2. 安徽农业大学动物科技学院, 合肥 230036
2. College of Animal Science and Technology, Anhui Agricultural University, Hefei 230036, China
肠道是动物体重要的消化器官,是消化食物和吸收营养物质的主要场所[1]。同时,肠道又是机体十分重要的防御屏障,可有效阻止细菌及内毒素等有害物质透过肠黏膜进入体循环,从而减少机体炎症反应的发生[2]。肠道功能受损可引起肠道菌群紊乱、蠕动减弱和免疫力降低及肠黏膜通透性升高,从而导致肠道消化吸收功能降低和炎症反应发生,最终造成畜禽生长性能下降[3-5]。因此,维护肠道健康对于促进营养物质的消化吸收及提高动物生产性能和养殖效益具有重要的意义。
饲粮中添加的硒源主要分为无机硒和有机硒,常用无机硒源为亚硒酸钠(sodium selenite, SS),有机硒源为酵母硒(selenium yeast,SY)。范秋丽等[6]研究表明,SY较SS可显著降低21日龄黄羽肉鸡空肠黏膜丙二醛(malondialdehyde, MDA)含量。Lynch等[7]的研究发现,SY减轻镉诱导的猪空肠上皮细胞DNA损伤的效果优于SS。Muhammad等[8]在23周龄罗曼蛋鸡饲粮中添加SS和SY进行16周的饲养试验,结果发现,SY较SS显著提高了回肠绒毛高度(villus height, VH)和空肠绒毛高度与隐窝深度的比值(villus height/crypt depth ratio, V/C)。
酵母硒的主效成分为硒代蛋氨酸(selenomethionine,SM),但含量不稳定[9]。因此,近年来化工合成的SM产品逐渐应用到饲粮中。与SS相比,SM在动物体内具有吸收率高、生物活性强、毒性低、抗氧化功能强和环境污染小等特点[10-11]。已有研究结果表明,SM在提高肉鸡肝脏、肾脏、胰脏和肌肉的抗氧化能力方面优于SS[12-14],而SM与肉鸡肠道抗氧化、免疫和细胞凋亡之间的关系却鲜有报道。为此,本试验以岭南黄鸡为研究对象,以无机硒源SS为对照,研究SM对肉鸡生长性能和肠道组织形态及抗氧化功能、免疫功能和细胞凋亡的影响,为SM在肉鸡生产上的应用提供理论依据。
1 材料与方法 1.1 试验材料亚硒酸钠(货号:10102-18-8),纯度99%,购自某化学试剂有限公司;SM(货号:259960000),纯度99%,购自北京某科技有限公司。
1.2 试验设计与饲粮 1.2.1 试验设计本试验经浙江农林大学动物伦理委员会批准,该委员会按照试验动物护理和使用指南来管理试验过程中所有动物的使用。选取1日龄体重相近的岭南黄肉雏鸡(购自浙江群大畜牧养殖有限公司)540只,按照单因素试验设计随机分为3组,每组6个重复,每个重复30只鸡(公母各占1/2)。试验分组如下:对照组(CON组),饲喂基础饲粮;SS组和SM组分别饲喂在基础饲粮中添加0.15 mg/kg(以硒计)SS和SM的试验饲粮,SS和SM的添加剂量根据本课题组前期的试验结果[13, 15]确定。饲养试验为期56 d,分为1~21日龄和22~56日龄2个饲养阶段。
1.2.2 基础饲粮基础饲粮参照我国农业行业标准《黄羽肉鸡营养需要量》(NY/T 3645—2020)[16]进行配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
准确称取饲粮样品2.0 g,根据GB/T 13883—2008的方法处理样品,采用氢化物原子荧光光谱法测定饲粮中硒含量,所用仪器为AF-610A型原子荧光光度计(北京北分瑞利分析仪器有限责任公司)。每个样品6个重复。各组饲粮中硒含量见表 2。
|
|
表 2 各组饲粮中硒含量(实测值) Table 2 Selenium content in diets of different groups (measured values) |
试验鸡采用网上平养,自由采食和饮水,按正常免疫程序免疫,其他饲养管理按照常规程序进行。每日以重复为单位记录耗料量和死淘鸡数。于1、21和56日龄以重复为单位对空腹12 h肉鸡称重,并准确统计各重复各阶段的耗料量和死亡数,同时以重复单位计算1~21日龄、22~56日龄和1~56日龄的平均日增重(average daily gain, ADG)、平均日采食量(average daily feed intake, ADFI)和料重比(feed/gain, F/G)。
1.4 样品采集与指标测定 1.4.1 样品采集饲养试验结束后,每组选取12只体重接近平均体重的健康公鸡(每重复2只),共计36只,给水不给料,禁食12 h,翅静脉采血于促凝管中,3 000 r/min(4 ℃)离心10 min制备血清。采血后,用戊巴比妥钠(50 mg/kg)对鸡进行麻醉并解剖,打开腹腔,将消化道取出,按组织学把小肠分为十二指肠、空肠和回肠。取十二指肠和空肠中段约2 cm,用预冷的生理盐水将其冲洗干净,而后平铺在滤纸上将液体吸干,置于4%多聚甲醛溶液固定。剩余十二指肠和空肠肠段迅速取黏膜。血清和肠黏膜样品于-80 ℃保存,用于后续指标的测定。
