2. 湖南今汉药业有限公司, 长沙 410014;
3. 中南大学湘雅药学院, 长沙 410013;
4. 湖南文凤禽业有限公司, 娄底 417700
2. Geneham Pharmaceutical Co., Ltd., Changsha 410014, China;
3. Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, China;
4. Hunan Wenfeng Poultry Co., Ltd., Loudi 417700, China
大肠杆菌是导致畜禽许多疾病的病原菌之一,其使畜禽生产性能降低,且对抗生素的耐药性不断增加[1]。因此,一些替代抗生素的植物提取物的探索、开发与利用开始兴起,给畜牧业的发展带来了新的突破点。迷迭香具有许多活性成分,是很多酚类抗氧化剂的提取来源,具有抗氧化、抗炎、抗血糖等作用[2-4]。目前关于迷迭香及其提取物对家禽以及其他畜禽生产性能影响的研究较少,大多为人类疾病治疗等药物性研究[5-6]。本团队前期探究了迷迭香提取物对蛋鸡生产性能的影响,发现200~400 mg/kg的迷迭香提取物对其有积极作用[7]。为了探究迷迭香提取物对肉鸡的影响,本试验以黄羽肉鸡为试验对象,探究迷迭香提取物对大肠杆菌攻毒肉鸡生长性能、抗氧化能力、肠道健康等方面的影响,为迷迭香提取物作为新型、绿色、潜在替抗饲料添加剂的研究与应用提供理论依据。
1 材料与方法 1.1 试验材料本试验采用的植物添加剂迷迭香提取物的主要成分为迷迭香酸(≥5%)、熊果酸(≥3%)、叶绿素(≥4%)和黄酮类物质(主要是橙皮苷)(≥16%)。攻毒采用大肠杆菌K88,浓度为1.0×109 CFU/mL。
1.2 试验动物与试验设计试验选取健康状况良好、其他条件相近的7日龄黄羽肉鸡144羽(全母),随机分为3组,每组6个重复,每个重复8只鸡。对照组(CON组)和大肠杆菌攻毒组(EC组)饲喂基础饲粮,迷迭香提取物+大肠杆菌攻毒组(ECRE组)在基础饲粮中添加400 mg/kg迷迭香提取物。试验期为28 d(8~35日龄)。在29~35日龄进行为期7 d的攻毒试验。
大肠杆菌攻毒试验:借鉴Wang等[8]的攻毒方法,考虑到攻毒日龄的不同,在试验前用大肠杆菌K88进行攻毒预试验,即选取非试验用鸡,通过口腔灌喂2 mL浓度分别为1.0×107、1.0×108和1.0×109 CFU/mL的大肠杆菌K88,观察每日攻毒肉鸡的健康状况和腹泻情况,确定最合适浓度为1.0×109 CFU/mL。最终确立的灌喂攻毒操作为:29日龄开始,CON组每只鸡灌服2 mL无菌生理盐水,EC组和ECRE组每只鸡灌服2 mL浓度为1.0×109 CFU/mL的大肠杆菌K88菌液,连续灌服7 d。
1.3 基础饲粮本试验所用基础饲粮参照《黄羽肉鸡营养需要量》(NY/T 647—2020)进行配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of basal diets (DM basis) |
整个试验期采用3层笼养的方式饲养,每笼8只鸡,每1笼为1个重复,保持每个组的饲养环境条件相同。鸡舍定期消毒和清洁,保持通风,鸡舍温度保持在(24±2) ℃,鸡只自由采食和饮水。鸡舍固定光照时间:在肉鸡1~3日龄时每天光照时间为24 h,4日龄以后每天光照时间控制在18 h。
1.5 测定指标 1.5.1 生长性能和腹泻率于肉鸡8、29和35日龄空腹12 h(停料不停水)后称重,每天计录各组每个重复的采食量,从而计算出8~28日龄及29~35日龄阶段的平均日增重(ADG)、平均日采食量(ADFI)和料重比(F/G)。试验期间,每天观察鸡健康状况。大肠杆菌攻毒期间(29~35日龄时),正常鸡的粪便为柱状螺旋圆锥形,颜色浅灰绿色,在鸡粪表面附有白色带状尿酸盐,柔软蓬松;当鸡腹泻时,表现多样,有白色、红色、绿色等稀便。参照赵宪臣[9]的方法,通过观察肉鸡肛门的肮脏度及粪盘中拉稀粪便占比,记录每天每组鸡腹泻情况,计算每天的腹泻率,最终取7 d腹泻率平均值进行统计分析。
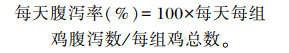
|
于35日龄空腹12 h后在每组的每个重复中随机选取1只健康的肉鸡,颈静脉采血,离心机分离血清,-80 ℃保存,进行后续的血清生化指标和抗氧化指标的测定。血清生化指标具体测定操作按试剂盒(南京建成生物工程研究所)说明书的方法实施,采用迈瑞BS200全自动生化分析仪(迈瑞医疗,深圳)检测血清中甘油三酯(TG,单试剂GPO-PAP法)、总胆固醇(TC,单试剂GPO-PAP法)、总蛋白(TP,考马斯亮蓝法)、葡萄糖(GLU,己糖激酶法)、白蛋白(ALB,溴甲酚绿法)、尿素(UREA,脲酶法)、高密度脂蛋白-胆固醇(HDL-C,双试剂直接法)、低密度脂蛋白-胆固醇(LDL-C,双试剂直接法)含量及谷草转氨酶(AST,赖氏法),谷丙转氨酶(ALT,赖氏法)、乳酸脱氢酶(LDH,微板法)、碱性磷酸酶(ALP,微量酶标法)活性。
1.5.3 血清抗氧化指标用上述保存的血清进行抗氧化指标的测定,具体操作按试剂盒(南京建成生物工程研究所)说明书的方法实施,采用酶标仪或分光光度计测定血清中总超氧化物歧化酶(T-SOD)、过氧化物酶(POD)、一氧化氮合成酶(NOS)、谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)活性以及总抗氧化能力(T-AOC)与一氧化氮(NO)、丙二醛(MDA)含量。
1.5.4 器官指数将放血后的肉鸡屠宰和剖解,分出胸腺、脾脏、法氏囊和肝脏等器官,用滤纸擦去血渍,去除脂肪后称重,用于计算器官指数。
1.5.5 肠道形态结构解剖肉鸡打开腹腔,每只鸡尽量控制在相同位置采取空肠、回肠肠段各1~2 cm,用镊子夹取样品肠段,用0.9%生理盐水冲洗肠道内容物,然后固定于4%多聚甲醛溶液中。将肠道组织样品从固定液中取出,经过修整、脱水、透明、浸蜡包埋、脱蜡染色制作肠道组织切片,并根据切片和染色效果选取切片,利用中性树脂进行封片。选择空肠和回肠组织形态完整、走向清晰的6个视野切片,使用显微镜图像处理软件(Image-Pro Plus 6.0)测量其绒毛高度(VH)和对应的隐窝深度(CD),并计算绒隐比(绒毛高度与隐窝深度的比值)。
1.6 数据分析试验原始数据用Excel 2010预处理,采用统计软件SPSS 21.0通过一般线性模型进行单因素方差分析,利用Duncan氏法进行多重比较,结果均以“平均值±标准差”表示,P<0.05为差异显著。
