酵母培养物是酵母菌经特定条件下发酵形成的一种微生态制品,主要成分为酵母和代谢产物如肽、醇、酯和有机酸等[1]。研究发现,酵母培养物作为饲料添加剂可以改善动物的生长性能和健康。酵母培养物可以调节肠道微生物,提高蛋鸡的蛋品质和繁殖性能[2],降低蛋鸡肠道沙门氏菌的水平和肠炎的发病率[3]。He等[4]研究发现,饲粮中添加酵母培养物改善了育肥猪的生长性能和肠道健康。此外,酵母培养物还可以通过Toll样受体2(Toll-like receptor 2,TLR2)通路增强鲫鱼的免疫力和抗病能力[5]。不过,饲粮中添加酵母培养物对肉鸡免疫功能和肠道健康的影响研究较少。因此,本研究通过研究饲粮添加不同水平酵母培养物对肉鸡生长性能、免疫功能和盲肠微生物区系的影响,旨在为酵母培养物在肉鸡上的应用提供参考。
1 材料与方法 1.1 试验材料酵母培养物:褐色粉末,pH为4.3,购自浙江某生物科技股份有限公司。
主要成分:底物组成为黄芪、党参、陈皮、香附、白芍、山药、乌梅、甘草、黄芪浸膏、豆粕及谷物副产物;菌株为乳酸菌和酵母菌(活菌数为1×106 CFU/g);粗蛋白质含量≥16%,小肽比例30%~40%(占蛋白质比例),甘露聚糖含量≥0.5%,总酸含量≥10%,中药多糖含量5%,水分含量≤12%。
发酵工艺:液体发酵、灭菌熟化、冷却接种、连续好氧、深度厌氧和滚筒+气流式干燥。
1.2 试验设计及饲粮试验选取360只1日龄黄羽肉鸡公雏,随机分为3个组,每组8个重复,每个重复15羽。对照组(YC0组)饲喂基础饲粮,试验组分别在基础饲粮中添加1 000(YC1000组)和2 000 mg/kg(YC2000组)酵母培养物。试验期70 d。基础饲粮参照《黄羽肉鸡营养需要量》(NY/T 3645—2020)配制,为玉米-豆粕型饲粮,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
试验鸡群采用笼养方式饲养,饲养管理和常规免疫按黄羽肉鸡饲养管理技术规程进行,试验期间鸡只自由采食、自由饮水。本试验经浙江农林大学动物伦理委员会批准。
1.4 测定指标及方法 1.4.1 生长性能试验期间,每天观察鸡群健康状况,记录死淘数,分别在肉鸡35和70日龄以各重复为单位对肉鸡进行空腹称重,并统计各重复肉鸡试验期间的耗料量,计算平均体重(ABW)、平均日增重(ADG)、平均日采食量(ADFI)和料重比(F/G)。
1.4.2 免疫器官指数70日龄时,每个重复选择1羽接近平均体重的鸡进行屠宰,解剖取出胸腺、脾脏和法氏囊称重,计算免疫器官指数:
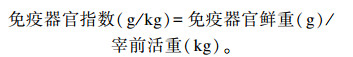
|
70日龄时,每个重复选择1羽接近平均体重的鸡,颈静脉采血,室温放置30 min,4 ℃、6 000 r/min离心10 min,收集血清。采用酶联免疫吸附试验(ELISA)法测定血清免疫球蛋白A(IgA)、免疫球蛋白Y(IgY)和免疫球蛋白M(IgM)含量以及血清白细胞介素-1β(IL-1β)、白细胞介素-2(IL-2)、白细胞介素-6(IL-6)、白细胞介素-10(IL-10)和肿瘤坏死因子-α(TNF-α)含量;采用微板法测定血清谷胱甘肽过氧化物酶(GSH-Px)和总超氧化物歧化酶(T-SOD)活性、总抗氧化能力(T-AOC)以及丙二醛(MDA)含量,采用比色法测定血清过氧化氢酶(CAT)活性。上述指标测定所用试剂盒均购自南京奥青生物技术有限公司。
1.4.4 盲肠微生物区系70日龄时,每个重复选择1羽接近平均体重的鸡进行屠宰,分离盲肠,收集盲肠食糜置于无菌冻存管中,液氮速冻后于-80 ℃保存待送样检测。样品委托上海美吉生物医药科技有限公司进行16S rDNA测序,基于Illumina NovaSeq测序平台对V3~V4高变区进行双末端测序,然后基于有效数据进行操作分类单元(OTU)聚类和物种分类分析。根据OTU聚类结果,对每个OTU的代表序列做物种注释,得到对应的物种信息和基于物种的丰度分布情况。
1.5 数据处理与统计分析使用Excel 2019对所得数据进行初步整理后,采用SPSS 26统计分析软件进行单因素方差分析和Duncan氏多重比较,结果以“平均值±标准差”表示,以P<0.05作为差异显著性判断标准。
