2. 内蒙古昶辉生物科技股份有限公司, 通辽 028400;
3. 湖南农业大学动物科学技术学院, 长沙 410128
2. Inner Mongolia Ever Brilliance Biotechnology Co., Ltd., Tongliao 028400, China;
3. College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China
目前,我国禁止在畜禽饲料中添加抗生素,因此各种植物添加剂的开发愈加重要。水飞蓟素是从水飞蓟干燥成熟果实中提取得到的黄酮类天然化合物,其主要成分是水飞蓟宾、异水飞蓟宾、水飞蓟亭和水飞蓟宁[1]。药理学研究发现,水飞蓟素具有抗氧化[2]、增强免疫功能[3-4]、调节脂质代谢[5]等作用。近年来,水飞蓟素在养殖业上的用途愈加广泛,如饲粮中添加水飞蓟素胶束可以通过提高母猪血清中催乳素浓度以提高母猪产奶量,从而提升断奶仔猪的窝重、仔猪个体重量和平均日增重[6]。肉鸡饲粮中添加250 mg/kg的水飞蓟素可调节流感病毒抗体滴度和绵羊红细胞(SRBC)抗体滴度[7]。黄羽肉鸡是我国优质肉鸡品类,但目前缺少水飞蓟素对黄羽肉鸡的作用探索,因此,本试验旨在研究饲粮中添加不同剂量水飞蓟素对黄羽肉鸡抗氧化能力、免疫功能及肠道菌群的影响,从而为水飞蓟素作为黄羽肉鸡饲料添加剂提供理论参考和技术支持。
1 材料与方法 1.1 试验材料水飞蓟素由内蒙古某生物科技股份有限公司提供,有效含量≥80%。
1.2 试验动物与设计试验选取288羽1日龄体质健康的快速型黄羽肉鸡,随机分成4组,每组6个重复,每个重复12羽。对照组(CON组)饲喂基础饲粮,低剂量组(S250组)、中剂量组(S500组)、高剂量组(S750组)在基础饲粮的基础上分别添加250、500、750 mg/kg水飞蓟素。试验期56 d,1~28日龄为试验前期,29~56日龄为试验后期。
1.3 试验饲粮基础饲粮以玉米、豆粕为主要原料,参照《鸡饲养标准》(NY/T 33—2004)进行配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
试验鸡采用3层笼养。试验前对鸡舍进行充分冲洗和严格消毒,入雏前24 h将鸡舍升温至32~35 ℃,此后温度每周降低2~3 ℃,直至保持在22~24 ℃为止。试验期舍内光照、湿度和温度根据常规饲养管理要求进行控制,鸡只按正常免疫程序进行免疫。整个试验期鸡自由采食和饮水。
1.5 样品采集于试验29和57日龄(空腹8 h),每个重复选体重相近的1只肉鸡进行屠宰取样。采集适量肝脏、空肠黏膜和盲肠食糜,液氮中速冻后,于-80 ℃冰箱中保存待测。
1.6 测定指标 1.6.1 肝脏抗氧化指标测定使用南京建成生物工程研究所试剂盒进行肝脏总抗氧化能力(T-AOC)及超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷胱甘肽过氧化物酶(GSH-Px)活性的测定。
1.6.2 空肠黏膜分泌型免疫球蛋白(SIgA)含量测定使用江苏雨桐生物科技有限公司酶联免疫吸附检测试剂盒(CK-E60068)测定空肠黏膜SIgA含量。
1.6.3 实时荧光定量PCR(RT-qPCR)测定将部分肝脏组织与空肠黏膜RNA裂解液匀浆后,按照SteadyPure通用型RNA提取试剂盒(AG21022,购自湖南艾科瑞生物工程有限公司)说明书操作进行肝脏RNA提取。得到RNA后,按照Evo M-MLV反转录试剂盒(AG11706,购自湖南艾科瑞生物工程有限公司)说明书操作进行,反转录得到cDNA,采用SYBR Green(AG11701,购自湖南艾科瑞生物工程有限公司)嵌合荧光法进行RT-qPCR检测,引物序列见表 2。目的基因包括核因子E2相关因子2(Nrf2)、谷氨酸半胱氨酸连接酶催化亚基(GCLC)、NAD(P)H醌氧化还原酶1(NQO1)、干扰素-γ(IFN-γ)、肿瘤坏死因子-α(TNF-α)、白细胞介素-6(IL-6)、免疫球蛋白G(IgG)和免疫球蛋白M(IgM)。以β-肌动蛋白(β-actin)作为内参基因,根据2-ΔΔCt法对定量结果进行计算分析。
|
|
表 2 RT-qPCR引物序列 Table 2 RT-qPCR primer sequences |
采用16S的测序方法测定盲肠微生物的组成及结构(上海派森诺生物科技有限公司)。
1.7 数据统计分析数据经Excel 2007整理后,采用SPSS 22.0软件进行单因素方差分析和Duncan氏法多重比较,结果以平均值和均值标准误(SEM)表示,以P<0.05为显著性水平。
2 结果 2.1 水飞蓟素对黄羽肉鸡肝脏抗氧化指标的影响如表 3所示,与CON组相比,S250组、S500组、S750组肉鸡前期和后期肝脏T-AOC显著升高(P<0.05);S250组、S500组、S750组肉鸡前期肝脏SOD活性显著升高(P<0.05),S750组肉鸡后期肝脏SOD活性显著升高(P<0.05);S500组、S750组肉鸡前期肝脏CAT活性显著升高(P<0.05),S250组肉鸡后期肝脏CAT活性显著升高(P<0.05);S250组、S500组肉鸡前期和后期肝脏GSH-Px活性显著升高(P<0.05)。
|
|
表 3 水飞蓟素对黄羽肉鸡肝脏抗氧化指标的影响 Table 3 Effects of silymarin on liver antioxidant indices of yellow feather broilers |
如表 4所示,与CON组相比,S250组、S500组、S750组肉鸡前期和后期肝脏GCLC mRNA表达量显著升高(P<0.05);S250组、S500组、S750组肉鸡前期肝脏Nrf2 mRNA表达量显著升高(P<0.05),S500组、S750组肉鸡后期肝脏Nrf2 mRNA表达量显著降低(P<0.05);S750组肉鸡前期肝脏NQO1 mRNA表达量显著升高(P<0.05),S500组、S750组肉鸡后期肝脏NQO1 mRNA表达量显著升高(P<0.05)。