2 结果与分析 2.1 迷迭香提取物对大肠杆菌攻毒肉鸡生长性能和腹泻的影响由表 2可知,饲粮中添加迷迭香提取物对8~28日龄肉鸡的平均日采食量、平均日增重和料重比均无显著影响(P>0.05),对8~35日龄(全期)肉鸡的平均日采食量、平均日增重和料重比也均无显著影响(P>0.05)。29~35日龄,与CON组相比,EC组肉鸡平均日采食量和平均日增重显著降低(P < 0.05),腹泻率显著升高(P < 0.05);而与EC组相比,ECRE组肉鸡的平均日增重显著升高(P < 0.05),料重比显著降低(P < 0.05),腹泻率也显著降低(P < 0.05),平均日采食量2组之间没有显著差异(P>0.05);甚至与CON组相比,ECRE组肉鸡的料重比也显著降低(P < 0.05)。
|
|
表 2 迷迭香提取物对大肠杆菌攻毒肉鸡生长性能和腹泻的影响 Table 2 Effects of rosemary extract on growth performance and diarrhea of broilers challenged with Escherichia coli |
由表 3可知,与CON组相比,大肠杆菌攻毒显著降低了肉鸡的脾脏指数(P < 0.05),显著升高了肝脏指数(P < 0.05);与EC组相比,饲粮中添加迷迭香提取物显著降低了大肠杆菌攻毒肉鸡的肝脏指数(P < 0.05),对胸腺指数、法氏囊指数和脾脏指数没有显著影响(P>0.05)。
|
|
表 3 迷迭香提取物对大肠杆菌攻毒肉鸡器官指数的影响 Table 3 Effects of rosemary extract on organ indexes of broilers challenged with Escherichia coli |
由表 4可知,与CON组相比,大肠杆菌攻毒使肉鸡血清中UREA含量和LDH活性显著升高(P < 0.05),GLU含量显著降低(P < 0.05);与EC组比,饲粮中添加迷迭香提取物使大肠杆菌攻毒肉鸡血清中GLU含量和AST活性显著升高(P < 0.05),UREA含量和LDH活性虽无显著变化(P>0.05),但分别下降0.4 mmol/L和161.91 U/L。大肠杆菌攻毒及在饲粮中添加迷迭香提取物对肉鸡血清中TP、ALB、TG、TC、HDL-C和LDL-C含量与ALT、ALP活性均无显著影响(P>0.05)。
|
|
表 4 迷迭香提取物对大肠杆菌攻毒肉鸡血清生化指标的影响 Table 4 Effects of rosemary extract on serum biochemical indices of broilers challenged with Escherichia coli |
由表 5可知,与CON组相比,大肠杆菌攻毒显著降低了肉鸡血清中GSH-Px、CAT活性(P < 0.05),而显著升高了血清中NO含量(P < 0.05);与EC组相比,饲粮中添加迷迭香提取物使大肠杆菌攻毒肉鸡血清中GSH-Px、CAT活性与T-AOC显著升高(P < 0.05);且与CON组相比,ECRE组肉鸡血清中T-AOC显著升高(P < 0.05)。大肠杆菌攻毒以及在饲粮中添加迷迭香提取物对肉鸡血清中SOD、POD、NOS活性及MDA含量均没有显著影响(P>0.05)。
|
|
表 5 迷迭香提取物对大肠杆菌攻毒肉鸡血清抗氧化指标的影响 Table 5 Effects of rosemary extract on serum antioxidant indices of broilers challenged with Escherichia coli |
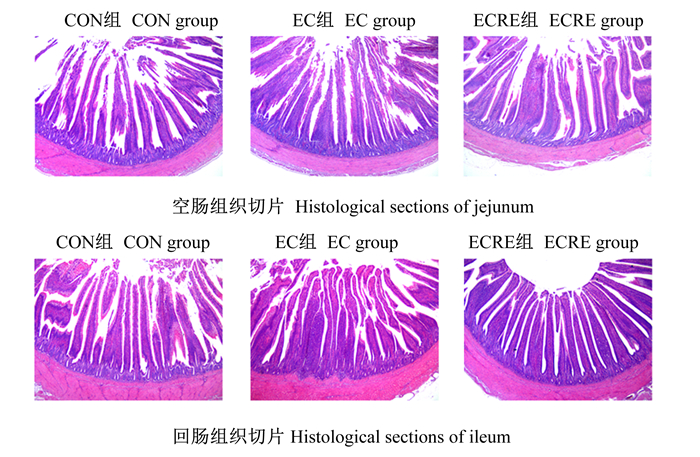
由表 6可知,与CON组相比,大肠杆菌攻毒使得肉鸡的回肠隐窝深度显著升高(P < 0.05),回肠绒隐比显著降低(P < 0.05);与EC组相比,饲粮中添加迷迭香提取物使得大肠杆菌攻毒回肠隐窝深度显著降低(P < 0.05),绒隐比显著升高(P < 0.05)。大肠杆菌攻毒以及在饲粮中添加迷迭香提取物对肉鸡空肠形态结构和回肠绒毛高度均无显著影响(P>0.05)。空肠、回肠切片如图 1所示。
|
|
表 6 迷迭香提取物对大肠杆菌攻毒肉鸡肠道形态结构的影响 Table 6 Effects of rosemary extract on intestinal morphology of broilers challenged with Escherichia coli |
|
图 1 肉鸡空肠、回肠组织切片(HE染色) Fig. 1 Histological sections of jejunum and ileum of broilers (HE staining, 400×) |
大肠杆菌产生的肠毒素会导致动物腹泻,从而降低动物的生长性能[10]。研究表明,迷迭香提取物有缓解动物大肠杆菌的感染损伤,降低腹泻率的作用[11-12]。本试验结果与上述研究结果一致。本试验中400 mg/kg的迷迭香提取物降低了肉鸡腹泻率,这可能与其活性成分的抗菌作用有关。研究表明,迷迭香酸能通过破坏细菌细胞和其细胞蛋白[13],或诱导机体自噬途径发挥巨噬细胞杀菌活性[3],有效抑制大肠杆菌等有害菌的增殖。熊果酸会增加大肠杆菌细胞内的电子传递链活性引起氧化应激,从而诱导细菌死亡[14]。除了降低腹泻率外,本试验结果还显示迷迭香提取物在降低料重比上也有显著作用。有研究指出,迷迭香精油能显著提高新西兰兔的饲料利用率[15]。Yesilbag等[16]试验也发现,虽然饲粮中添加迷迭香、迷迭香挥发油对肉鸡的采食量没有显著影响,但对饲料转化率和肉鸡体重具有积极作用。迷迭香提取物能降低料重比、提高饲料利用率可能与消化酶的分泌有关。植物提取物可以促进消化酶的分泌,从而提高不同营养物质的消化率[17]。Youssef等[18]试验表明,含有迷迭香的精油能改善肉鸡肠道发育和增加蛋白酶的活性,从而提高蛋白质的消化率和膳食营养素的吸收,进而促进其生长性能的提高。
综上所述,大肠杆菌攻毒对肉鸡生长性能有负面影响,而迷迭香提取物能增加大肠杆菌攻毒肉鸡的饲料转化率、降低腹泻率,在一定程度上改善大肠杆菌攻毒肉鸡的生长性能。