2 结果与分析 2.1 饲粮添加酵母培养物对黄羽肉鸡生长性能的影响由表 2可知,与YC0组(对照组)相比,YC1000组黄羽肉鸡70日龄ABW显著提高(P<0.05)。1~35日龄和36~70日龄,饲粮添加不同水平酵母培养物对黄羽肉鸡ABW、ADG、ADFI和F/G均无显著影响(P>0.05),但YC2000组1~35日龄F/G显著高于YC1000组(P<0.05)。1~70日龄,与YC0组相比,YC1000组ADG有提高趋势,但差异不显著(P>0.05);YC1000组F/G显著降低(P<0.05),YC2000组F/G组显著差异(P>0.05)。
|
|
表 2 饲粮添加酵母培养物对黄羽肉鸡生长性能的影响 Table 2 Effects of dietary yeast culture on growth performance of yellow-feathered broilers |
由表 3可知,与YC0组相比,YC1000组和YC2000组黄羽肉鸡脾脏指数和法氏囊指数均有一定程度的提高,但差异不显著(P>0.05)。
|
|
表 3 饲粮添加酵母培养物对黄羽肉鸡免疫器官指数的影响 Table 3 Effects of dietary yeast culture on immune organ indices of yellow-feathered broilers |
由表 4可知,与YC0组相比,YC1000组和YC2000组黄羽肉鸡血清IgA和IgY含量均显著提高(P<0.05),血清IgM含量无显著差异(P>0.05)。
|
|
表 4 饲粮添加酵母培养物对黄羽肉鸡血清免疫球蛋白含量的影响 Table 4 Effects of dietary yeast culture on serum immunoglobulin content of yellow-feathered broilers |
由表 5可知,与YC0组相比,YC1000组和YC2000组黄羽肉鸡血清IL-1β、IL-2、IL-6和TNF-α含量显著降低(P<0.05),血清IL-10含量显著提高(P<0.05)。与YC2000组相比,YC1000组血清IL-1β含量显著降低(P<0.05),血清IL-10含量显著提高(P<0.05)。
|
|
表 5 饲粮添加酵母培养物对黄羽肉鸡血清炎症因子含量的影响 Table 5 Effects of dietary yeast culture on serum inflammatory factor content of yellow-feathered broilers |
由表 6可知,与YC0组相比,YC1000组和YC2000组黄羽肉鸡血清GSH-Px、T-SOD、CAT活性和T-AOC均显著提高(P<0.05),血清MDA含量显著降低(P<0.05)。与YC2000组相比,YC1000组血清GSH-Px、T-SOD活性和T-AOC显著提高(P<0.05),血清MDA含量显著降低(P<0.05)。
|
|
表 6 饲粮添加酵母培养物对黄羽肉鸡血清抗氧化指标的影响 Table 6 Effects of dietary yeast culture on serum antioxidant indices of yellow-feathered broilers |
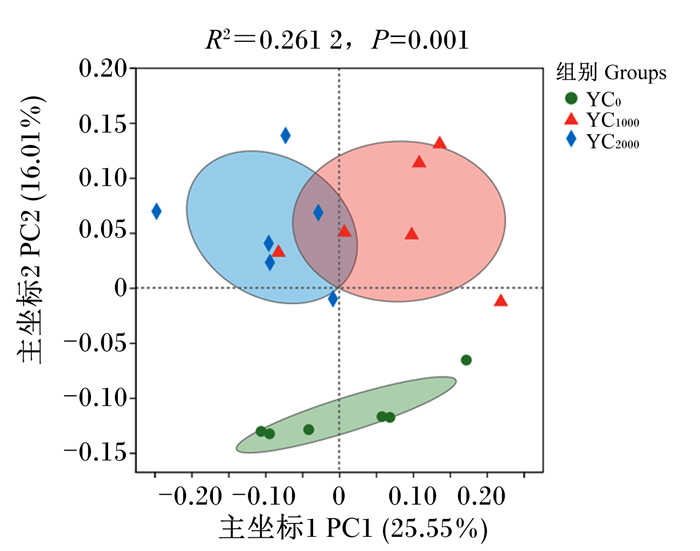
由表 7可知,与YC0组相比,YC2000组黄羽肉鸡盲肠微生物Chao指数和Shannon指数均显著提高(P<0.05)。如图 1所示,以主坐标分析(PCoA)为代表的beta多样性分析结果表明,YC1000组和YC2000组盲肠微生物之间有聚类现象,存在更为相似的微生物群落;在相似性分析(ANOSIM)中发现,R2=0.261 2、P=0.001,表明组间差异显著大于组内差异。
|
|
表 7 饲粮添加酵母培养物对黄羽肉鸡盲肠微生物alpha多样性的影响 Table 7 Effects of dietary yeast culture on alpha diversity of cecal microbiota of yellow-feathered broilers |
|
图 1 主坐标分析 Fig. 1 PCoA |
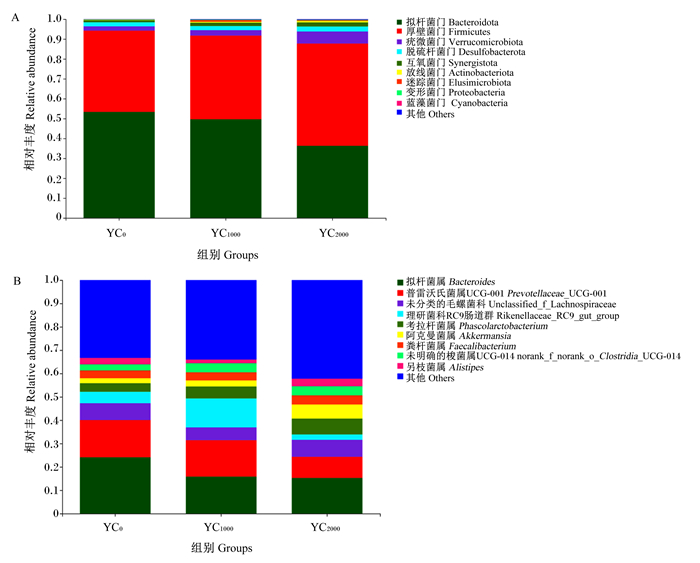
如图 2-A所示,在门水平上,黄羽肉鸡盲肠微生物中以拟杆菌门(Bacteroidota)、厚壁菌门(Firmicutes)、疣微菌门(Verrucomicrobiota)、脱硫杆菌门(Desulfobacterota)和互氧菌门(Synergistota)为优势菌门。
|