1.4.2 肠道形态的测定取出经4%多聚甲醛溶液固定后的十二指肠和空肠样品,经脱水、石蜡包埋、修块、切片、苏木精-伊红(HE)染色及封片固定后放于光学显微镜下拍照,用Image软件测量VH和隐窝深度(crypt depth,CD),每张切片选取5根完整肠绒毛测量VH和其对应位置CD,并计算V/C。
1.4.3 抗氧化指标的测定用商品化试剂盒(南京建成生物工程研究所)测定十二指肠和空肠黏膜NAD(P)H: 醌氧化还原酶1[NAD(P)H:dehydrogenase quinone 1, NQO1]、硫氧还蛋白还原酶(thioredoxin reductase, TrxR)、血红素加氧酶-1(heme oxygenase-1, HO-1)、过氧化氢酶(catalase, CAT)、总超氧化物歧化酶(total superoxide dismutase, T-SOD)和谷胱甘肽过氧化物酶(glutathione peroxidase, GPx)活性,具体操作步骤按照试剂盒说明书严格执行。
1.4.4 氧化损伤指标的测定采用商品化试剂盒(南京建成生物工程研究所)测定十二指肠和空肠黏膜蛋白羰基(protein carbonyl, PC)、MDA和8-羟基脱氧鸟苷(8-hydroxydeoxyguanosine, 8-OHdG)含量,操作步骤按照试剂盒说明书进行。
1.4.5 免疫指标的测定采用酶联免疫吸附试验(ELISA)法测定血清中免疫球蛋白A(immunoglobulin A,IgA)、免疫球蛋白M(immunoglobulin M,IgM)和免疫球蛋白G(immunoglobulin G,IgG)含量及十二指肠和空肠黏膜分泌型免疫球蛋白A(secretory immunoglobulin A,sIgA)含量,所用试剂盒购自北京方程生物科技有限公司。
1.4.6 细胞凋亡指标的测定采用ELISA法测定十二指肠和空肠黏膜半胱氨酸天冬氨酸蛋白酶-3(cysteine-aspartic acid protease-3, Caspase-3)和B淋巴细胞瘤-2(B-cell lymphoma-2, Bcl-2)的含量,所用试剂盒购自圣地亚哥生物技术公司。
1.4.7 肠黏膜蛋白含量的测定十二指肠和空肠黏膜蛋白含量测定采用南京建成生物工程研究所生产的试剂盒以考马斯亮兰法测定。
1.5 数据统计与分析试验数据采用SPSS 21.0软件进行单因素方差分析(one-way ANOVA),并采用Duncan氏法进行多重比较,结果以平均值±标准差表示,P<0.05表示差异显著。
2 结果 2.1 不同硒源对黄羽肉鸡生长性能的影响由表 3可知,各组之间肉鸡各生长阶段ADFI均无显著差异(P>0.05)。与CON组相比,饲粮中添加SS可显著提高56日龄肉鸡均重及22~56日龄和1~56日龄肉鸡ADG(P<0.05);饲粮中添加SM可显著提高21和56日龄肉鸡体重及各生长阶段肉鸡ADG(P<0.05),并显著降低各生长阶段肉鸡F/G(P<0.05)。此外,SM组56日龄肉鸡体重及22~56日龄和1~56日龄肉鸡ADG较SS组显著提高(P<0.05),22~56日龄和1~56日龄肉鸡F/G较SS组显著降低(P<0.05)。
|
|
表 3 不同硒源对黄羽肉鸡生长性能的影响 Table 3 Effects of different selenium sources on growth performance of yellow-feathered broilers |
各组肉鸡肠道组织切片见图 1。由表 4可知,与CON组相比,饲粮添加SS和SM均显著提高了十二指肠和空肠VH和V/C(P<0.05),显著降低了十二指肠和空肠CD(P<0.05)。与SS组相比,SM组十二指肠V/C以及空肠VH和V/C显著提高(P<0.05),空肠CD显著降低(P<0.05)。
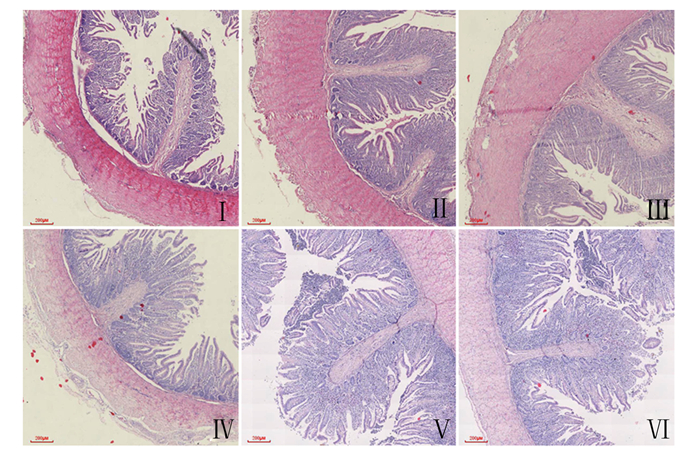
|
A:十二指肠duodenum;B:空肠jejunum。 图 1 黄羽肉鸡肠道组织切片图 Fig. 1 Slice maps of intestinal tissue of yellow-feathered broilers (40×) |
|
|
表 4 不同硒源对黄羽肉鸡肠道形态的影响 Table 4 Effects of different selenium sources on intestinal morphology of yellow-feathered broilers |
由表 5可知,与CON组相比,SS组和SM组十二指肠和空肠黏膜GPx和TrxR活性显著提高(P<0.05)。SM组空肠黏膜GPx和TrxR活性显著低于SS组(P<0.05)。SS组十二指肠黏膜HO-1和CAT活性及空肠黏膜NQO1活性显著高于CON组(P<0.05)。SM组十二指肠和空肠黏膜NQO1、HO-1、CAT和T-SOD活性比CON组显著提高(P<0.05)。SM组十二指肠和空肠黏膜NQO1和CAT活性及十二指肠黏膜HO-1和T-SOD活性较SS组显著提高(P<0.05)。
|
|
