近年来,我国奶牛养殖业快速发展,规模化水平和产奶量逐年提高。但是,优质粗饲料短缺的现状,使得生产中不得不饲喂含有大量谷物或脂肪的高能饲粮,以满足高产奶牛的能量需求。饲粮的精粗比影响反刍动物胃肠道健康和生产性能,当精料比例过低时,产生的丙酸不足,葡萄糖异生受限,机体会动用大量的体脂提供能量,诱发酮病和妊娠毒血症[1];当精料比例过高时,则会引起瘤胃pH过低或波动增加,造成胃肠道发酵参数和菌群结构改变,诱发亚急性瘤胃酸中毒(subacute ruminal acidosis, SARA)和乳脂合成抑制(milk fat depression, MFD)[2-3]。同时,高精料饲粮可损伤反刍动物后肠道的肠道屏障和黏膜上皮,造成上皮细胞凋亡增加,紧密连接蛋白表达异常, 诱发局部炎症反应[4-5]。
饲粮中添加适量脂肪可以改善奶牛的能量供给,提高泌乳性能和繁殖性能[6-8]。研究显示,在饲粮中添加1.5%或3.0%的大豆油可以促进奶牛胰岛素和胰岛素样生长因子Ⅰ的分泌,抑制胰高血糖素和瘦素的分泌,从而起到提高葡萄糖利用效率和产奶量的作用[9]。饲粮中油脂的种类和含量也可影响动物的胃肠道菌群组成,如饲喂含棕榈油的饲粮可使小鼠结肠中疣微菌门的相对丰度显著上升[10]。当饲粮中油脂含量过高时,尤其是含有大量不饱和脂肪酸时,会在饲料颗粒和微生物细胞表面形成疏水涂层,从而破坏微生物细胞膜,抑制反刍动物瘤胃内营养物质的降解[11-12]。研究表明,饲粮中补充玉米油会降低绵羊瘤胃中酸性洗涤纤维(ADF)和脂肪酸的消化率[13]。
目前,关于饲粮淀粉或油脂含量对奶牛影响的相关研究主要集中在瘤胃方面,对于后肠道发酵和菌群多样性变化的研究相对较少。此外,以往试验饲粮大多只添加淀粉或油脂,二者共同添加如何影响后肠道发酵和菌群变化目前尚不明确。因此,本试验通过给奶牛饲喂高淀粉高油脂饲粮,研究其对奶牛后肠道发酵参数和菌群多样性的影响,为科学配制饲粮和提高奶牛的生产性能提供数据支撑。
1 材料与方法 1.1 试验动物及饲养管理选取来自广东省某奶牛场的8头荷斯坦奶牛[泌乳天数:(215±34) d,体重:(575±23) kg]作为试验动物。采用全混合日粮(TMR)饲喂,日饲喂2次(07:00和17:00),保证5%~10%的剩料量;每天的06:30和16:30分2次机器挤奶。奶牛拴系饲养,自由饮水,躺卧区铺有橡胶垫和细沙,日清粪2次。
1.2 试验设计正式试验开始前进行10 d的预饲,在此期间所用奶牛均饲喂基础饲粮。正试期采用2×2反转试验设计,将8头奶牛随机分为2组,每组4头,进行2期的饲喂试验,每期23 d,第2期对照组和试验组对调。每期的前16 d,对照组奶牛饲喂基础饲粮,试验组奶牛饲喂高淀粉高油脂饲粮[在基础饲粮基础上添加266 g/kg DM粉碎玉米(过1.2~1.5 mm筛)和46 g/kg DM大豆油];后7 d为恢复期,2组奶牛均饲喂基础饲粮。试验组奶牛在每期试验的前4 d进行饲粮过渡。饲粮组成及营养水平见表 1。
|
|