3.2 迷迭香提取物对大肠杆菌攻毒肉鸡器官指数的影响脾脏、胸腺与法氏囊是家禽重要的免疫器官,免疫器官指数的大小能够反映家禽免疫功能情况[19]。Rostami等[20]试验发现,迷迭香与维生素E相互作用显著提高了肉鸡胸腺指数和脾脏指数,从而提高了机体免疫能力。Farouk等[11]试验则发现,大肠杆菌感染肉鸡后使其肝脏、肾脏、肠道、脾脏、法氏囊和胸腺组织受损,而迷迭香能改善部分组织的损伤。与以上结果一致,本试验结果也表明,迷迭香提取物对大肠杆菌攻毒肉鸡的脾脏损伤的改善有积极作用。迷迭香提取物能提高家禽的免疫功能,可能与其活性物质促进免疫细胞的分泌有关。研究显示,熊果酸能通过激活细胞免疫降低脾脏和肝脏的损伤[21]。
家禽肝脏在代谢以及机体解毒过程中起着重要的作用[22-23]。Bao等[24]试验表明,大肠杆菌会引起鸡的肝脏损伤,使得其肝脏指数显著增加。而有研究显示,迷迭香对肝脏有保护作用,对大鼠肝病有预防和治疗作用[25]。本试验结果也证实在饲粮中添加迷迭香提取物能有效减少大肠杆菌攻毒导致的肉鸡肝脏指数升高。迷迭香其提取物中的多种活性成分都具有护肝作用。迷迭香酸被发现能通过发挥抗菌作用以减少家禽免疫损伤[26]、调节淋巴细胞亚群的比例以增强家禽免疫器官及肝脏的功能[27]。熊果酸被发现对肝脏再生有积极作用,对部分肝脏切除的大鼠灌喂熊果酸,其血浆中肝细胞生长因子的水平上升[28]。综合上述研究结果推测,迷迭香提取物中的活性成分可能通过缓解机体大肠杆菌感染毒性反应、调节机体代谢等改善大肠杆菌攻毒肉鸡的肝脏指数。
综上可知,大肠杆菌攻毒对肉鸡器官指数造成影响,而迷迭香提取物能通过提高其脾脏指数和缓解肝脏损伤改善大肠杆菌攻毒对肉鸡造成的不利影响。
3.3 迷迭香提取物对大肠杆菌攻毒肉鸡血清生化指标的影响血清中TP、ALB、UREA含量可反映机体中的氮代谢情况,也是肝脏功能损伤的标志物[29-31]。研究表明,大肠杆菌攻毒导致大鼠血清中TP、ALB、UREA含量显著升高[32]。而本试验结果与前人结果相似,大肠杆菌攻毒对肉鸡血清TP含量没有显著影响,但显著提高了肉鸡血清中UREA含量,且对血清中ALB含量有提高的趋势。本试验结果来看,迷迭香提取物一定程度降低了大肠杆菌攻毒肉鸡血清中UREA含量,这与Hassanen等[33]的研究结果类似,其研究得出,迷迭香粉能使大鼠血清的UREA含量下降。
此外,大肠杆菌会使鸡血清中其他肝脏损伤标志物如ALT、AST活性增加[24]。据研究报道,迷迭香精油能通过诱导肝脏损伤大鼠血清中AST、ALT活性降低而发挥护肝作用[34],Yu等[35]也证实,迷迭香酸有降低血液中ALT、AST活性的作用。但Jameel[36]研究发现,不同剂量的迷迭香叶对鸡血清中AST与ALT活性的影响不同,一定剂量下的迷迭香叶甚至有升高AST、ALT活性的可能性。本试验结果与Jameel[36]的研究结果类似,迷迭香提取物使得大肠杆菌攻毒肉鸡血清中AST活性增加,但与CON组相比无显著差异,结合前面肝脏指数的结果,表明迷迭香提取物没有对肝脏造成明显损伤。
血清GLU的含量能反映机体血糖的变化情况,当家禽机体受损时,其血清GLU含量会降低。Jiang等[37]研究发现,脂多糖攻毒蛋鸡其血清GLU含量降低。血清中GLU含量可能与能量的摄入有关,饲粮能量水平升高其血清中GLU含量升高[38]。而大肠杆菌使机体能量摄入降低,迷迭香精油或提取物可能通过提高机体能量摄入,缓解大肠杆菌引起的血清GLU含量降低[16]。本试验结果与之一致,大肠杆菌攻毒使得肉鸡血清中GLU含量下降,而迷迭香提取物使得大肠杆菌攻毒肉鸡血清GLU含量上升。
LDH是厌氧代谢途径的重要酶,在肝脏功能受损时LDH活性会升高[39]。Tang等[40]研究发现,纯化的迷迭香提取物能降低热应激肉鸡的血清LDH活性。El-Demerdash等[41]也表示,杂酚油诱导大鼠肝脏中毒,使其LDH活性升高,而迷迭香能使其恢复到接近正常水平。本试验结果与上述研究结果类似,肉鸡血清中LDH活性在大肠杆菌攻毒的作用下显著升高,而在饲粮中添加迷迭香提取物使得大肠杆菌攻毒肉鸡中血清LDH活性有降低趋势,即迷迭香提取物能在一定程度上改善大肠杆菌攻毒导致的肉鸡肝脏损伤。经分析,其发挥作用的主要成分可能是迷迭香酸,前人报道,迷迭香酸在改善乙醇诱导的大鼠肝脏损伤时,血清中LDH活性降低[42]。
综上可知,大肠杆菌改变了肉鸡血清部分生化指标,而迷迭香提取物在一定程度上调节了肉鸡血清生化指标,从而缓解了大肠杆菌对肉鸡的不利影响。
3.4 迷迭香提取物对大肠杆菌攻毒肉鸡抗氧化能力的影响机体发生氧化应激也是家禽生产性能受影响的一个重要原因,因此,改善家禽的抗氧化能力有助于提高家禽的生产性能。研究显示,大肠杆菌感染会导致肉种鸡体内自由基含量增加[43]。NO是反映机体氧化应激的一种自由基[44]。本试验结果显示,大肠杆菌使肉鸡血清NO含量升高,而使SOD、GSH-Px、CAT等抗氧化酶活性降低,即机体氧化与抗氧化平衡遭到破坏,说明大肠杆菌引起了家禽氧化应激。但本试验结果显示,虽然迷迭香提取物未使得大肠杆菌攻毒肉鸡血清NO含量下降,但提高了抗氧化酶活性。据报道,迷迭香提取物中的抗氧化成分是提高机体内抗氧化酶活性和缓解机体氧化损伤的关键[45]。研究显示,迷迭香酸在机体多数器官中都能升高其抗氧化酶活性,如Heidari等[46]发现,迷迭香酸能够增加胃组织内的SOD、CAT活性与谷胱甘肽(GSH)含量;Wang等[47]研究表明,迷迭香酸减弱了大鼠缺血脑组织中的SOD、CAT活性与GSH含量的下降;Oǧuz等[48]试验表明,迷迭香酸能降低大鼠肝脏、肺脏中的氧化应激等。熊果酸和橙皮苷在治疗糖尿病大鼠时,被发现其也显著提高了机体SOD、GSH-Px等抗氧化酶的活性[49-50]。本试验结果与前人研究结果一致,迷迭香提取物提高了肉鸡血清中T-AOC,减弱因大肠杆菌攻毒导致的SOD、GSH-Px、CAT活性降低,可能是迷迭香中的各抗氧化活性成分发挥了作用。
综上可知,大肠杆菌造成了肉鸡机体一定程度上的氧化损伤,而迷迭香提取物主要通过增强体内抗氧化酶的活性来提高大肠杆菌攻毒肉鸡的抗氧化能力。