表 5 不同硒源对黄羽肉鸡肠黏膜抗氧化指标的影响 Table 5 Effects of different selenium sources on intestinal mucosal antioxidant indices of yellow-feathered broilers |
由表 6可知,与CON组相比,SS组十二指肠和空肠黏膜MDA和PC含量及十二指肠黏膜8-OHdG含量显著降低(P<0.05)。SM组十二指肠和空肠黏膜MDA、PC和8-OHdG含量显著低于SS组和CON组(P<0.05)。
|
|
表 6 不同硒源对黄羽肉鸡肠黏膜氧化损伤指标的影响 Table 6 Effects of different selenium sources on intestinal mucosal oxidative damage indices of yellow-feathered broilers |
由表 7可知,与CON组相比,SS组血清IgA和IgG含量及空肠黏膜sIgA含量显著增加(P<0.05)。SM组血清IgA、IgM和IgG含量及十二指肠和空肠黏膜sIgA含量较CON组和SS组显著增加(P<0.05)。
|
|
表 7 不同硒源对黄羽肉鸡血清和肠黏膜免疫指标的影响 Table 7 Effects of different selenium sources on serum and intestinal mucosal immune indices of yellow-feathered broilers |
由表 8可知,与CON组相比,SS组十二指肠黏膜Caspase-3含量显著降低(P<0.05),空肠Bcl-2含量显著提高(P<0.05);SM组十二指肠和空肠黏膜Caspase-3含量显著降低(P<0.05),十二指肠和空肠黏膜Bcl-2含量显著提高(P<0.05)。与SS组相比,SM组十二指肠和空肠黏膜Bcl-2含量显著提高(P<0.05),空肠黏膜Caspase-3含量显著降低(P<0.05)。
|
|
表 8 不同硒源对肉鸡肠黏膜细胞凋亡指标的影响 Table 8 Effects of different selenium sources on apoptosis indexes of intestinal mucosal in yellow-feathered broilers |
硒是动物生长必需的微量元素,饲粮中补硒可提高动物的生产性能[17]。本试验结果发现,饲粮添加SS和SM显著提高了22~56日龄和1~56日龄黄羽肉鸡的ADG,添加SM还显著降低了各生长阶段黄羽肉鸡的F/G。李建柱等[18]在饲粮中添加0.2 mg/kg的SS和SY进行9周的饲养试验,发现SY组淮南麻鸭的ADG较SS组显著提高。Arnaut等[19]在科宝肉鸡上的研究发现,与SS组相比,SY组ADG显著提高。本试验结果表明,SM组与SS组22~56日龄和1~56日龄黄羽肉鸡ADG及F/G的差异达到显著水平,这与以上研究结果大体一致。造成此结果的原因在于SM较SS显著提高了肠黏膜抗氧化和免疫功能,减轻了肠黏膜氧化损伤和细胞凋亡,改善了肠道形态,进而可能促进了营养物质的消化和吸收,导致了生长性能的提高。
3.2 不同硒源对黄羽肉鸡肠道形态的影响小肠是消化食物和吸收营养物质的主要场所,其VH、CD和V/C是衡量小肠消化吸收功能的重要指标。在本试验中,与CON组相比,饲粮中添加SS或SM均显著提高了十二指肠和空肠VH和V/C,显著降低了十二指肠和空肠CD,这与He等[20]的研究结果相似。Muhammad等[8]研究表明,与SS组相比,SY组39周龄罗曼蛋鸡回肠VH和空肠V/C显著提高。本试验结果与上述试验结果基本一致,本研究发现,与SS组相比,SM组十二指肠V/C以及空肠VH和V/C显著提高,空肠CD显著降低。对此结果的解释是SM比SS减轻了肠黏膜细胞凋亡,进而引起肠道VH和V/C的升高及CD的降低。
3.3 不同硒源对黄羽肉鸡肠黏膜抗氧化指标的影响硒在体内的生物学功能主要是以硒蛋白的形式表现,硒以硒代半胱氨酸(Sec)的形式共价结合在硒蛋白中[21]。目前在人和动物体内比较重要的抗氧化硒蛋白主要有GPx家族和TrxR家族[22]。GPx可特异性地清除有害的过氧化氢(H2O2),并阻止脂质过氧化物的形成[23]。TrxR具有抗氧化和还原核苷酸参与DNA合成等功能[24]。本试验结果表明,与CON组相比,饲粮中添加SS或SM均显著提高了十二指肠和空肠黏膜GPx和TrxR活性,这与前人研究报道[25-26]相似。另外,本试验结果还发现,在提高空肠黏膜GPx和TrxR活性方面,SS优于SM;但在提高十二指肠黏膜GPx和TrxR活性方面,SS与SM效果相当。这与前人试验结果[27-29]基本一致。无机硒在提高GPx和TrxR活性方面优于有机硒,其可能的原因有2种:1)不同硒源在体内均须转化为Sec后才能合成硒蛋白,而SS转化为Sec的效率显著高于SM[30],因此SS合成硒蛋白的效率更高;2)蛋氨酸(Met)-tRNA无法区分SM和Met化学形式上的不同,使得SM可直接替代Met用于机体蛋白质合成[9]。White等[31]的研究发现,SM中的硒小鼠成纤维细胞中首先合成体蛋白质然后再合成硒蛋白,而SS中的硒则以很快的速度直接合成硒蛋白。因此,SM和Met竞争性的合成体蛋白质也影响SM合成硒蛋白的效率。