图 2 黄羽肉鸡盲肠微生物在门水平和属水平上的相对丰度 Fig. 2 Relative abundance of cecal microbiota at phylum and genus levels of yellow-feathered broilers |
如图 2-B所示,在属水平上,盲肠微生物中以拟杆菌属(Bacteroides)、普雷沃氏菌属UCG-001(Prevotellaceae_UCG-001)、未分类的毛螺菌科(unclassified_f_Lachnospiraceae)、理研菌科RC9肠道群(Rikenellaceae_RC9_gut_group)和考拉杆菌属(Phascolarctobacterium)为优势菌属。
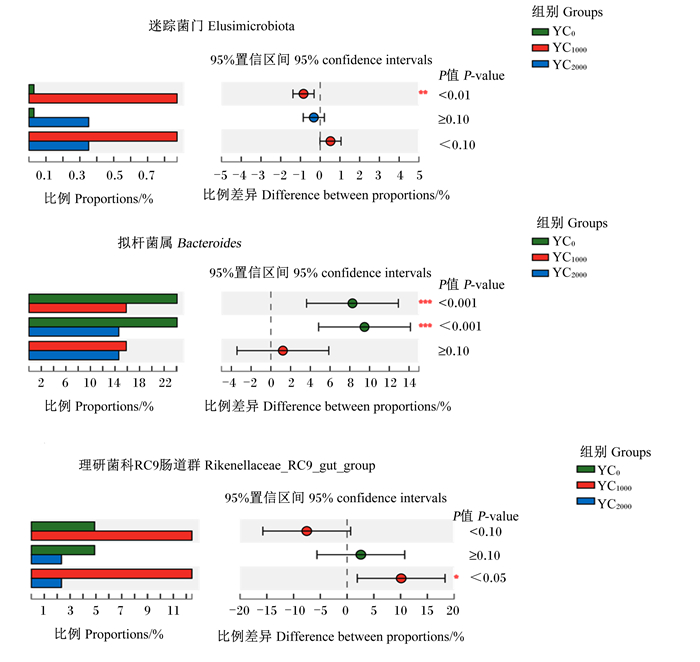
如图 3所示,在门水平上,与YC0组相比,YC1000组黄羽肉鸡盲肠迷踪菌门(Elusimicrobiota)相对丰度显著提高(P<0.05)。在属水平上,与YC0组相比,YC1000组和YC2000组盲肠拟杆菌属相对丰度显著降低(P<0.05);与YC2000组相比,YC1000组盲肠理研菌科RC9肠道群相对丰度显著提高(P<0.05)。
|
*表示P<0.05,* *表示P<0.01,* * *表示P<0.001。 * mean P < 0.05, * * mean P < 0.01, and * * * mean P < 0.001. 图 3 黄羽肉鸡盲肠微生物在门水平和属水平上的差异菌群(Kruskal-Wallis检验) Fig. 3 Differential microbiota at phylum and genus levels in cecum of yellow-feathered broilers (Kruskal-Wallis test) |
本研究结果显示,与YC0组相比,YC1000组黄羽肉鸡70日龄ABW显著提高,1~70日龄F/G显著降低。研究表明,饲粮添加酵母培养物能够提高畜禽的生长性能[6];陈脊宇等[7]研究表明,饲粮添加0.2%~0.8%酵母培养物能不同程度地提高五黑鸡生长性能和蛋品质;补充酵母产品能够提高犊牛的ADG和饲料转化率(FCR),改善犊牛胃肠道的发育[8];饲粮添加酵母培养物能提高肉鸡ADG和FCR[9]。本研究结果与前人的研究结果基本一致,这可能是由于饲粮添加酵母培养物后,肉鸡对营养物质的利用率提高,从而生长性能得到提高。
3.2 饲粮添加酵母培养物对黄羽肉鸡免疫功能的影响免疫球蛋白的含量反映了动物的免疫状态。IgA、IgY和IgM是肉鸡体内3种主要的免疫球蛋白,对鸡免疫系统的调节具有重要的作用[10]。酵母培养物含有不同的免疫刺激剂(β-葡聚糖、甘露糖蛋白、几丁质和核苷酸),可以产生更普遍的免疫反应,改善动物的免疫状态[11]。王斌星等[12]研究表明,饲粮添加300 mL/kg酿酒酵母发酵液可显著提高断奶仔猪十二指肠、空肠和回肠黏膜IgA、免疫球蛋白G(IgG)和IgM含量。周苗等[13]研究发现,酵母培养物可显著提高固始鸡血清IgM含量。本研究结果显示,饲粮添加不同水平酵母培养物均能显著提高黄羽肉鸡血清IgA和IgY含量,但对免疫器官指数无显著影响,这可能是由于酵母培养物的添加不足以影响免疫器官相对重量的变化。
细胞因子在机体的调节中起着至关重要的作用[14],研究表明,促炎因子大量释放会破坏肠道完整性和肠道屏障功能[15]。McCaffrey等[16]研究表明,饲粮添加酵母细胞壁能显著下调肉鸡血清促炎细胞因子IL-1β、白细胞介素-12(IL-12)和白细胞介素-18(IL-18)mRNA表达水平,减少细胞坏死。Browne等[17]研究表明,酵母可以下调HT-29细胞IL-1β mRNA表达水平。饲粮添加酵母培养物可以降低围产期奶牛血清IL-6和白细胞介素-8(IL-8)含量,下调子宫IL-6 mRNA表达水平[18]。本研究结果显示,饲粮添加酵母培养物能显著降低黄羽肉鸡血清IL-1β、IL-2、IL-6和TNF-α含量,显著提高血清IL-10含量,这表明酵母培养物可以降低肉鸡血清中炎症因子的含量,且添加1 000 mg/kg酵母培养物的效果要优于添加2 000 mg/kg。