3.5 迷迭香提取物对大肠杆菌攻毒肉鸡肠道形态结构的影响肠绒毛形态结构在不同物种之间存在差异,作为重要的消化器官,肠道形态结构与家禽的营养吸收息息相关[51]。研究表明,肠道绒毛高度增加,隐窝深度变浅,绒隐比越大,其肠道的消化与吸收能力越强[52]。绒毛高度增加,肠道吸收养分的表面积越大,而隐窝深度代表细胞的生成率,隐窝深度越浅,其细胞成熟度越好,分泌功能越强[53]。大肠杆菌会破坏肠道形态结构。Liu等[54]研究发现,大肠杆菌感染仔猪,使得回肠的绒毛高度和绒隐比都降低;Zhang等[55]研究发现,大肠杆菌感染肉鸡,使得其14 d的空肠绒毛高度降低,7、14和21 d的空肠隐窝深度增加。本试验结果与上述研究结果相似,大肠杆菌攻毒对肉鸡空肠结构形态无显著影响,但显著增加了回肠隐窝深度,降低了回肠绒隐比。迷迭香提取物及其活性成分具有改善肠道形态结构的作用。Yang等[12]在饲粮中添加100、200或400 mg/kg迷迭香提取物饲喂断奶仔猪,发现其空肠和回肠的绒毛高度增加、隐窝深度降低以及绒隐比增加。用橙皮苷饲喂蛋鸡能提高其回肠的绒毛高度与绒隐比,并能降低十二指肠、空肠和回肠的隐窝深度[56]。本试验结果与上述研究结果相似,迷迭香提取物能通过改善回肠隐窝深度和绒隐比来改善肉鸡的肠道形态结构。
综上可知,大肠杆菌改变了肉鸡回肠形态结构,而迷迭香提取物可能通过抗氧化作用,在一定程度上改善大肠杆菌攻毒肉鸡的回肠形态结构,进而改善生长性能。
4 结论大肠杆菌攻毒对肉鸡的生长性能有负面影响,而迷迭香提取物主要通过增加其饲料转化率、降低腹泻率等改善大肠杆菌攻毒黄羽肉鸡的生长性能;此外,迷迭香提取物还可以通过改善大肠杆菌攻毒肉鸡的器官指数、调节血清生化指标、提高机体抗氧化能力和改善肠道形态结构来缓解大肠杆菌攻毒对肉鸡机体产生的负面影响。
| [1] |
ROTH N, KÄSBOHRER A, MAYRHOFER S, et al. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview[J]. Poultry Science, 2019, 98(4): 1791-1804. DOI:10.3382/ps/pey539 |
| [2] |
BARON D C, MARKO D M, TSIANI E, et al. Rosemary extract increases neuronal cell glucose uptake and activates AMPK[J]. Applied Physiology, Nutrition, and Metabolism, 2021, 46(2): 141-147. DOI:10.1139/apnm-2020-0014 |
| [3] |
BIANCHIN M, PEREIRA D, ALMEIDA J D F, et al. Antioxidant properties of lyophilized rosemary and sage extracts and its effect to prevent lipid oxidation in poultry Pátê[J]. Molecules, 2020, 25(21): 5160. DOI:10.3390/molecules25215160 |
| [4] |
GONÇALVES C, FERNANDES D, SILVA I, et al. Potential anti-inflammatory effect of Rosmarinus officinalis in preclinical in vivo models of inflammation[J]. Molecules, 2022, 27(3): 609. DOI:10.3390/molecules27030609 |
| [5] |
GHASEMZADEH RAHBARDAR M, HOSSEINZADEH H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders[J]. Iranian Journal of Basic Medical Sciences, 2020, 23(9): 1100-1112. |
| [6] |
SATOH T, TRUDLER D, OH C K, et al. Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer's disease, Parkinson's disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome[J]. Antioxidants, 2022, 11(1): 124. DOI:10.3390/antiox11010124 |
| [7] |
符小琴, 范晴, 勾丹, 等. 迷迭香提取物对黑羽绿壳蛋鸡生产性能、蛋品质和抗氧化功能的影响[J]. 动物营养学报, 2022, 34(1): 329-339. FU X Q, FAN Q, GOU D, et al. Effects of Rosmarinus officinalis extract on performance, egg quality and antioxidant function of black feather green shell laying hens[J]. Chinese Journal of Animal Nutrition, 2022, 34(1): 329-339 (in Chinese). |
| [8] |
WANG S, PENG Q, JIA H M, et al. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1[J]. Poultry Science, 2017, 96(8): 2576-2586. DOI:10.3382/ps/pex061 |
| [9] |
赵宪臣. 鸡腹泻的初步诊断、中西疗法与预防措施[J]. 现代畜牧科技, 2019(10): 115-116. ZHAO X C. Preliminary diagnosis, Chinese and western therapies and preventive measures of chicken diarrhea[J]. Modern Animal Husbandry Science & Technology, 2019(10): 115-116 (in Chinese). DOI:10.19369/j.cnki.2095-9737.2019.10.059 |
| [10] |