超氧化物歧化酶(SOD)可催化超氧阴离子转化为H2O2和氧(O2);CAT可分解H2O2产生O2和H2O;NQO1可阻止环境胁迫剂对DNA造成的氧化损伤,还可通过维持泛醌和α-生育酚的还原形式,保护体内内源性抗氧化剂[32];HO-1是一种抗炎、抗氧化和具有神经保护作用的诱导酶[32];SOD、CAT、NQO1和HO-1活性的提高均可增强机体的抗氧化能力。本试验结果显示,从综合效果来看,饲粮中添加SS和SM均可提高十二指肠和空肠黏膜T-SOD、CAT、NQO1和HO-1活性,且以SM效果较佳,这与前人研究报道[33-34]基本一致。由此可见,本试验条件下饲粮中补硒可提高黄羽肉鸡肠道抗氧化能力,且SM的效果优于SS。补硒能提高肠道抗氧化能力的原因在于补硒可增加肠黏膜硒沉积。因为硒是GPx和TrxR的活性成分,补硒直接导致GPx和TrxR活性升高,进而使H2O2和其他活性氧自由基(ROS)的降解作用加强,节省了SOD、CAT、NQO1和HO-1,最终提高了肠道的抗氧化能力。有机硒在提高肠道抗氧化能力方面优于无机硒,可能原因是SM较SS显著激活了抗氧化相关信号通路,但具体的作用机理还有待进一步研究。
3.4 不同硒源对黄羽肉鸡肠黏膜氧化损伤的影响PC、8-OHdG和MDA分别是蛋白质、DNA和脂质氧化损伤的产物[35]。在本研究中,补硒降低了黄羽肉鸡十二指肠和空肠黏膜PC、8-OHdG和MDA含量,这与前人研究报道[36-37]一致。鞠耿越[38]在江南白鹅上的研究显示,SM组肝脏和血浆MDA含量极显著低于SS组。在本试验中,与SS组相比,SM组十二指肠和空肠黏膜PC、8-OHdG和MDA含量显著降低,这与上述试验结果一致。本试验结果提示,SM较SS降低十二指肠和空肠黏膜氧化损伤,这与SM较SS能提高肠黏膜的抗氧化功能有关。
3.5 不同硒源对黄羽肉鸡血清和肠黏膜免疫指标的影响硒是优化免疫应答的重要元素之一,可促进免疫器官的生长发育和淋巴细胞的增殖及免疫球蛋白的合成[39]。在本研究中,2个补硒组黄羽肉鸡血清IgA和IgG含量及空肠黏膜sIgA含量显著高于CON组,这与前人试验结果[20, 40]一致。此外,本试验数据还显示,SM组黄羽肉鸡血清IgA、IgM和IgG含量及十二指肠和空肠黏膜sIgA含量均显著高于SS组,这表明,在提高肉鸡肠黏膜免疫功能方面,SM的效果优于SS。当机体内活化的免疫细胞代谢增加时,产生的ROS增多。过多的ROS又可损伤免疫活性细胞,降低免疫功能,因此需要强大的抗氧化系统清除ROS。SM比SS能显著增强肠黏膜抗氧化能力,进而提高肠黏膜免疫力。
3.6 不同硒源对黄羽肉鸡肠黏膜细胞凋亡指标的影响Caspase-3可通过对蛋白激酶、核酸酶及细胞骨架的裂解,激活特定信号系统,使细胞核皱缩,导致细胞凋亡[41]。Bcl-2可抑制细胞凋亡,能增强细胞对大多数DNA损伤因子的抵抗性[42]。本研究结果显示,补硒显著降低了黄羽肉鸡十二指肠黏膜Caspase-3含量,提高了空肠黏膜Bcl-2含量,这与前人研究结果[43-44]相似。此外,本研究结果还发现,与SS相比,SM显著提高了十二指肠和空肠黏膜Bcl-2含量及降低了空肠黏膜Caspase-3含量。本试验结果提示,在降低十二指肠和空肠黏膜细胞凋亡方面,SM效果优于SS,推测与SM较SS能提高肠黏膜的抗氧化能力和免疫功能有关。因为肠黏膜抗氧化能力和免疫功能下降会导致ROS过量蓄积,诱发细胞毒性和DNA损伤,导致细胞凋亡[45]。
4 结论本试验条件下,饲粮添加SM和SS均可提高黄羽肉鸡的生长性能,改善肠道形态,增强肠道抗氧化功能和免疫功能,减少肠道氧化损伤和细胞凋亡,且SM效果优于SS。
| [1] |
李祥, 何金环, 潘春梅, 等. 丁酸钠对肉鸡肠道形态与消化吸收功能影响的研究进展[J]. 中国畜牧兽医, 2021, 48(5): 1603-1612. LI X, HE J H, PAN C M, et al. Research progress on effect of sodium butyrate on intestinal morphology, digestion and absorption function of broilers[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(5): 1603-1612 (in Chinese). DOI:10.16431/j.cnki.1671-7236.2021.05.011 |
| [2] |
AHERN P P, MALOY K J. Understanding immune-microbiota interactions in the intestine[J]. Immunology, 2020, 159(1): 4-14. DOI:10.1111/imm.13150 |
| [3] |
