast and white striping myopathies in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(10): 2329-2340 (in Chinese). DOI:10.11843/j.issn.0366-6964.2020.10.001 |
| [44] |
HOSOTANI M, KAWASAKI T, HASEGAWA Y, et al. Physiological and pathological mitochondrial clearance is related to pectoralis major muscle pathogenesis in broilers with wooden breast syndrome[J]. Frontiers in Physiology, 2020, 11: 579. DOI:10.3389/fphys.2020.00579 |
| [45] |
LAKE J A, BRANNICK E M, PAPAH M B, et al. Blood gas disturbances and disproportionate body weight distribution in broilers with wooden breast[J]. Frontiers in Physiology, 2020, 11: 304. DOI:10.3389/fphys.2020.00304 |
| [46] |
SIHVO H K, AIRAS N, LINDÉN J, et al. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy[J]. Journal of Comparative Pathology, 2018, 161: 1-10. DOI:10.1016/j.jcpa.2018.04.002 |
| [47] |
CÔNSOLO N R B, SAMUELSSON L M, BARBOSA L C G S, et al. Characterization of chicken muscle disorders through metabolomics, pathway analysis, and water relaxometry: a pilot study[J]. Poultry Science, 2020, 99(11): 6247-6257. DOI:10.1016/j.psj.2020.06.066 |
| [48] |
ABASHT B, MUTRYN M F, MICHALEK R D, et al. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens[J]. PLoS One, 2016, 11(4): e0153750. DOI:10.1371/journal.pone.0153750 |
| [49] |
LAKE J A, ABASHT B. Glucolipotoxicity: a proposed etiology for wooden breast and related myopathies in commercial broiler chickens[J]. Frontiers in Physiology, 2020, 11: 169. DOI:10.3389/fphys.2020.00169 |
| [50] |
KAWASAKI T, IWASAKI T, YAMADA M C, et al. Rapid growth rate results in remarkably hardened breast in broilers during the middle stage of rearing: a biochemical and histopathological study[J]. PLoS One, 2018, 13(2): e0193307. DOI:10.1371/journal.pone.0193307 |
| [51] |
BROTHERS B, ZHUO Z, PAPAH M B, et al. RNA-Seq analysis reveals spatial and sex differences in pectoralis major muscle of broiler chickens contributing to difference in susceptibility to wooden breast disease[J]. Frontiers in Physiology, 2019, 10: 764. DOI:10.3389/fphys.2019.00764 |
| [52] |
KLIP A, VOLCHUK A, HE L J, et al. The glucose transporters of skeletal muscle[J]. Seminars in Cell & Developmental Biology, 1996, 7(2): 229-237. |
| [53] |
TOKUSHIMA Y, TAKAHASHI K, SATO K, et al. Glucose uptake in vivo in skeletal muscles of insulin-injected chicks[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2005, 141(1): 43-48. DOI:10.1016/j.cbpc.2005.01.008 |
| [54] |
GOMEZ-CAPILLA J A, LANGSLOW D R. Insulin action on glucose utilisation by chicken adipocytes[J]. International Journal of Biochemistry, 1977, 8(6): 417-420. DOI:10.1016/0020-711X(77)90039-8 |
| [55] |
HAZELWOOD R L, LORENZ F W. Effects of fasting and insulin on carbohydrate metabolism of the domestic fowl[J]. The American Journal of Physiology, 1959, 197(1): 47-51. DOI:10.1152/ajplegacy.1959.197.1.47 |
| [56] |
KONO T, NISHIDA M, NISHIKI Y, et al. Characterisation of glucose transporter (GLUT) gene expression in broiler chickens[J]. British Poultry Science, 2005, 46(4): 510-515. DOI:10.1080/00071660500181289 |
| [57] |
MAHARJAN P, BEITIA A, WEIL J, et al. Woody breast myopathy broiler show age-dependent adaptive differential gene expression in Pectoralis major and altered in-vivo triglyceride kinetics in adipogenic tissues[J]. Poultry Science, 2021, 100(7): 101092. DOI:10.1016/j.psj.2021.101092 |
| [58] |
PAPAH M B, ABASHT B. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of wooden breast myopathy in modern broiler chickens[J]. Scientific Reports, 2019, 9(1): 17170. DOI:10.1038/s41598-019-53728-8 |