3.3 饲粮添加酵母培养物对黄羽肉鸡血清抗氧化指标的影响抗氧化系统的关键酶,如超氧化物歧化酶(SOD)、GSH-Px和CAT,可以清除机体过量的自由基,对机体的健康起着至关重要的作用。Bu等[11]研究发现,饲粮添加10 g/kg的酵母培养物可以增强乌苏里鲶鱼肝脏SOD和GSH-Px活性,降低肝脏MDA含量。饲粮添加酵母培养物能够提高肉鸡空肠和血清SOD和GSH-Px活性,降低血清MDA含量[19-20]。本研究结果显示,饲粮添加酵母培养物能够显著提高黄羽肉鸡血清GSH-Px、T-SOD、CAT活性和T-AOC,降低血清MDA含量,与前人的研究结果相似。由此可见,酵母培养物具有较强的抗氧化能力,且添加1 000 mg/kg酵母培养物的效果要优于添加2 000 mg/kg。
3.4 饲粮添加酵母培养物对黄羽肉鸡盲肠微生物区系的影响肠道菌群是体内最大、最复杂的微生态系统,微生物群通过竞争排斥和产生抑菌和杀菌物质来减少和防止肠道有害菌的定植[21]。本试验中,alpha多样性分析表明,饲粮添加酵母培养物能改善肉鸡盲肠微生物的多样性。在门水平上,黄羽肉鸡盲肠以拟杆菌门和厚壁菌门相对丰度较高,与前人的研究结果[22]相似;但也有研究发现,厚壁菌门在肉鸡盲肠微生物中的相对丰度最高[23],这可能是由于试验肉鸡品种、日龄不同,盲肠内的微生物丰度存在差异。
本试验中,与YC0组相比,在门水平上,YC1000组黄羽肉鸡盲肠迷踪菌门相对丰度显著提高;在属水平上,YC1000组和YC2000组盲肠拟杆菌属相对丰度显著降低,且与YC2000组相比,YC1000组盲肠理研菌科RC9肠道群相对丰度显著提高,这与前人的研究结果[24-25]相似。Berry等[26]研究表明,拟杆菌属能降解黏液寡糖,从而破坏肠黏膜屏障,引起肠道炎症。研究发现,理研菌科RC9肠道群可以促进肠道中碳水化合物的消化和吸收[26],与促炎细胞因子和其他损伤因素呈负相关[27],且能够通过增加丁酸盐水平以保护黏膜屏障功能[28],而丁酸盐在肠道生理和宿主健康中起重要作用。结果提示,酵母培养物可以通过增加有益菌,减少有害菌来改善黄羽肉鸡肠道健康,从而促进其生长性能的提高。
4 结论饲粮添加酵母培养物能够降低黄羽肉鸡F/G,增强血清免疫和抗氧化功能,降低盲肠拟杆菌属相对丰度,提高盲肠理研菌科RC9肠道群相对丰度,且以添加1 000 mg/kg效果为佳。
| [1] |
SHURSON G C. Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods[J]. Animal Feed Science and Technology, 2018, 235: 60-76. DOI:10.1016/j.anifeedsci.2017.11.010 |
| [2] |
LIU Y C, CHENG X, ZHEN W R, et al. Yeast culture improves egg quality and reproductive performance of aged breeder layers by regulating gut microbes[J]. Frontiers in Microbiology, 2021, 12: 633276. DOI:10.3389/fmicb.2021.633276 |
| [3] |
SUGANUMA K, HAMASAKI T, HAMAOKA T. Effect of dietary direct-fed microbial and yeast cell walls on cecal digesta microbiota of layer chicks inoculated with nalidixic acid resistant Salmonella Enteritidis[J]. Poultry Science, 2021, 100(10): 101385. DOI:10.1016/j.psj.2021.101385 |
| [4] |
HE W, GAO Y N, GUO Z Q, et al. Effects of fermented wheat bran and yeast culture on growth performance, immunity, and intestinal microflora in growing-finishing pigs[J]. Journal of Animal Science, 2021, 99(11): skab308. DOI:10.1093/jas/skab308 |
| [5] |