|
|
表 4 水飞蓟素对黄羽肉鸡肝脏Nrf2、GCLC和NQO1 mRNA表达量的影响 Table 4 Effects of silymarin on mRNA expression levels of Nrf2, GCLC and NQO1 in liver of yellow feather broilers |
如表 5所示,与CON组相比,S500组、S750组肉鸡前期空肠黏膜IFN-γ mRNA表达量显著升高(P<0.05),S250组、S500组肉鸡后期空肠黏膜IFN-γ mRNA表达量显著升高(P<0.05);S500组肉鸡前期空肠黏膜TNF-α mRNA表达量显著升高(P<0.05),S250组、S500组肉鸡后期空肠黏膜TNF-α mRNA表达量显著升高(P<0.05);S250组、S500组、S750组肉鸡前期和后期空肠黏膜IL-6 mRNA表达量显著升高(P<0.05);S250组、S500组、S750组肉鸡前期空肠黏膜IgG mRNA表达量显著降低(P<0.05),S250组、S500组、S750组肉鸡后期空肠黏膜IgG mRNA表达量显著升高(P<0.05);S500组、S750组肉鸡前期空肠黏膜IgM mRNA表达量显著升高(P<0.05),S250组、S500组、S750组肉鸡后期空肠黏膜IgM mRNA表达量显著降低(P<0.05)。
|
|
表 5 水飞蓟素对黄羽肉鸡空肠黏膜免疫相关基因mRNA表达量的影响 Table 5 Effects of silymarin on mRNA expression levels of immune-related genes in jejunum mucosa of yellow feather broilers |
如表 6所示,与CON组相比,S500组肉鸡后期空肠黏膜SIgA含量显著升高(P<0.05)。
|
|
表 6 水飞蓟素对黄羽肉鸡空肠黏膜SIgA含量的影响 Table 6 Effects of silymarin on jejunum mucosa SIgA content of yellow feather broilers |
如图 1所示,与CON组相比,S250组、S500组、S750组肉鸡前期盲肠菌群Chao1、Goods_coverage、Shannon、Simpson和Observed_species指数均显著升高(P<0.05)。
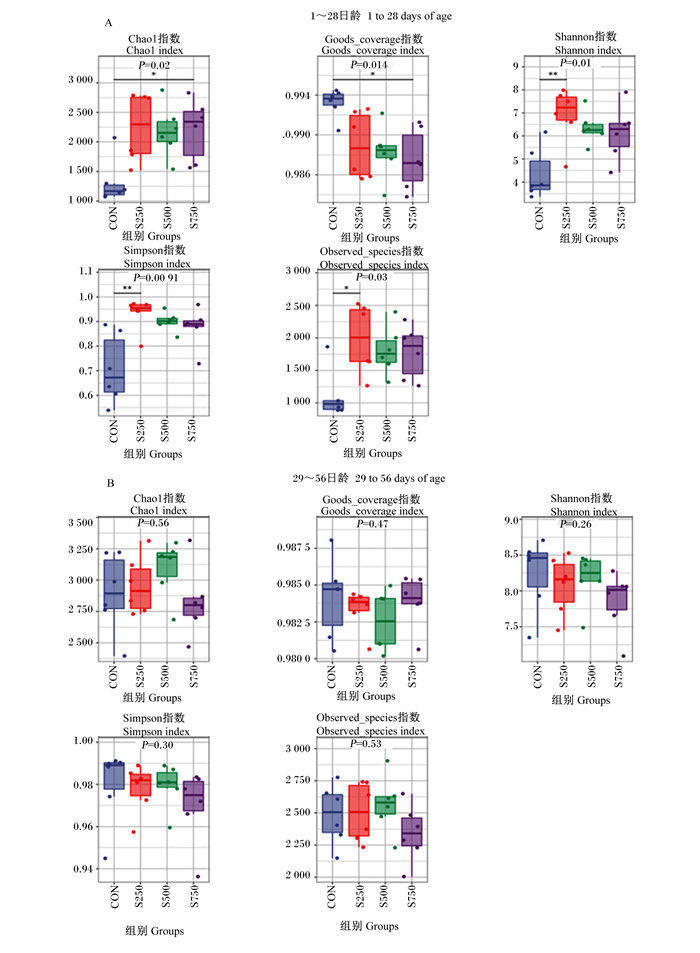
|
*表示与对照组相比差异显著(P<0.05)。 * mean significant difference compared with the control group (P < 0.05). 图 1 水飞蓟素对黄羽肉鸡盲肠菌群α多样性的影响 Fig. 1 Effects of silymarin on cecal microflora α diversity of yellow feather broilers |
如表 7所示,各组肉鸡前期和后期盲肠优势菌群均为厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、变形菌门(Proteobacteria)和软壁菌门(Tenericutes)。与CON组相比,S250组、S500组、S750组肉鸡前期盲肠Firmicutes门相对丰度均显著升高(P<0.05),盲肠Bacteroidetes门相对丰度显著降低(P<0.05);S750组肉鸡后期盲肠Tenericutes门相对丰度显著降低(P<0.05)。
|
|
表 7 盲肠菌群在门水平上的物种组成 Table 7 Species composition of cecal microbiota at phylum level |
如表 8所示,与CON组相比,S250组、S500组、S750组肉鸡前期盲肠粪杆菌属(Faecalibacterium)相对丰度显著升高(P<0.05),盲肠另枝菌属(Alistipes)、AF12相对丰度显著降低(P<0.05);S500组肉鸡后期盲肠布劳特氏菌属(Blautia)相对丰度显著升高(P<0.05)。
|
|
表 8 盲肠菌群在属水平上的物种组成 Table 8 Species composition of cecal microbiota at genus level |
如图 2所示,试验前期,CON组肉鸡盲肠的优势菌属为另枝菌属(Alistipes)、HB2_32_21、AF12和科尔韦尔氏菌属(Colwellia),S250组肉鸡盲肠的优势菌属为粪杆菌属(Faecalibacterium)和颤螺菌属(Oscillospira),S500组肉鸡盲肠的优势菌属为乳杆菌属(Lactobacillus),S750组肉鸡盲肠没有出现优势菌属。试验后期,CON组、S750组肉鸡盲肠没有出现优势菌属,S250组肉鸡盲肠的优势菌属为双歧杆菌属(Bifidobacterium),S500组肉鸡盲肠的优势菌属为Anaerofustis。
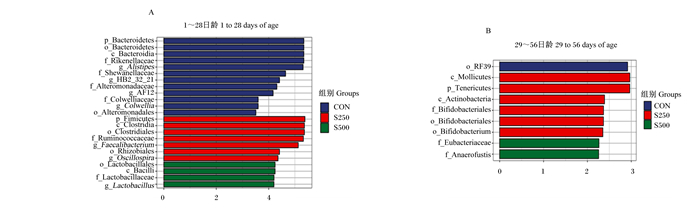
|