| [59] |
PAPAH M B, BRANNICK E M, SCHMIDT C J, et al. Gene expression profiling of the early pathogenesis of wooden breast disease in commercial broiler chickens using RNA-sequencing[J]. PLoS One, 2018, 13(12): e0207346. DOI:10.1371/journal.pone.0207346 |
| [60] |
ZHANG X, ANTONELO D, HENDRIX J, et al. Proteomic characterization of normal and woody breast meat from broilers of five genetic strains[J]. Meat and Muscle Biology, 2020, 4(1): 1-17. |
| [61] |
VELLEMAN S G, CLARK D L, TONNIGES J R. The effect of the wooden breast myopathy on sarcomere structure and organization[J]. Avian Diseases, 2018, 62(1): 28-35. DOI:10.1637/11766-110217-Reg.1 |
| [62] |
ABASHT B, PAPAH M B, QIU J. Evidence of vascular endothelial dysfunction in wooden breast disorder in chickens: insights through gene expression analysis, ultra-structural evaluation and supervised machine learning methods[J]. PLoS One, 2021, 16(1): e0243983. DOI:10.1371/journal.pone.0243983 |
| [63] |
EMAMI N K, CAUBLE R N, DHAMAD A E, et al. Hypoxia further exacerbates woody breast myopathy in broilers via alteration of satellite cell fate[J]. Poultry Science, 2021, 100(7): 101167. DOI:10.1016/j.psj.2021.101167 |
| [64] |
ZHANG C C, CHENG N X, QIAO B K, et al. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration[J]. Journal of Cachexia, Sarcopenia and Muscle, 2020, 11(5): 1291-1305. DOI:10.1002/jcsm.12584 |
| [65] |
TAN X M, HUANG Y, CHAI T J, et al. Differential gut microbiota and fecal metabolites related with the clinical subtypes of myasthenia gravis[J]. Frontiers in Microbiology, 2020, 11: 564579. DOI:10.3389/fmicb.2020.564579 |
| [66] |
MAHARJAN P, HILTON K, WEIL J, et al. Characterizing woody breast myopathy in a meat broiler line by heat production, microbiota, and plasma metabolites[J]. Frontiers in Veterinary Science, 2019, 6: 497. |
| [67] |
ZHANG X, ZHANG L, LI X F, et al. Cecal microbiota contribute to the development of woody breast myopathy[J]. Poultry Science, 2021, 100(6): 101124. DOI:10.1016/j.psj.2021.101124 |
| [68] |
VIEIRA S L, SIMÕES C T, KINDLEIN L, et al. Progressive in vivo detection of wooden breast in broilers as affected by dietary energy and protein[J]. Poultry Science, 2021, 100(6): 101120. DOI:10.1016/j.psj.2021.101120 |
| [69] |
MELOCHE K J, FANCHER B I, EMMERSON D A, et al. Effects of reduced dietary energy and amino acid density on pectoralis major myopathies in broiler chickens at 36 and 49 days of age1[J]. Poultry Science, 2018, 97(5): 1794-1807. DOI:10.3382/ps/pex454 |
| [70] |
MAHARJAN P, MARTINEZ D A, WEIL J, et al. Review: physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production[J]. Animal: an International Journal of Animal Bioscience, 2021, 15(Suppl.1): 100284. |
| [71] |
张道静, 周存六. L-精氨酸和L-赖氨酸在肉及肉制品中的应用研究进展[J]. 肉类研究, 2020, 34(6): 96-102. ZHANG D J, ZHOU C L. A review of the effects of L-arginine and L-lysine on the physicochemical properties of meat and meat products[J]. Meat Research, 2020, 34(6): 96-102 (in Chinese). |
| [72] |
MELOCHE K J, FANCHER B I, EMMERSON D A, et al. Effects of reduced digestible lysine density on myopathies of the pectoralis major muscles in broiler chickens at 48 and 62 days of age[J]. Poultry Science, 2018, 97(9): 3311-3324. DOI:10.3382/ps/pey171 |
| [73] |
ZAMPIGA M, SOGLIA F, PETRACCI M, et al. Effect of different arginine-to-lysine ratios in broiler chicken diets on the occurrence of breast myopathies and meat quality attributes[J]. Poultry Science, 2019, 98(6): 2691-2697. DOI:10.3382/ps/pey608 |