ZHANG P Y, CAO S P, ZOU T, et al. Effects of dietary yeast culture on growth performance, immune response and disease resistance of gibel carp (Carassius auratus gibelio CAS Ⅲ)[J]. Fish & Shellfish Immunology, 2018, 82: 400-407. |
| [6] |
ESPINOSA C D, LAGOS L V, STEIN H H. Effect of torula yeast on growth performance, diarrhea incidence, and blood characteristics in weanling pigs[J]. Journal of Animal Science, 2020, 98(10): skaa307. DOI:10.1093/jas/skaa307 |
| [7] |
陈脊宇, 何航, 王芬, 等. 酵母培养物对五黑鸡生产性能、蛋品质及血清生化指标的影响[J]. 动物营养学报, 2022, 34(4): 2314-2323. CHEN J Y, HE H, WANG F, et al. effects of yeast culture on performance, egg quality and serum biochemical indices of Wuhei chickens[J]. Chinese Journal of Animal Nutrition, 2022, 34(4): 2314-2323 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.04.027 |
| [8] |
SILVA L G T, COOKE R F, SCHUBACH K M, et al. Supplementing a yeast-derived product to enhance productive and health responses of beef steers[J]. Animal, 2018, 12(8): 1576-1583. DOI:10.1017/S1751731117003585 |
| [9] |
SUN Z, WANG T, DEMELASH N, et al. Effect of yeast culture (Saccharomyces cerevisiae) on broilers: a preliminary study on the effective components of yeast culture[J]. Animals, 2019, 10(1): 68. DOI:10.3390/ani10010068 |
| [10] |
LANZARINI N M, BENTES G A, DE MELLO-VOLOTÃO E, et al. Use of chicken immunoglobulin Y in general virology[J]. Journal of Immunoassay and Immunochemistry, 2018, 39(3): 235-248. DOI:10.1080/15321819.2018.1500375 |
| [11] |
BU X Y, LIAN X Q, WANG Y, et al. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-κB signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis)[J]. Fish & Shellfish Immunology, 2019, 84: 711-718. |
| [12] |
王斌星, 王蜀金, 郭春华, 等. 酿酒酵母发酵液对断奶仔猪生长性能、小肠发育及小肠黏膜免疫功能的影响[J]. 动物营养学报, 2016, 28(12): 4014-4022. WANG B X, WANG S J, GUO C H, et al. Effects of sccharomyces cerevisiae fermentation broth on growth performance, small intestine development and small intestinal mucosal immune function of weaned piglets[J]. Chinese Journal of Animal Nutrition, 2016, 28(12): 4014-4022 (in Chinese). |