以线性判别分析评分>2为差异筛选阈值。 p_Bacteroidetes:拟杆菌门;o_Bacteroidetes:拟杆菌目;c_Bacteroidia:拟杆菌纲;f_Rikenellaceae:理研菌科;g_Alistipes:另枝菌属;f_Shewanellaceae:希瓦氏菌科;f_Alteromonadaceae:交替单胞菌科;f_Colwelliaceae:科尔韦氏菌科;g_Colwellia:科尔韦尔氏菌属;o_Alteromonadales:交替单胞菌目;p_Fimicutes:厚壁菌门;c_Clostridia:梭菌纲;o_Clostridiales:梭菌目;f_Ruminococcaceae:瘤胃菌科;g_Faecalibacterium:粪杆菌属;o_Rhizobiales:根瘤菌目;g_Oscillospira:颤螺菌属;o_Lactobacillales:乳杆菌目;c_Bacilli:芽孢杆菌纲;f_Lactobacillaceae:乳杆菌科;g_Lactobacillus:乳杆菌属;c_Mollicutes:柔膜菌纲;p_Tenericutes:软壁菌门;c_Actinobacteria:放线菌纲;f_Bifidobacteriales:双歧杆菌科;o_Bifidobacterium:双歧杆菌目;f_Eubacteriaceae:优杆菌科。 LDA score > 2 was used as the difference screening threshold. 图 2 线性判别分析分布柱状图 Fig. 2 Histogram of LDA distribution |
T-AOC反映动物机体抗氧化能力;SOD、CAT、GSH-Px是机体主要的抗氧化酶,能够清除过多的自由基[8],其活性的高低可以评定动物体抗氧化功能。Yu等[9]研究发现,水飞蓟素可提高赭曲霉毒素诱导的鸡原代肝细胞的SOD活性;Oskoueian等[10]研究发现,水飞蓟素可提高热应激鸡肝细胞中SOD和CAT活性。本试验中,水飞蓟素可以提高黄羽肉鸡肝脏T-AOC,改善SOD、CAT、GSH-Px活性,与Yu等[9]、Oskoueian等[10]研究结果一致。此外,本试验结果还显示,在黄羽肉鸡生长前期,随着水飞蓟素添加量的增加,黄羽肉鸡肝脏抗氧化能力增强;在黄羽肉鸡生长后期,随着水飞蓟素添加量的增加,水飞蓟素增强黄羽肉鸡肝脏抗氧化能力的效果减弱。
Nrf2是调控机体抗氧化能力的重要转录因子,能介导下游基因GCLC、NQO1的转录[11],具有调节SOD、CAT等抗氧化酶表达的作用[12]。胡俊等[13]研究发现,水飞蓟素能提高HepG2细胞Nrf2 mRNA表达量;李亮[14]研究发现,水飞蓟素可以提高PC12细胞Nrf2、GCLC mRNA表达量,且与剂量呈正相关。本试验结果显示,水飞蓟素可以提高肝脏GCLC、NQO1 mRNA表达量,与前人研究结果一致。且本试验中,在黄羽肉鸡生长前期,肝脏GCLC、NQO1 mRNA表达量与水飞蓟素添加量呈正相关;在黄羽肉鸡生长后期,随着水飞蓟素添加量的增加,水飞蓟素增强黄羽肉鸡肝脏GCLC、NQO1 mRNA表达量的效果逐渐减弱。水飞蓟素对肝脏GCLC、NQO1 mRNA表达量的影响与对肝脏SOD、CAT、GSH-Px活性的作用一致,表明水飞蓟素通过上调黄羽肉鸡肝脏GCLC、NQO1 mRNA的表达量以改善SOD、CAT、GSH-Px活性,进而发挥抗氧化作用。
3.2 水飞蓟素对黄羽肉鸡免疫功能的影响细胞因子IFN-γ和TNF-α具有免疫调节活性[15-16]。IFN-γ、TNF-α由Th1细胞分泌,可诱导T、B淋巴细胞分化增殖,从而介导细胞免疫应答[16-17]。本试验中,饲粮中添加中剂量水飞蓟素提高了肉鸡前期空肠黏膜中IFN-γ、TNF-α mRNA表达量,添加低、中剂量水飞蓟素提高了肉鸡后期空肠黏膜中IFN-γ、TNF-α mRNA表达量,与Rodríguez-Flores等[18]研究结果相似。IL-6由Th17细胞产生[17],Th17细胞参与促炎反应,调节性T细胞(Treg)能减轻炎症,Treg/Th17细胞的平衡状态对于机体免疫功能具有重要影响[19]。本试验中,饲粮中添加3种不同剂量的水飞蓟素均提高了肉鸡空肠黏膜IL-6 mRNA表达量,可能是因为水飞蓟素促进了初始T细胞分化为Treg[4],为维持机体Treg/Th17细胞平衡,水飞蓟素增加Th17细胞数量从而使得空肠黏膜中IL-6含量升高。免疫球蛋白是B淋巴细胞受抗原刺激之后合成并分泌于体液中能够与抗原产生特异性反应的球蛋白[20]。张洪[21]通过水飞蓟素治疗淋巴瘤,发现治疗后患者血清IgG和IgM含量升高。本试验中,饲粮中添加中、高剂量水飞蓟素能上调肉鸡前期空肠黏膜IgM mRNA表达量,添加低、中、高剂量水飞蓟素均能上调肉鸡后期空肠黏膜IgG mRNA表达量,与前人研究结果一致。因此,水飞蓟素或是在一定程度上刺激肉鸡空肠B淋巴细胞增殖,从而提高IgG、IgM mRNA表达量。此外,在试验前期添加水飞蓟素使肉鸡空肠黏膜IgG mRNA表达量下降,试验后期添加水飞蓟素使IgM mRNA表达量下降,可能是因为肉鸡空肠黏膜中已经积聚大量的IgG或IgM,肉鸡空肠黏膜中的IgG或IgM已经能够满足机体的需要。
SIgA是由呼吸道和消化道黏膜固有层B淋巴细胞分泌的一种抗体[22],其是黏膜免疫的主要效应因子。本试验中,饲粮中添加中剂量水飞蓟素提高了肉鸡后期空肠黏膜SIgA含量。郭秀丽等[23]也得出了类似的结论,其通过给大鼠饲喂高脂饮食并添加水飞蓟素,发现水飞蓟素能提升高脂饮食大鼠小肠SIgA含量。有研究表明,水飞蓟素能促进脂多糖(LPS)诱导的B淋巴细胞增殖[24]。因此,水飞蓟素或是通过促进肉鸡B淋巴细胞增殖继而促进空肠黏膜SIgA含量的升高。
综上所述,水飞蓟素对黄羽肉鸡空肠黏膜免疫功能具有一定的调节作用。