| [74] |
CRUZ R F A, VIEIRA S L, KINDLEIN L, et al. Occurrence of white striping and wooden breast in broilers fed grower and finisher diets with increasing lysine levels[J]. Poultry Science, 2017, 96(2): 501-510. DOI:10.3382/ps/pew310 |
| [75] |
SIMÕES C T, VIEIRA S L, STEFANELLO C, et al. An in vivo evaluation of the effects of feed restriction regimens on wooden breast using ultrasound images as a predictive tool[J]. British Poultry Science, 2020, 61(5): 583-589. DOI:10.1080/00071668.2020.1764909 |
| [76] |
RADAELLI G, PICCIRILLO A, BIROLO M, et al. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction[J]. Poultry Science, 2017, 96(2): 309-319. DOI:10.3382/ps/pew270 |
| [77] |
PEDERSEN I J, TAHAMTANI F M, FORKMAN B, et al. Effects of environmental enrichment on health and bone characteristics of fast growing broiler chickens[J]. Poultry Science, 2020, 99(4): 1946-1955. DOI:10.1016/j.psj.2019.11.061 |
| [78] |
WANG J, CLARK D L, JACOBI S K, et al. Effect of early posthatch supplementation of vitamin E and omega-3 fatty acids on the severity of wooden breast, breast muscle morphological structure, and gene expression in the broiler breast muscle[J]. Poultry Science, 2020, 99(11): 5925-5935. DOI:10.1016/j.psj.2020.08.043 |
| [79] |
WANG J, CLARK D L, JACOBI S K, et al. Effect of vitamin E and alpha lipoic acid on intestinal development associated with wooden breast myopathy in broilers[J]. Poultry Science, 2021, 100(3): 100952. DOI:10.1016/j.psj.2020.12.049 |
| [80] |
LIVINGSTON M L, LANDON C D, BARNES H J, et al. Dietary potassium and available phosphorous on broiler growth performance, carcass characteristics, and wooden breast[J]. Poultry Science, 2019, 98(7): 2813-2822. DOI:10.3382/ps/pez015 |
| [81] |
FATEMI S A, ALQHTANI A, ELLIOTT K E C, et al. Effects of the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 in Ross 708 broilers subsequently fed commercial or Calcium and phosphorus-restricted diets.Ⅰ.Performance, carcass characteristics, and incidence of woody breast myopathy1, 2, 3[J]. Poultry Science, 2021, 100(8): 101220. DOI:10.1016/j.psj.2021.101220 |
| [82] |
SIRRI F, MAIORANO G, TAVANIELLO S, et al. Effect of different levels of dietary zinc, manganese, and copper from organic or inorganic sources on performance, bacterial chondronecrosis, intramuscular collagen characteristics, and occurrence of meat quality defects of broiler chickens[J]. Poultry Science, 2016, 95(8): 1813-1824. DOI:10.3382/ps/pew064 |
| [83] |
LI Z M, LIANG H, XIN J P, et al. Effects of dietary guanidinoacetic acid on the feed efficiency, blood measures, and meat quality of Jinjiang bulls[J]. Frontiers in Veterinary Science, 2021, 8: 684295. DOI:10.3389/fvets.2021.684295 |
| [84] |
CÓRDOVA-NOBOA H A, OVIEDO-RONDÓN E O, SARSOUR A H, et al. Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products[J]. Poultry Science, 2018, 97(7): 2494-2505. DOI:10.3382/ps/pey097 |
| [85] |
GREENE E, FLEES J, DADGAR S, et al. Quantum blue reduces the severity of woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens[J]. Frontiers in Physiology, 2019, 10: 1251. DOI:10.3389/fphys.2019.01251 |
| [86] |
KUTTAPPAN V A, MANANGI M, BEKKER M, et al. Nutritional intervention strategies using dietary antioxidants and organic trace minerals to reduce the incidence of wooden breast and other carcass quality defects in broiler birds[J]. Frontiers in Physiology, 2021, 12: 663409. DOI:10.3389/fphys.2021.663409 |
| [87] |