| [13] |
周苗, 朱胜澜, 王俊峰, 等. 凝结芽孢杆菌-酵母培养物对固始鸡生长性能、血清生化、抗氧化及免疫功能的影响[J]. 饲料研究, 2021, 44(15): 117-122. ZHOU M, ZHU S L, WANG J F, et al. Effect of bacillus coagulans-yeast culture on growth performance, serum biochemical, antioxidant and immune functions of Gushi chicken[J]. Feed Research, 2021, 44(15): 117-122 (in Chinese). DOI:10.13557/j.cnki.issn1002-2813.2021.15.027 |
| [14] |
魏庆, 魏朝阳, 王克玮, 等. 植物精油和柠檬酸复合物对肉鸡肠道屏障功能及炎症反应的影响[J]. 中国畜牧兽医, 2021, 48(11): 4014-4024. WEI Q, WEI C Y, WANG K W, et al. Effects of plant essential oil-citric acid complex on intestinal barrier function and inflammatory response of broilers[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(11): 4014-4024 (in Chinese). DOI:10.16431/j.cnki.1671-7236.2021.11.011 |
| [15] |
AL-SADI R, BOIVIN M, MA T. Mechanism of cytokine modulation of epithelial tight junction barrier[J]. Frontiers in Bioscience, 2009, 14: 2765-2778. |
| [16] |
MCCAFFREY C, CORRIGAN A, MOYNAGH P, et al. Effect of yeast cell wall supplementation on intestinal integrity, digestive enzyme activity and immune traits of broilers[J]. British Poultry Science, 2021, 62(5): 771-782. DOI:10.1080/00071668.2021.1929070 |
| [17] |
BROWNE N, TRAYNOR A, HORGAN K. Mannan rich fraction from yeast modulates inflammatory responses in intestinal cells (HT-29) exposed to Escherichia coli[J]. Journal of Applied Animal Nutrition, 2019, 72(1): 248-254. |
| [18] |
YUAN K, MENDONÇA L G D, HULBERT L E, et al. Yeast product supplementation modulated humoral and mucosal immunity and uterine inflammatory signals in transition dairy cows[J]. Journal of Dairy Science, 2015, 98(5): 3236-3246. DOI:10.3168/jds.2014-8469 |
| [19] |
WANG T, CHENG K, YU C Y, et al. Effects of a yeast-derived product on growth performance, antioxidant capacity, and immune function of broilers[J]. Poultry Science, 2021, 100(9): 101343. DOI:10.1016/j.psj.2021.101343 |