DING L Y, CHEN X T, QIAN K, et al. Probiotics on intestinal flora disturbance and bacterial translocation in mice with inflammatory bowel disease[J]. Indian Journal of Pharmaceutical Sciences, 2021, 83(6): 1174-1180. |
| [4] |
KOGUT M H, GENOVESE K J, SWAGGERTY C L, et al. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal[J]. Poultry Science, 2018, 97(7): 2339-2346. DOI:10.3382/ps/pey087 |
| [5] |
QIN L S, JI W, WANG J L, et al. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets[J]. Food & Function, 2019, 10(5): 2359-2371. |
| [6] |
范秋丽, 蒋守群, 林厦菁, 等. 维生素E和不同来源硒对1~21日龄黄羽肉鸡生长性能和肠道功能的影响[J]. 饲料研究, 2018(5): 39-44. FAN Q L, JIANG S Q, LIN S J, et al. Effects of vitamin E and different sources of selenium on growth performance and intestinal function of yellow-feathered broilers aged from 1 to 21 days[J]. Feed Research, 2018(5): 39-44 (in Chinese). DOI:10.13557/j.cnki.issn1002-2813.2018.05.009 |
| [7] |
LYNCH S J, HORGAN K A, WHITE B, et al. Selenium source impacts protection of porcine jejunal epithelial cells from cadmium-induced DNA damage, with maximum protection exhibited with yeast-derived selenium compounds[J]. Biological Trace Element Research, 2017, 176(2): 311-320. DOI:10.1007/s12011-016-0828-7 |
| [8] |
MUHAMMAD A I, MOHAMED D A, CHWEN L T, et al. Effect of selenium sources on laying performance, egg quality characteristics, intestinal morphology, microbial population and digesta volatile fatty acids in laying hens[J]. Animals, 2021, 11(6): 1681. DOI:10.3390/ani11061681 |
| [9] |
SCHRAUZER G N. The nutritional significance, metabolism and toxicology of selenomethionine[J]. Advances in Food and Nutrition Research, 2003, 47: 73-112. |
| [10] |
FALK M, BERNHOFT A, REINOSO-MASET E, et al. Beneficial antioxidant status of piglets from sows fed selenomethionine compared with piglets from sows fed sodium selenite[J]. Journal of Trace Elements in Medicine and Biology, 2020, 58: 126439. DOI:10.1016/j.jtemb.2019.126439 |
| [11] |
XU X J, ZHANG D G, ZHAO T, et al. Dietary selenium sources differentially regulate selenium concentration, mRNA and protein expression of representative selenoproteins in various tissues of yellow catfish Pelteobagrus fulvidraco[J]. British Journal of Nutrition, 2022, 127(4): 490-502. DOI:10.1017/S000711452100194X |
| [12] |