表 1 饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of diets (DM basis) |
试验期每天记录每头牛的采食量和剩料量,计算干物质采食量(DMI),并于每期的第13~15天采集TMR和饲料原料,65 ℃烘干后粉碎,参照《饲料分析及饲料质量检测技术》中的方法测定干物质(DM)、有机物(OM)、粗蛋白质(CP)、粗脂肪(EE)、中性洗涤纤维(NDF)和酸性洗涤纤维(ADF)含量,采用比色法测定淀粉含量[14]。
1.3.2 粪便样品采集和发酵参数测定试验期第13~15天记录每头牛的粪便评分,粪便评分参考Lawrence等[15]的1~5分评分方法。采用直肠取样法,分别于每个试验期的第15天晨饲后0、4、8和12 h采集新鲜粪便样品(300~500 g),将采集的粪便样品分别进行如下处理:1)将每个时间点采集的粪便取10 g加入20 mL的重蒸水,充分涡旋后测定pH(PB-10 pH计,Sartorius,德国),将稀释后的粪便以10 845×g离心15 min分离上清液,上清液按照5 ∶ 1的比例加入25%偏磷酸,用于氨态氮(NH3-N)和挥发性脂肪酸(VFA)浓度的测定,NH3-N浓度参考冯宗慈等[16]的方法测定,VFA浓度参照Cao等[17]的方法采用气相色谱仪(Agilent 7890B,美国)测定,并计算总挥发性脂肪酸(TVFA)浓度;2)取晨饲后4 h采集的粪便样品分装至2 mL冻存管,-80 ℃冰箱保存,用于DNA提取和高通量测序分析。
1.3.3 粪便菌群多样性检测对采集的粪便样品进行16S rRNA基因V3~V4区域高通量测序,分析菌群多样性。DNA提取采用TIANGEN DNA Stool Mini Kit,具体抽提程序参照其说明书。提取的DNA通过紫外分光光度计检测其浓度和纯度。PCR扩增采用的上游引物序列为5’-ACTCCTACGGGAGGCAGCA-3’,下游引物序列为5’-GGACTACHVGGG TWTCTAAT-3’。PCR反应步骤为98 ℃预变性2 min,98 ℃变性15 s,55 ℃退火30 s,25个循环,72 ℃延伸30 s,最后于72 ℃延长5 min。通过对原始测序序列进行过滤、双端拼接,得到;将优化序列进行聚类,划分操作分类单元(OTU),并根据OTU的序列组成得到其物种分类。使用Qiime 2软件生成不同分类水平上的物种相对丰度表,用R软件绘制成样品各分类水平上的群落结构图。
1.4 统计分析数据采用Excel 2016进行初步处理,采用SAS 9.4中的MIXED程序进行处理计算。奶牛的DMI、粪便评分、菌群相对丰度及其代谢通路采用以下分析模型:
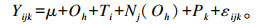
|
式中:Yijk=试验牛在不同处理下的因变量值;μ=总体均值;Oh=次序效应(h=1, 2);Ti=饲粮i的处理效应(i=1,2);Nj=嵌套次序的试验牛j的随机效应(j=1, 2, 3, …, 8);Pk=时期效应(k=1, 2);εijk=随机误差效应。