BIN P, TANG Z Y, LIU S J, et al. Intestinal microbiota mediates enterotoxigenic Escherichia coli-induced diarrhea in piglets[J]. BMC Veterinary Research, 2018, 14(1): 385. DOI:10.1186/s12917-018-1704-9 |
| [11] |
FAROUK S M, ABDEL-RAHMAN H G, ABDALLAH O A, et al. Comparative immunomodulatory efficacy of rosemary and fenugreek against Escherichia coli infection via suppression of inflammation and oxidative stress in broilers[J]. Environmental Science and Pollution Research, 2022, 29(26): 40053-40067. DOI:10.1007/s11356-021-18358-6 |
| [12] |
YANG M, YIN Y X, WANG F, et al. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs[J]. Journal of Animal Science, 2021, 99(9): skab237. DOI:10.1093/jas/skab237 |
| [13] |
ZHANG J H, CUI X, ZHANG M, et al. The antibacterial mechanism of perilla rosmarinic acid[J/OL]. Biotechnology and Applied Biochemistry. https://doi.org/10.1002/bab.2248.DOI: 10.1002/bab.2248.
|
| [14] |
OLOYEDE H O B, AJIBOYE H O, SALAWU M O, et al. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid[J]. Microbial Pathogenesis, 2017, 111: 338-344. DOI:10.1016/j.micpath.2017.08.012 |
| [15] |
ELAZAB M A, KHALIFAH A M, ELOKIL A A, et al. Effect of dietary rosemary and ginger essential oils on the growth performance, feed utilization, meat nutritive value, blood biochemicals, and redox status of growing NZW rabbits[J]. Animals, 2022, 12(3): 375. DOI:10.3390/ani12030375 |
| [16] |
YESILBAG D, EREN M, AGEL H, et al. Effects of dietary rosemary, rosemary volatile oil and vitamin E on broiler performance, meat quality and serum SOD activity[J]. British Poultry Science, 2011, 52(4): 472-482. DOI:10.1080/00071668.2011.599026 |
| [17] |
MAHGOUB S A M, EL-HACK M E A, SAADELDIN I M, et al. Impact of Rosmarinus officinalis cold-pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail[J]. Poultry Science, 2019, 98(5): 2139-2149. DOI:10.3382/ps/pey568 |
| [18] |
YOUSSEF I M I, MÄNNER K, ZENTEK J. Effect of essential oils or saponins alone or in combination on productive performance, intestinal morphology and digestive enzymes' activity of broiler chickens[J]. Journal of Animal Physiology and Animal Nutrition, 2021, 105(1): 99-107. DOI:10.1111/jpn.13431 |
| [19] |
SHU G, KONG F L, XU D, et al. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers[J]. Scientific Reports, 2020, 10(1): 12324. DOI:10.1038/s41598-020-69010-1 |
| [20] |
ROSTAMI H, SEIDAVI A, DADASHBEIKI M, et al. Supplementing dietary rosemary (Rosmarinus officinalis L.) powder and vitamin E in broiler chickens: evaluation of humoral immune response, lymphoid organs, and blood proteins[J]. Environmental Science and Pollution Research International, 2018, 25(9): 8836-8842. DOI:10.1007/s11356-018-1209-x |
| [21] |