3.3 水飞蓟素对黄羽肉鸡盲肠菌群的影响本研究对肉鸡盲肠菌群进行了16S rRNA测序。α多样性分析用于研究特定环境内或单一样本中的多样性,常用的指标有Goods_coverage、Chao1、Shannon、Simpson和Observed_species指数。Goods_coverage指数主要反映操作分类单元(OTU)覆盖情况,其值高于98%说明覆盖率高、数据量够,Observed_species、Chao1指数与菌群中物种数目成正比,Shannon、Simpson指数与菌群中物种丰度和均匀度成正比[25]。本试验中,试验前期和后期盲肠菌群Goods_coverage指数均高于98%,说明本次盲肠菌群测样数据符合统计学要求;饲粮中添加3种不同剂量水飞蓟素均显著提高了肉鸡前期的盲肠菌群Chao1、Shannon、Simpson和Observed_species指数,表明饲粮中添加水飞蓟素能显著提升肉鸡前期盲肠菌群中物种数量、丰度和均匀度,优化菌群结构。
本试验中,我们从门水平和属水平上探讨了饲粮中添加水飞蓟素对肉鸡盲肠菌群物种组成的影响,结果表明,试验前期,饲粮中添加3种不同剂量水飞蓟素均显著增加了Firmicutes相对丰度,Firmicutes相对丰度的改变主要是由于Faecalibacterium相对丰度的增加;饲粮中添加3种不同剂量水飞蓟素降低了Bacteroidetes相对丰度,Bacteroidetes相对丰度的改变主要是由于Alistipes相对丰度的降低。Faecalibacterium具有肠黏膜保护作用,能通过上调闭锁小带蛋白-1(ZO-1)的表达修复肠黏膜屏障结构[26]。有研究显示,肠道中Alistipes相对丰度与血清IL-6含量呈正相关[27],表明Alistipes参与机体炎症性疾病的发生。此外,Alistipes与促进回肠炎症有关[28]。因此,饲粮中添加水飞蓟素或能通过提高盲肠Faecalibacterium相对丰度改善肠黏膜屏障,减少Alistipes相对丰度,抑制炎症。本试验中,饲粮中添加水飞蓟素降低了AF12相对丰度,AF12是理研菌科(Rikenellaceae)的细菌,有研究表明,Rikenellaceae相对丰度与银屑病病理特征呈正相关,且与丙酸含量呈负相关[29]。肠道微生物来源的丙酸可通过增加黏蛋白和杯状细胞分泌产物来加强肠道黏液屏障,因此,水飞蓟素能通过抑制肠道Rikenellaceae中AF12相对丰度从而增加肠道环境中丙酸含量,进而维护肠道健康。试验后期,饲粮中添加中剂量水飞蓟素提高了Blautia相对丰度,有研究表明,Blautia是广泛存在于动物粪便和肠道中的益生菌[30],其能通过增加肠道Treg数量和产生短链脂肪酸维持肠道环境平衡和预防炎症[31],因此,饲粮中添加中剂量水飞蓟素能通过促进Blautia生长继而有益于肠道健康。此外,试验后期,饲粮中添加高剂量水飞蓟素降低了Tenericutes相对丰度,Tenericutes可以上调糖苷水解酶的活性以促进多糖的降解,并具有产生氢气的潜力,是一种有益菌[32]。因此,本试验结果提示,试验后期饲粮中添加高剂量水飞蓟素会降低盲肠有益菌数量,对肠道产生一定程度的不利影响。
对饲粮中添加水飞蓟素的肉鸡盲肠菌群进行了线性判别分析分析,结果发现,试验前期CON组出现优势菌属Alistipes、HB2_32_21、AF12和Colwellia,S250组出现优势菌属Faecalibacterium和Oscillospira,S500组出现优势菌属Lactobacillus;试验后期S250组出现优势菌属Bifidobacterium,S500组出现优势菌属Anaerofustis。HB2_32_21和Colwellia多存在于海洋湖泊中[33-34],目前有关二者的功能性研究较少,HB2_32_21和Colwellia对于肠道的影响还有待探讨。Oscillospira在肠道可产生丁酸为主的各种短链脂肪酸[35],肠道微生物产生的丁酸介导小肠发育和黏膜屏障完整性增强,促进鸡肠上皮细胞增殖[36],因而饲粮中添加低剂量水飞蓟素可通过提高前期肉鸡盲肠优势菌属Oscillospira的相对丰度继而增强肠黏膜屏障。Lactobacillus可激活Wnt/β-连环蛋白(β-catenin)途径诱导肠干细胞向潘氏细胞分化,增强抗菌肽的表达以抑制有害菌的肠道定植[37];Bifidobacterium的代谢产物通过激活芳香烃受体和羟基羧基受体3以剂量依赖性的方式调控CD4+T细胞和单核细胞,继而参与肠道免疫反应[38];Anaerofustis是肠道的产丁酸菌[39],因此,其也可以同Oscillospira一样通过产生丁酸刺激鸡肠上皮细胞增殖和调节肠道黏液屏障的修复[36]。因而,水飞蓟素可通过增加盲肠Lactobacillus、Bifidobacterium和Anaerofustis相对丰度提高肉鸡肠道免疫功能。
综上所述,饲粮中添加水飞蓟素可促进盲肠有益菌生长,抑制有害菌繁殖,优化肉鸡肠道菌群组成。
4 结论饲粮中添加水飞蓟素可以提高快速型黄羽肉鸡肝脏抗氧化能力和空肠黏膜免疫功能,优化盲肠菌群结构。试验前期和后期均以饲粮中添加中剂量(500 mg/kg)水飞蓟素为宜。
| [1] |
贾睿, 曹丽萍, 杜金梁, 等. 水飞蓟素对四氯化碳致鲫肝(细胞)损伤的保护和抗氧化作用[J]. 中国水产科学, 2013, 20(3): 551-560. JIA R, CAO L P, DU J L, et al. Protective and antioxidant effects of silymarin on liver (cell) injury of crucian carp induced by carbon tetrachloride[J]. Journal of Fishery Science of China, 2013, 20(3): 551-560 (in Chinese). |
| [2] |
AGHASHAHI M, MOMENI H R, DARBANDI N. Impact of aluminium toxicity on vital human sperm parameters-protective effects of silymarin[J]. Andrologia, 2020, 52(10): e13742. |