WANG Y X, ZHAN X A, ZHANG X W, et al. Comparison of different forms of dietary selenium supplementation on growth performance, meat quality, selenium deposition, and antioxidant property in broilers[J]. Biological Trace Element Research, 2011, 143(1): 261-273. DOI:10.1007/s12011-010-8839-2 |
| [13] |
WANG Y X, XIAO X, ZHAN X A. Antagonistic effects of different selenium sources on growth inhibition, oxidative damage, and apoptosis induced by fluorine in broilers[J]. Poultry Science, 2018, 97(9): 3207-3217. DOI:10.3382/ps/pey192 |
| [14] |
IBRAHIM D, KISHAWY A T Y, KHATER S I, et al. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in Ross broiler chickens[J]. Animals, 2019, 9(6): 342. DOI:10.3390/ani9060342 |
| [15] |
WANG Y, WANG H, ZHAN X. Effects of different DL-selenomethionine and sodium selenite levels on growth performance, immune functions and serum thyroid hormones concentrations in broilers[J]. Journal of Animal Physiology and Animal Nutrition, 2016, 100(3): 431-439. DOI:10.1111/jpn.12396 |
| [16] |
中华人民共和国农业农村部. 黄羽肉鸡营养需要量: NY/T 3645-2020[S]. 北京: 中国农业出版社, 2020. Ministry of Agriculture and Rural Affairs of the People's Republic of China. Nutrient requirements of yellow-feathered broilers: NY/T 3645-2020[S]. Beijing: China Agriculture Press, 2020. (in Chinese) |
| [17] |
PECORARO B M, LEAL D F, FRIAS-DE-DIEGO A, et al. The health benefits of selenium in food animals: a review[J]. Journal of Animal Science and Biotechnology, 2022, 13(1): 58. DOI:10.1186/s40104-022-00706-2 |
| [18] |
李建柱, 唐雪峰, 赵云焕, 等. 不同硒源对淮南麻鸭1~9周龄生长性能及免疫功能的影响[J]. 饲料研究, 2015(10): 35-39. LI J Z, TANG X F, ZHAO Y H, et al. Effects of different selenium source on growth performance and immune function of Huainan partridge ducks aged from 1 to 9 weeks[J]. Feed Research, 2015(10): 35-39 (in Chinese). DOI:10.13557/j.cnki.issn1002-2813.2015.10.010 |
| [19] |
ARNAUT P R, DA SILVA VIANA G, DA FONSECA L, et al. Selenium source and level on performance, selenium retention and biochemical responses of young broiler chicks[J]. BMC Veterinary Research, 2021, 17(1): 151. DOI:10.1186/s12917-021-02855-4 |
| [20] |