DE JESUS J A, LAURENTI M D, ANTONANGELO L, et al. Related pentacyclic triterpenes have immunomodulatory activity in chronic experimental visceral leishmaniasis[J]. Journal of Immunology Research, 2021, 2021: 6671287. |
| [22] |
WANG X G, SHAO F, YU J F, et al. MicroRNA-122 targets genes related to liver metabolism in chickens[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2015, 184: 29-35. DOI:10.1016/j.cbpb.2015.02.002 |
| [23] |
XU T, GAO X J, LIU G W. The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver[J]. Biological Trace Element Research, 2016, 169(2): 365-373. DOI:10.1007/s12011-015-0422-4 |
| [24] |
BAO J L, ZHANG Y, ZHANG L C, et al. Therapeutic effect of schisandrin A on avian colibacillosis through gut-liver axis[J]. Poultry Science, 2021, 100(10): 101371. DOI:10.1016/j.psj.2021.101371 |
| [25] |
MARTÍNEZ-RODRÍGUEZ J L, GUTIÉRREZ-HERNÁNDEZ R, REYES-ESTRADA C A, et al. Hepatoprotective, antihyperlipidemic and radical scavenging activity of hawthorn (Crataegus oxyacantha) and rosemary (Rosmarinus officinalis) on alcoholic liver disease[J]. Alternative Therapies in Health and Medicine, 2019, 25(4): 54-63. |
| [26] |
IQBAL H, WRIGHT C L, JONES S, et al. Extracts of Sida cordifolia contain polysaccharides possessing immunomodulatory activity and rosmarinic acid compounds with antibacterial activity[J]. BMC Complementary Medicine and Therapies, 2022, 22(1): 27. DOI:10.1186/s12906-022-03502-7 |
| [27] |
CAO W, MO K, WEI S J, et al. Effects of rosmarinic acid on immunoregulatory activity and hepatocellular carcinoma cell apoptosis in H22 tumor-bearing mice[J]. The Korean Journal of Physiology & Pharmacology, 2019, 23(6): 501-508. |
| [28] |
ŽALOUDKOVÁ L, TICHÁ A, NEKVINDOVÁ J, et al. Different forms of ursolic acid and their effect on liver regeneration[J]. Evidence-Based Complementary and Alternative Medicine, 2020, 2020: 4074068. |
| [29] |
LI F, NING H, DUAN X, et al. Effect of dietary L-arginine of broiler breeder hens on embryonic development, apparent metabolism, and immunity of offspring[J]. Domestic Animal Endocrinology, 2021, 74: 106537. DOI:10.1016/j.domaniend.2020.106537 |
| [30] |
ELAHI U, WANG J, MA Y B, et al. The response of broiler chickens to dietary soybean meal reduction with glycine and cysteine inclusion at marginal sulfur amino acids (SAA) deficiency[J]. Animals, 2020, 10(9): 1686. DOI:10.3390/ani10091686 |
| [31] |
BIGOT A, TCHAN M C, THOREAU B, et al. Liver involvement in urea cycle disorders: a review of the literature[J]. Journal of Inherited Metabolic Disease, 2017, 40(6): 757-769. DOI:10.1007/s10545-017-0088-5 |
| [32] |
ISMAIL H T H. The ameliorative efficacy of Thymus vulgaris essential oil against Escherichia coli O157:H7-induced hematological alterations, hepatorenal dysfunction and immune-inflammatory disturbances in experimentally infected rats[J]. Environmental Science and Pollution Research, 2022, 29(27): 41476-41491. DOI:10.1007/s11356-022-18896-7 |