| [3] |
DUPUIS M L, CONTI F, MASELLI A, et al. The natural agonist of estrogen receptor β-silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis[J]. Frontiers in Immunology, 2018, 9: 1903. DOI:10.3389/fimmu.2018.01903 |
| [4] |
NAMDARI H, IZAD M, REZAEI F, et al. Differential regulation of CD4+ T cell subsets by silymarin in vitro and in ovalbumin immunized mice[J]. Daru Journal of Faculty of Pharmacy Tehran University of Medical Sciences, 2018, 26(2): 215-227. |
| [5] |
JIANG G Y, SUN C H, WANG X D, et al. Hepatoprotective mechanism of Silybum marianum on nonalcoholic fatty liver disease based on network pharmacology and experimental verification[J]. Bioengineered, 2022, 13(3): 5216-5235. DOI:10.1080/21655979.2022.2037374 |
| [6] |
ZHANG Q Q, AHN J M, KIM I H. Micelle silymarin supplementation to sows' diet from day 109 of gestation to entire lactation period enhances reproductive performance and affects serum hormones and metabolites[J]. Journal of Animal Science, 2021, 99(12): skab354. DOI:10.1093/jas/skab354 |
| [7] |
ZAKER-ESTEGHAMATI H, SEIDAVI A, BOUYEH M. The effects of Cynara scolymus and Silybum marianum on growth, carcass and organ characteristics, immunity, blood constitutes, liver enzymes, jejunum morphology, and fatty acid profile of breast meat in broilers[J]. Food Science & Nutrition, 2021, 9(12): 6692-6706. |
| [8] |
窦晓利, 张占军, 范茂盛, 等. 黄芪精油对良凤花鸡生长性能、免疫机能及抗氧化能力的影响[J]. 中国畜牧杂志, 2021, 57(6): 233-236. DOU X L, ZHANG Z J, FAN M S, et al. Effects of Astragalus essential oil on growth performance, immune function and antioxidant capacity of Liangfenghua broiler[J]. Chinese Journal of Animal Science, 2021, 57(6): 233-236 (in Chinese). DOI:10.19556/j.0258-7033.20201019-07 |
| [9] |
YU Z G, WU F, TIAN J, et al. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin a in primary chicken hepatocytes[J]. Molecular Medicine Reports, 2018, 18(3): 2551-2560. |
| [10] |
OSKOUEIAN E, ABDULLAH N, IDRUS Z, et al. Palm kernel cake extract exerts hepatoprotective activity in heat-induced oxidative stress in chicken hepatocytes[J]. BMC Complementary and Alternative Medicine, 2014, 14: 368. DOI:10.1186/1472-6882-14-368 |
| [11] |
王换换, 申正杰, 肖航, 等. 热应激对肝脏中Keap1-Nrf2信号通路及下游基因表达的影响[J]. 南京农业大学学报, 2017, 40(1): 151-156. WANG H H, SHEN Z J, XIAO H, et al. Effects of heat stress on Keap1-Nrf2-ARE signal pathway of liver in dairy cows[J]. Journal of Nanjing Agricultural University, 2017, 40(1): 151-156 (in Chinese). |
| [12] |