粪便发酵参数采用以下分析模型:
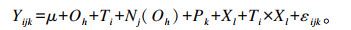
|
式中:Yijk=试验牛在不同处理下的因变量值;μ=总体均值;Oh=次序效应(h=1,2);Ti=饲粮i的处理效应(i=1,2);Nj=嵌套次序的试验牛j的随机效应(j=1, 2, 3, …, 8);Pk=时期效应(k=1,2);Xl=采样时间点(l=0,4,8,12);εijk=随机误差。
利用Tukey法进行组间差异比较,以P < 0.05作为差异显著性的判断标准。
2 结果 2.1 高淀粉高油脂饲粮对奶牛DMI和粪便评分的影响由表 2可知,与对照组相比,试验组奶牛的DMI和粪便评分显著降低(P < 0.05)。
|
|
表 2 高淀粉高油脂饲粮对奶牛DMI和粪便评分的影响 Table 2 Effects of high starch-high oil diet on DMI and fecal score of dairy cows |
由表 3可知,饲喂高淀粉高油脂饲粮后,奶牛粪便的pH、丙酸浓度、乙酸摩尔比显著低于对照组(P < 0.05),NH3-N浓度有降低趋势(P=0.07),而丁酸摩尔比显著高于对照组(P < 0.05)。
|
|
表 3 高淀粉高油脂饲粮对奶牛粪便发酵参数的影响 Table 3 Effects of high starch-high oil diet on fecal fermentation parameters of dairy cows |
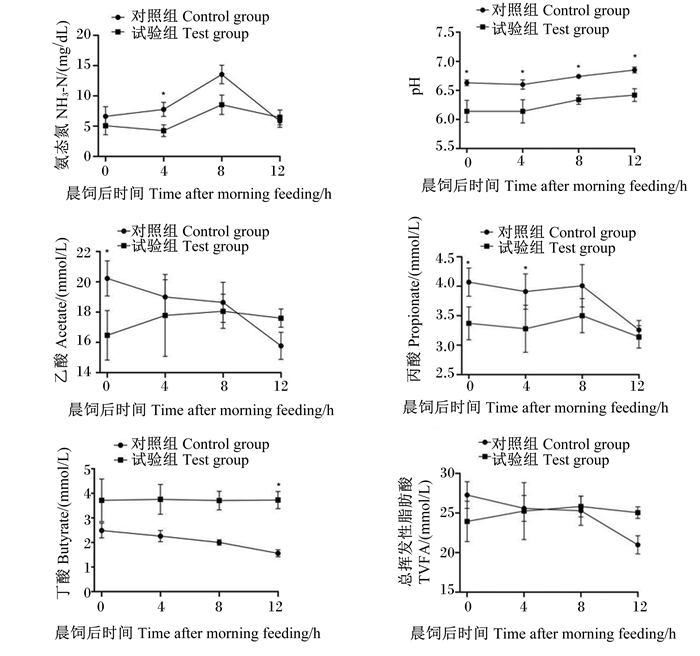
由图 1可知,1 d内,随着晨饲后时间的延长,2组奶牛粪便pH均呈逐渐上升趋势,各时间点试验组均显著低于对照组(P < 0.05);NH3-N浓度呈先升高后降低趋势,其中晨饲后4 h时试验组显著低于对照组(P < 0.05);对照组乙酸浓度呈逐渐下降趋势,试验组则呈先升高后降低趋势,其中晨饲后0 h时试验组显著低于对照组(P < 0.05);2组的丙酸浓度均呈逐渐下降趋势,其中晨饲后0和4 h时试验组显著低于对照组(P < 0.05);试验组丁酸浓度受晨饲后时间的影响较小,对照组则呈逐渐下降趋势,其中晨饲后12 h时试验组显著高于对照组(P < 0.05);对照组TVFA浓度呈逐渐下降趋势,试验组则呈先升高后降低趋势,但TVFA浓度未受饲粮处理的显著影响(P>0.05)。
|
数据点标注*表示试验组与对照组差异显著(P < 0.05)。 Data points with * mean significant difference between test group and control group (P < 0.05). 图 1 高淀粉高油脂饲粮对奶牛1 d内粪便发酵参数的影响 Fig. 1 Effects of high starch-high oil diet on daily fecal fermentation parameters of dairy cows |