| [20] |
MAVROMMATIS A, GIAMOURI E, MYRTSI E D, et al. Antioxidant status of broiler chickens fed diets supplemented with vinification by-products: a valorization approach[J]. Antioxidants, 2021, 10(8): 1250. DOI:10.3390/antiox10081250 |
| [21] |
CLAVIJO V, FLÓREZ M J V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review[J]. Poultry Science, 2018, 97(3): 1006-1021. DOI:10.3382/ps/pex359 |
| [22] |
彭钰筝, 王相科, 宋曼玲, 等. 混合型酸化剂对肉鸡小肠形态和盲肠微生物区系的影响[J]. 中国家禽, 2020, 42(2): 52-58. PENG Y Z, WANG X K, SONG M L, et al. Effect of mixed acidifier on intestinal morphology and caecum microbial flora of broilers[J]. China Poultry, 2020, 42(2): 52-58 (in Chinese). DOI:10.16372/j.issn.1004-6364.2020.02.010 |
| [23] |
WU Y P, ZHANG H R, ZHANG R Q, et al. Serum metabolome and gut microbiome alterations in broiler chickens supplemented with lauric acid[J]. Poultry Science, 2021, 100(9): 101315. DOI:10.1016/j.psj.2021.101315 |
| [24] |
MA Y B, WANG W W, ZHANG H J, et al. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens[J]. Scientific Reports, 2018, 8(1): 15358. DOI:10.1038/s41598-018-33762-8 |
| [25] |
CHEN F, ZHANG H, ZHAO N, et al. Effect of chlorogenic acid on intestinal inflammation, antioxidant status, and microbial community of young hens challenged with acute heat stress[J]. Animal Science Journal, 2021, 92(1): e13619. |
| [26] |
BERRY D, MADER E, LEE T K, et al. Tracking heavy water (D2O) incorporation for identifying and sorting active microbial cells[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(2): E194-E203. |
| [27] |
BIAN X Y, WU W R, YANG L Y, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice[J]. Frontiers in Microbiology, 2019, 10: 2259. DOI:10.3389/fmicb.2019.02259 |
| [28] |
CAO Y, REN G F, ZHANG Y H, et al. A new way for punicalagin to alleviate insulin resistance: regulating gut microbiota and autophagy[J]. Food & Nutrition Research, 2021, 65: 29219. |