张成, 王驰, 王孝成, 等. 白藜芦醇对热应激肉鸡肝脏抗氧化功能和Nrf2通路相关基因表达的影响[J]. 南京农业大学学报, 2021, 44(6): 1154-1161. ZHANG C, WANG C, WANG X C, et al. Effects of resveratrol on antioxidant function and the expression of Nrf2 signaling pathway-related genes in liver of heat-stressed broilers[J]. Journal of Nanjing Agricultural University, 2021, 44(6): 1154-1161 (in Chinese). |
| [13] |
胡俊, 饶紫兰, 陈江木, 等. 水飞蓟素通过调控Nrf2抗氧化通路抑制NLRP3炎症小体改善非酒精性脂肪性肝病[J]. 中国药理学通报, 2020, 36(7): 971-977. HU J, RAO Z L, CHEN J M, et al. Silymarin inhibits NLRP3 inflammasome to improve nonalcoholic fatty liver disease via regulating Nrf2 antioxidant pathway[J]. Chinese Pharmacological Bulletin, 2020, 36(7): 971-977 (in Chinese). DOI:10.3969/j.issn.1001-1978.2020.07.016 |
| [14] |
李亮. 水飞蓟素对丙烯酰胺所致PC12细胞氧化损伤的保护作用[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2017: 41-43. LI L. Protection of silymarin against acrylamide-induced oxidative damage in PC12 cells[D]. Master's Theses. Harbin: Northeast Agricultural University, 2017: 41-43. (in Chinese) |
| [15] |
谭艳, 宁丽常, 蹇孝丽, 等. 胶原诱导性关节炎大鼠血清TNF-α、IL-6、IFN-γ及IL-10水平变化[J]. 贵州医科大学学报, 2020, 45(1): 18-22. TAN Y, NING L C, JIAN X L, et al. Changes of serum TNF-α, IL-6, IFN-γ and IL-10 in collagen-induced arthritis rats[J]. Journal of Guizhou Medical University, 2020, 45(1): 18-22 (in Chinese). |
| [16] |
高焕, 王德云, 郭利伟, 等. 淫羊藿多糖脂质体对鸡淋巴细胞增殖及IL-2、IL-4和IFN-γ mRNA表达的影响[J]. 畜牧兽医学报, 2013, 44(1): 115-121. GAO H, WANG D Y, GUO L W, et al. Effect of epimedium polysaccharide liposome on lymphocyte proliferation, mRNA expression of IL-2, IL-4 and IFN-γ in chicken[J]. Chinese Journal of Animal and Veterinary Sciences, 2013, 44(1): 115-121 (in Chinese). |
| [17] |
薛力刚. 黄芪多糖联合新城疫疫苗胚胎免疫对雏鸡生长发育和免疫功能的影响及作用机制[D]. 博士学位论文. 长春: 吉林农业大学, 2021: 85-86. XUE L G. Effects and mechanisms of embryonic immunisation with newcastle disease vaccine combined with astragalus polysaccharide on the growth, development and immune function of chickens[D]. Ph. D. Thesis. Changchun: Jilin Agricultural University, 2021: 85-86. (in Chinese) |
| [18] |
RODRÍGUEZ-FLORES E M, MATA-ESPINOSA D, BARRIOS-PAYAN J, et al. A significant therapeutic effect of silymarin administered alone, or in combination with chemotherapy, in experimental pulmonary tuberculosis caused by drug-sensitive or drug-resistant strains: in vitro and in vivo studies[J]. PLoS One, 2019, 14(5): e0217457. DOI:10.1371/journal.pone.0217457 |
| [19] |