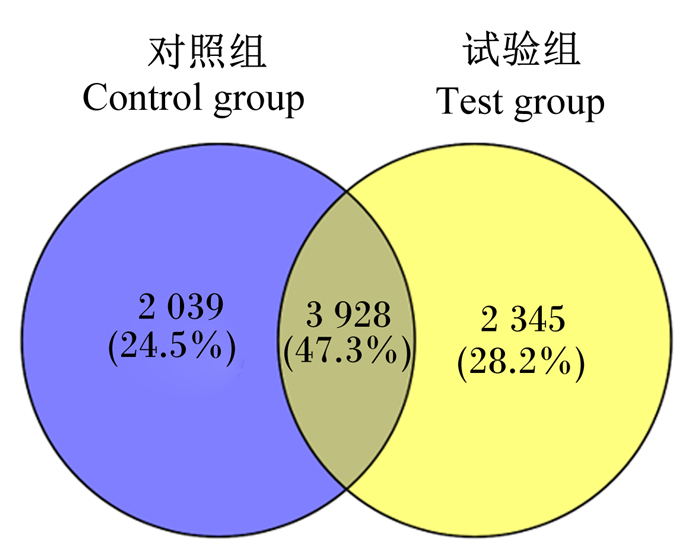
如图 2所示,在97%的相似水平上,2组共得到了8 312个OTU,2组间共有3 928个OTU,其中对照组和试验组独有的OTU数目分别为2 049和2 345个。
|
图 2 奶牛粪便菌群Venn图 Fig. 2 Venn diagram of fecal bacterial flora of dairy cows |
由表 4可知,试验组的ACE指数、Chao指数及Shannon指数均显著低于对照组(P < 0.05),说明饲喂高淀粉高油脂饲粮后粪便菌群的丰富度和多样性显著降低。
|
|
表 4 高淀粉高油脂饲粮对奶牛粪便菌群α多样性指数的影响 Table 4 Effects of high starch-high oil diet on α diversity indexes of fecal bacterial flora of dairy cows |
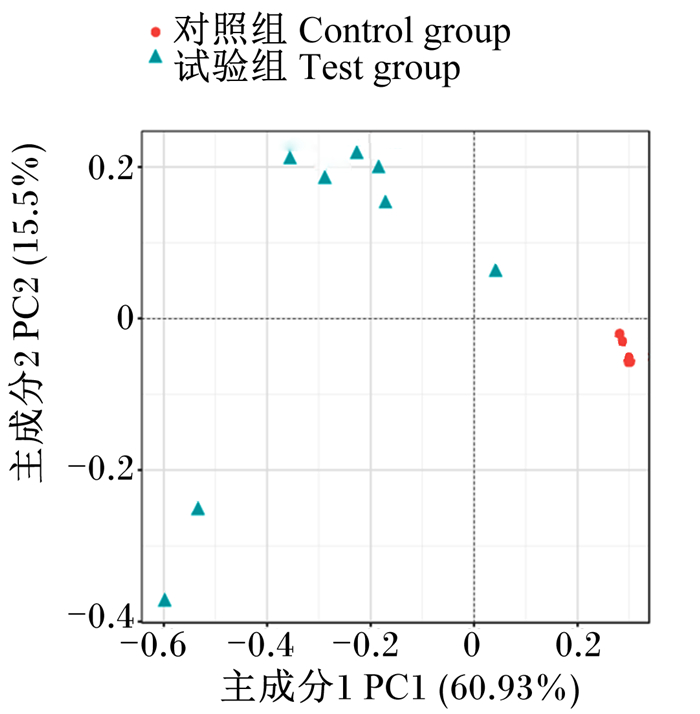
由图 3可知,主成分1(PC1)的贡献值为60.93%,主成分2(PC2)的贡献值为15.5%,对照组和试验组明显区分开,对照组奶牛粪便样点基本集中分布,试验组奶牛粪便样点相对分散。
|
图 3 奶牛粪便菌群主坐标分析图 Fig. 3 PCoA diagram of fecal bacterial flora of dairy cows |
本试验在奶牛的粪便中共鉴定出14个门,24个纲,37个目,63个科,119个属的细菌。相对丰度排名前10的菌门如表 4所示,厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)和放线菌门(Actinobacteria)这3个菌门均是2组粪便菌群中的优势菌门。由表 5可知,当饲喂高淀粉高油脂饲粮后,奶牛粪便中放线菌门、疣微菌门(Verrucomicrobia)和TM7的相对丰度显著降低(P < 0.05),变形菌门(Proteobacteria)的相对丰度显著升高(P < 0.05)。厚壁菌门、拟杆菌门、螺旋菌门(Spirochae- tes)和软壁菌门(Tenericutes)的相对丰度未受饲粮处理的显著影响(P>0.05)。
|
|
表 5 高淀粉高油脂饲粮对奶牛粪便菌群门水平相对丰度的影响 Table 5 Effects of high starch-high oil diet on relative abundance of fecal bacterial flora at phylum level of dairy cows |