HE X J, LIN Y C, LIAN S, et al. Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology, SIgA secretion, and GSH-Px activity[J]. Biological Trace Element Research, 2020, 197(2): 660-666. DOI:10.1007/s12011-019-02017-6 |
| [21] |
HA H Y, ALFULAIJ N, BERRY M J, et al. From selenium absorption to selenoprotein degradation[J]. Biological Trace Element Research, 2019, 192(1): 26-37. DOI:10.1007/s12011-019-01771-x |
| [22] |
ZHANG Y, ROH Y J, HAN S J, et al. Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review[J]. Antioxidants, 2020, 9(5): 383. DOI:10.3390/antiox9050383 |
| [23] |
HARIHARAN S, DHARMARAJ S. Selenium and selenoproteins: it's role in regulation of inflammation[J]. Inflammopharmacology, 2020, 28(3): 667-695. DOI:10.1007/s10787-020-00690-x |
| [24] |
XU J Q, FANG J G. How can we improve the design of small molecules to target thioredoxin reductase for treating cancer?[J]. Expert Opinion on Drug Discovery, 2021, 16(4): 331-333. DOI:10.1080/17460441.2021.1854220 |
| [25] |
PLACHA I, TAKACOVA J, RYZNER M, et al. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers[J]. British Poultry Science, 2014, 55(1): 105-114. DOI:10.1080/00071668.2013.873772 |
| [26] |
MENG T T, LIU Y L, XIE C Y, et al. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens[J]. Biological Trace Element Research, 2019, 189(2): 548-555. DOI:10.1007/s12011-018-1490-z |
| [27] |
CANTOR A H, LANGEVIN M L, NOGUCHI T, et al. Efficacy of selenium in selenium compounds and feedstuffs for prevention of pancreatic fibrosis in chicks[J]. The Journal of Nutrition, 1975, 105(1): 106-111. DOI:10.1093/jn/105.1.106 |
| [28] |
JIANG Z Y, LIN Y C, ZHOU G L, et al. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers[J]. Journal of Agricultural and Food Chemistry, 2009, 57(20): 9769-9772. DOI:10.1021/jf902411c |
| [29] |
LI K, JIANG L, WANG J, et al. Maternal dietary supplementation with different sources of selenium on antioxidant status and mortality of chicken embryo in a model of diquat-induced acute oxidative stress[J]. Animal Feed Science and Technology, 2020, 261: 114369. DOI:10.1016/j.anifeedsci.2019.114369 |
| [30] |
HENRY P R, AMMERMAN C B. Selenium biovailability[M]//AMMERMAN C B, BAKER D H, LEWIS A J. Bioavailability of nutrients for animals. San Diego: Academic Press, 1995: 303-336.