| [33] |
HASSANEN N H M, FAHMI A, SHAMS-ELDIN E, et al. Protective effect of rosemary (Rosmarinus officinalis) against diethylnitrosamine-induced renal injury in rats[J]. Biomarkers, 2020, 25(3): 281-289. DOI:10.1080/1354750X.2020.1737734 |
| [34] |
RAŠKOVIĆ A, MILANOVIĆ I, PAVLOVIĆ N, et al. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential[J]. BMC Complementary and Alternative Medicine, 2014, 14: 225. DOI:10.1186/1472-6882-14-225 |
| [35] |
YU Y, WU Y, YAN H Z, et al. Rosmarinic acid ameliorates acetaminophen-induced acute liver injury in mice via RACK1/TNF-α mediated antioxidant effect[J]. Pharmaceutical Biology, 2021, 59(1): 1284-1291. DOI:10.1080/13880209.2021.1974059 |
| [36] |
JAMEEL F R. Investigation of biochemical blood parameters, characteristics for carcass, and mineral composition in chicken meat when feeding on coriander seed and rosemary leaves[J]. Journal of Advanced Veterinary and Animal Research, 2019, 6(1): 33-43. DOI:10.5455/javar.2019.f309 |
| [37] |
JIANG J L, QI L N, WEI Q W, et al. Maternal stevioside supplementation ameliorates intestinal mucosal damage and modulates gut microbiota in chicken offspring challenged with lipopolysaccharide[J]. Food & Function, 2021, 12(13): 6014-6028. |
| [38] |
WANG Y C, WANG Q Y, DAI C P, et al. Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs[J]. Animal Nutrition, 2020, 6(4): 499-506. DOI:10.1016/j.aninu.2020.05.008 |
| [39] |
PRASANNA P L, RENU K, VALSALA GOPALAKRISHNAN A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity[J]. Life Sciences, 2020, 250: 117599. DOI:10.1016/j.lfs.2020.117599 |
| [40] |
TANG S, YIN B, XU J, et al. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 7014126. |
| [41] |
EL-DEMERDASH F M, ABBADY E A, BAGHDADI H H. Oxidative stress modulation by Rosmarinus officinalis in creosote-induced hepatotoxicity[J]. Environmental Toxicology, 2016, 31(1): 85-92. DOI:10.1002/tox.22024 |
| [42] |
HASANEIN P, SEIFI R. Beneficial effects of rosmarinic acid against alcohol-induced hepatotoxicity in rats[J]. Canadian Journal of Physiology and Pharmacology, 2018, 96(1): 32-37. DOI:10.1139/cjpp-2017-0135 |
| [43] |
DA ROSA G, ALBA D F, SILVA A D, et al. Impact of Escherichia coli infection in broiler breeder chicks: the effect of oxidative stress on weight gain[J]. Microbial Pathogenesis, 2020, 139: 103861. DOI:10.1016/j.micpath.2019.103861 |
| [44] |
MONTIEL-JAEN M G, MONSALVO-VILLEGAS A, ÁVILA G. Modulating ALDH2 reveals a differential dependence on ROS for hypertrophy and SR Ca2+ release in aldosterone-treated cardiac myocytes[J]. Biochemical and Biophysical Research Communications, 2021, 536: 7-13. DOI:10.1016/j.bbrc.2020.12.049 |