WANG J, CLARK D L, JACOBI S K, et al. Alpha-tocopherol acetate and alpha lipoic acid may mitigate the development of wooden breast myopathy in broilers at an early age[J]. British Poultry Science, 2021, 62(5): 749-758. DOI:10.1080/00071668.2021.1927985 |
| [88] |
ERINLE T J, OLADOKUN S, MACISAAC J, et al. Dietary grape pomace-effects on growth performance, intestinal health, blood parameters, and breast muscle myopathies of broiler chickens[J]. Poultry Science, 2022, 101(1): 101519. DOI:10.1016/j.psj.2021.101519 |
| [89] |
XU Q, SI W, MBA O I, et al. Research note: effects of supplementing cranberry and blueberry pomaces on meat quality and antioxidative capacity in broilers[J]. Poultry Science, 2021, 100(3): 100900. DOI:10.1016/j.psj.2020.11.069 |
| [90] |
JIA L N, ZHANG X, LI X F, et al. Bacitracin, Bacillus subtilis, and Eimeria spp. challenge exacerbates woody breast incidence and severity in broilers[J]. Poultry Science, 2022, 101(1): 101512. DOI:10.1016/j.psj.2021.101512 |
| [91] |
SOBOTIK E B, LEE J T, HAGERMAN S, et al. Evaluation of the use of phosphatidic acid in the diet on growth performance and breast meat yield in broilers[J]. Animals, 2018, 8(6): 87. DOI:10.3390/ani8060087 |
| [92] |
OLIVEIRA R F D, FÁVERO M S, MELLO J L M D, et al. Effects of storage on quality traits of sausages made with chicken breast meat affected by wooden breast[J]. Animals, 2021, 11(2): 513. DOI:10.3390/ani11020513 |
| [93] |
MADRUGA M S, DA ROCHA T C, DE CARVALHO L M, et al. The impaired quality of chicken affected by the wooden breast myopathy is counteracted in emulsion-type sausages[J]. Journal of Food Science and Technology, 2019, 56(3): 1380-1388. DOI:10.1007/s13197-019-03612-0 |
| [94] |
SANTOS M M F, LIMA D A S, DA SILVA ARAÚ JO Í B, et al. Effect of wooden breast myopathy on texture and acceptability of emulsified chicken patties[J]. Journal of Food Science and Technology, 2021, 58(10): 4062-4067. DOI:10.1007/s13197-021-05098-1 |
| [95] |
XING T, XU Y, QI J, et al. Effect of high intensity ultrasound on the gelation properties of wooden breast meat with different NaCl contents[J]. Food Chemistry, 2021, 347: 129031. DOI:10.1016/j.foodchem.2021.129031 |
| [96] |
DE CARVALHO L M, MADRUGA M S, ESTÉVEZ M, et al. Occurrence of wooden breast and white striping in Brazilian slaughtering plants and use of near-infrared spectroscopy and multivariate analysis to identify affected chicken breasts[J]. Journal of Food Science, 2020, 85(10): 3102-3112. DOI:10.1111/1750-3841.15465 |
| [97] |
CALDAS-CUEVA J P, MAUROMOUSTAKOS A, SUN X, et al. Use of image analysis to identify woody breast characteristics in 8-week-old broiler carcasses[J]. Poultry Science, 2021, 100(4): 100890. DOI:10.1016/j.psj.2020.12.003 |
| [98] |
CASTILHO V A R D, KOMIYAMA C M, BURBARELLI M F C, et al. Techniques for in vivo assessment of myopathies in broiler chicken breasts using a biopsy as a support tool[J]. Avian Pathology: Journal of the W.V.P.A, 2021, 50(6): 477-489. DOI:10.1080/03079457.2021.1970107 |
| [99] |
孙啸, 谢葛亮, 束婧婷, 等. 木质鸡胸肉生肉品质表征及检测方法研究[J]. 食品安全质量检测学报, 2020, 11(3): 745-751. SUN X, XIE G L, SHU J T, et al. Quality characterization and detection methods on raw breast fillets with woody breast condition[J]. Journal of Food Safety & Quality, 2020, 11(3): 745-751 (in Chinese). DOI:10.19812/j.cnki.jfsq11-5956/ts.2020.03.018 |
| [100] |
KONG F L, ZHAO G P, HE Z X, et al. Serum creatine kinase as a biomarker to predict wooden breast in vivo for chicken breeding[J]. Frontiers in Physiology, 2021, 12: 711711. DOI:10.3389/fphys.2021.711711 |