|
| [31] |
WHITE C L, HOEKSTRA W G. The metabolism of selenite and selenomethionine in mouse fibroblasts grown in tissue culture[J]. Biological Trace Element Research, 1979, 1(3): 243-257. DOI:10.1007/BF02783818 |
| [32] |
ZHANG Z H, QU J, ZHENG C, et al. Nrf2 antioxidant pathway suppresses numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis[J]. Cell Death & Disease, 2018, 9(2): 83. |
| [33] |
DALIA A M, LOH T C, SAZILI A Q, et al. The effect of dietary bacterial organic selenium on growth performance, antioxidant capacity, and selenoproteins gene expression in broiler chickens[J]. BMC Veterinary Research, 2017, 13(1): 254. DOI:10.1186/s12917-017-1159-4 |
| [34] |
LI K, CAO Z J, GUO Y, et al. Selenium yeast alleviates ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 4048706. |
| [35] |
WAZIR H, CHAY S Y, ZAREI M, et al. Effects of storage time and temperature on lipid oxidation and protein co-oxidation of low-moisture shredded meat products[J]. Antioxidants, 2019, 8(10): 486. DOI:10.3390/antiox8100486 |
| [36] |
郑世杰. 硒对雄鼠睾丸硒蛋白组表达及相关生殖功能的影响[D]. 硕士学位论文. 广州: 广东药学院, 2014: 34-35. ZHENG S J. Effects of selenium on the expression of male rat testis selenoproteome and related reproductive functions[D]. Master's Thesis. Guangzhou: Guangdong Pharmaceutical University, 2014: 34-35. (in Chinese) |
| [37] |
KORZENIOWSKA M, KRÓLICZEWSKA B, KOPEĆ W. Carbonyl and sulfhydryl groups of chicken meat proteins after dietary modulation with selenium[J]. Open Chemistry, 2015, 13(1): 1293-1302. |
| [38] |
鞠耿越. 不同硒源和硒水平对仔鹅生产性能、抗氧化性能和组织微量元素含量的影响[D]. 硕士学位论文. 扬州: 扬州大学, 2019: 37-38. JU G Y. Effects of different selenium sources and levels on production performance, antioxidant capacity and tissue trace elements content in goose[D]. Master's Thesis. Yangzhou: Yangzhou University, 2019: 37-38. (in Chinese) |
| [39] |
AVERY J C, HOFFMANN P R. Selenium, selenoproteins, and immunity[J]. Nutrients, 2018, 10(9): 1203. DOI:10.3390/nu10091203 |
| [40] |
SHI X, WANG W, ZHENG S F, et al. Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway[J]. Biological Trace Element Research, 2020, 194(2): 525-535. DOI:10.1007/s12011-019-01789-1 |
| [41] |
CHOUDHARY G S, AL-HARBI S, ALMASAN A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis[M]//MOR G, ALVERO A B. Apoptosis and cancer. New York: Humana Press, 2015: 1-9.
|
| [42] |
LIU H, YU Q F, FANG C K, et al. Effect of selenium source and level on performance, egg quality, egg selenium content, and serum biochemical parameters in laying hens[J]. Foods, 2020, 9(1): 68. DOI:10.3390/foods9010068 |
| [43] |
YANG J, ZHANG Y, HAMID S, et al. Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken[J]. Journal of Inorganic Biochemistry, 2017, 170: 17-25. DOI:10.1016/j.jinorgbio.2017.02.006 |
| [44] |
WANG X Y, AN Y, JIAO W Y, et al. Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys[J]. Biological Trace Element Research, 2018, 182(2): 354-363. DOI:10.1007/s12011-017-1097-9 |
| [45] |
MIAO K K, ZHANG L, YANG S Y, et al. Intervention of selenium on apoptosis and Fas/FasL expressions in the liver of fluoride-exposed rats[J]. Environmental Toxicology and Pharmacology, 2013, 36(3): 913-920. DOI:10.1016/j.etap.2013.08.003 |