| [45] |
RASOOLIJAZI H, NOROUZI OFOGH S, ABABZADEH S, et al. Comparing the effects of rosemary extract and treadmill exercise on the hippocampal function and antioxidant capacity in old rats[J]. Basic and Clinical Neuroscience, 2021, 12(3): 361-372. DOI:10.32598/bcn.12.3.2139.1 |
| [46] |
HEIDARI F, KOMEILI-MOVAHHED T, HAMIDIZAD Z, et al. The protective effects of rosmarinic acid on ethanol-induced gastritis in male rats: antioxidant defense enhancement[J]. Research in Pharmaceutical Sciences, 2021, 16(3): 305-314. DOI:10.4103/1735-5362.314829 |
| [47] |
WANG J J, WANG S Q, GUO H Y, et al. Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response[J]. Brain Research, 2021, 1757: 147336. DOI:10.1016/j.brainres.2021.147336 |
| [48] |
OǦUZ A, BÖYVK A, EKINCI A, et al. Investigation of antioxidant effects of rosmarinic acid on liver, lung and kidney in rats: a biochemical and histopathological study[J]. Folia Morphologica, 2020, 79(2): 288-295. DOI:10.5603/FM.a2019.0087 |
| [49] |
BACANLI M, AYDIN S, ANLAR H G, et al. Protective effects of ursolic acid in the kidneys of diabetic rats[J]. Turkish Journal of Pharmaceutical Sciences, 2018, 15(2): 166-170. DOI:10.4274/tjps.49469 |
| [50] |
AKSU E H, KANDEMIR F M, KVÇVKLER S. Ameliorative effect of hesperidin on streptozotocin-diabetes mellitus-induced testicular DNA damage and sperm quality degradation in Sprague-Dawley rats[J]. Journal of Food Biochemistry, 2021, 45(10): e13938. |
| [51] |
HUYCKE T R, TABIN C J. Chick midgut morphogenesis[J]. The International Journal of Developmental Biology, 2018, 62(1/2/3): 109-119. |
| [52] |
XIE Y Q, LIU J, WANG H, et al. Effects of fermented feeds and ginseng polysaccharides on the intestinal morphology and microbiota composition of Xuefeng black-bone chicken[J]. PLoS One, 2020, 15(8): e0237357. DOI:10.1371/journal.pone.0237357 |
| [53] |
CASAS G A, BLAVI L, CROSS T W L, et al. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance[J]. Journal of Animal Science, 2020, 98(1): skz372. DOI:10.1093/jas/skz372 |
| [54] |
LIU Y, SONG M, CHE T M, et al. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli[J]. Journal of Animal Science, 2013, 91(11): 5294-5306. DOI:10.2527/jas.2012-6194 |
| [55] |
ZHANG L, ZHANG L L, ZHAN X A, et al. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88[J]. Journal of Animal Science and Biotechnology, 2016, 7: 3. DOI:10.1186/s40104-016-0061-4 |
| [56] |
ZHU A N, ZHANG K Y, WANG J P, et al. Effect of different concentrations of neohesperidin dihydrochalcone on performance, egg quality, serum biochemistry and intestinal morphology in laying hens[J]. Poultry Science, 2021, 100(7): 101097. DOI:10.1016/j.psj.2021.101097 |