张敏, 于成功. 普拉梭菌及其上清液对葡聚糖酸钠诱导的结肠炎小鼠Treg/Th17平衡的影响[J]. 胃肠病学和肝病学杂志, 2017, 26(12): 1396-1400. ZHANG M, YU C G. Effect of Faecalibacterium prausnitzii and its supernatant on the balance of Treg/Th17 in dextran sulfate sodium-induced colitis mice[J]. Chinese Journal of Gastroenterology and Hepatology, 2017, 26(12): 1396-1400 (in Chinese). DOI:10.3969/j.issn.1006-5709.2017.12.018 |
| [20] |
YASUMA R, CICATIELLO V, MIZUTANI T, et al. Intravenous immune globulin suppresses angiogenesis in mice and humans[J]. Signal Transduction and Targeted Therapy, 2016, 1: 15002. DOI:10.1038/sigtrans.2015.2 |
| [21] |
张洪. 芩黄合剂治疗非霍奇金淋巴瘤的临床评价及初步机制研究[D]. 硕士学位论文. 上海: 上海交通大学, 2018: 51-62. ZHANG H. Clinical evaluation of Qinhuang mixture in the treatment of non-Hodgkin lymphoma and its preliminary mechanism study[D]. Master's Theses. Shanghai: Shanghai Jiaotong University, 2018: 51-62. (in Chinese) |
| [22] |
ZHANG L, CAO G T, ZENG X F, et al. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88[J]. Poultry Science, 2014, 93(1): 46-53. DOI:10.3382/ps.2013-03412 |
| [23] |
郭秀丽, 徐有青, 杨昭徐. 水飞蓟素对非酒精性脂肪性肝炎大鼠小肠组织抗氧化作用的实验研究[J]. 实用肝脏病杂志, 2011, 14(6): 407-409. GUO X L, XU Y Q, YANG Z X. Anti-oxidant effects of silymarin on intestinal tissues in rats with non-alcoholic steatohepatitis[J]. Journal of Clinical Hepatology, 2011, 14(6): 407-409 (in Chinese). DOI:10.3969/j.issn.1672-5069.2011.06.003 |
| [24] |
JOHNSON V J, HE Q R, OSUCHOWSKI M F, et al. Physiological responses of a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: Ⅲ.Silymarin inhibits T-lymphocyte function at low doses but stimulates inflammatory processes at high doses[J]. Planta Medica, 2003, 69(1): 44-49. DOI:10.1055/s-2003-37023 |
| [25] |
马作霖. 乳清粉调节D-半乳糖诱导衰老小鼠肠道菌群结构及抗衰老作用研究[D]. 博士学位论文. 兰州: 甘肃农业大学, 2021: 47-59. MA Z L. A study on intestinal microflora structure regulation and anti-aging effect of whey powder in D-galactose induced aging mice[D]. Ph. D. Thesis. Lanzhou: Gansu Agricultural University, 2021: 47-59. (in Chinese) |
| [26] |
XU J H, LIANG R R, ZHANG W, et al. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression[J]. Journal of Diabetes, 2020, 12(3): 224-236. DOI:10.1111/1753-0407.12986 |
| [27] |
康永波. 魔芋葡甘露聚糖通过调控肠道微生物治疗肥胖的机制研究[D]. 博士学位论文. 昆明: 昆明理工大学, 2018: 81-84. KANG Y B. Mechanism of konjac glucomannan treating obesity by regulating gut microbiota[D]. Ph. D. Thesis. Kunming: Kunming University of Science and Technology, 2018: 81-84. (in Chinese) |
| [28] |
PARKER B J, WEARSCH P A, VELOO A C M, et al. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health[J]. Frontiers in Immunology, 2020, 11: 906. DOI:10.3389/fimmu.2020.00906 |
| [29] |
LU W W, DENG Y D, FANG Z F, et al. Potential role of probiotics in ameliorating psoriasis by modulating gut microbiota in imiquimod-induced psoriasis-like mice[J]. Nutrients, 2021, 13(6): 2010. DOI:10.3390/nu13062010 |
| [30] |
LIU X M, MAO B Y, GU J Y, et al. Blautia-a new functional genus with potential probiotic properties?[J]. Gut Microbes, 2021, 13(1): 1-21. |
| [31] |
KIM C H, PARK J, KIM M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation[J]. Immune Network, 2014, 14(6): 277-288. DOI:10.4110/in.2014.14.6.277 |
| [32] |
ZHENG R K, LIU R, SHAN Y Q, et al. Characterization of the first cultured free-living representative of Candidatus Izemoplasma uncovers its unique biology[J]. The ISME Journal, 2021, 15(9): 2676-2691. DOI:10.1038/s41396-021-00961-7 |
| [33] |
SHEU D S, SHEU S Y, LIN K R, et al. Planctobacterium marinum gen.nov., sp.nov., a new member of the family Alteromonadaceae isolated from seawater[J]. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(4): 974-980. DOI:10.1099/ijsem.0.001726 |
| [34] |
CHRISTIANSEN L, BECH P K, SCHULTZ-JOHANSEN M, et al. Colwellia echini sp.nov., an agar-and carrageenan-solubilizing bacterium isolated from sea urchin[J]. International Journal of Systematic and Evolutionary Microbiology, 2018, 68(2): 687-691. DOI:10.1099/ijsem.0.002568 |
| [35] |
KONIKOFF T, GOPHNA U. Oscillospira: a central, enigmatic component of the human gut microbiota[J]. Trends in Microbiology, 2016, 24(7): 523-524. DOI:10.1016/j.tim.2016.02.015 |
| [36] |
ZHANG Y J, WANG Z X, DONG Y L, et al. Blue light alters the composition of the jejunal microbiota and promotes the development of the small intestine by reducing oxidative stress[J]. Antioxidants (Basel), 2022, 11(2): 274. DOI:10.3390/antiox11020274 |
| [37] |
WU H Q, XIE S, MIAO J F, et al. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa[J]. Gut Microbes, 2020, 11(4): 997-1014. DOI:10.1080/19490976.2020.1734423 |
| [38] |
LAURSEN M F, SAKANAKA M, VON BURG N, et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut[J]. Nature Microbiology, 2021, 6(11): 1367-1382. DOI:10.1038/s41564-021-00970-4 |
| [39] |
林萍. 基于"中药多糖-产丁酸菌"研究健脾方参苓白术散的微生态机制[D]. 硕士学位论文. 南昌: 江西中医药大学, 2021: 44-51. LIN P. Research on the micro-ecological mechanism of the jianpi recipe shenlingbaizhusan based on"traditional chinese medicine polysaccharides-butyric acid producing bacteria"[D]. Master's Thesis. Nanchang: Jiangxi University of Chinese Medicine, 2021: 44-51. (in Chinese) |