由表 6可知,在属水平上,2组奶牛粪便菌群中的优势菌属均为瘤胃球菌科未分类属(unclassified_Ruminococcaceae)和拟杆菌目未分类属(unclassified_Bacteroidales)。与对照组相比,高淀粉高油脂饲粮显著降低了粪便中瘤胃球菌科未分类属、拟杆菌目未分类属和梭菌目未分类属(unclassified_Clostridiales)的相对丰度(P < 0.05),显著提高了毛螺旋菌科未分类属(unclassified_Lachnospiraceae)和琥珀酸弧菌属(Succinivibrio)的相对丰度(P < 0.05)。奶牛粪便中CF231、双歧杆菌属(Bifidobacterium)、S24-7菌科未分类属(unclassified_S24-7)和罗氏菌属(Roseburia)的相对丰度未受饲粮处理的显著影响(P>0.05)。
|
|
表 6 高淀粉高油脂饲粮对奶牛粪便菌群属水平相对丰度的影响 Table 6 Effects of high starch-high oil diet on relative abundance of fecal bacterial flora at genus level of dairy cows |
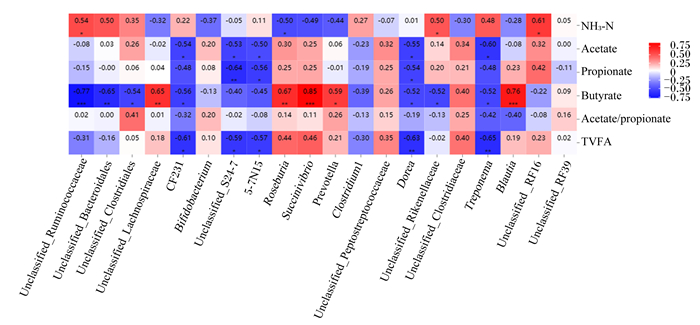
图 4展示了奶牛粪便菌群属水平相对丰度(排名前20的菌属)与粪便发酵参数的相关性。NH3-N浓度与瘤胃球菌科未分类属(P=0.030)、理研菌科未分类属(unclassified_Rikenellaceae)(P=0.049)和RF16菌群未分类属(unclassified_RF16)(P=0.012)的相对丰度呈显著正相关,与罗氏菌属(P=0.047)的相对丰度呈显著负相关;乙酸浓度与CF231(P=0.032)、S24-7菌科未分类属(P=0.033)、多尔氏菌属(Dorea)(P=0.026)和密螺旋体属(Treponema)(P=0.014)的相对丰度呈显著负相关;丙酸浓度与S24-7菌科未分类属(P=0.007)、5-7N15(P=0.023)和多尔氏菌属(P=0.032)的相对丰度呈显著负相关;丁酸浓度与毛螺旋菌科未分类属(P=0.007)、罗氏菌属(P=0.050)、琥珀酸弧菌属(P < 0.001)、普雷沃氏菌属(Prevotella)(P=0.016)和布劳特氏属(Blautia)(P < 0.001)的相对丰度呈显著正相关,与瘤胃球菌科未分类属(P < 0.001)、拟杆菌目未分类属(P=0.007)、CF231(P=0.025)、多尔氏菌属(P=0.040)、理研菌科未分类属(P=0.039)和密螺旋体属(P=0.038)的相对丰度呈显著负相关;乙酸/丙酸与CF231(P=0.012)、S24-7菌科未分类属(P=0.016)、5-7N15(P=0.022)、多尔氏菌属(P=0.009)和密螺旋体属(P=0.007)的相对丰度呈显著负相关。
|
Unclassified_Ruminococcaceae:瘤胃球菌科未分类属;Unclassified_Bacteroidales:拟杆菌目未分类属;Unclassified_Clostridiales:梭菌目未分类属;Unclassified_Lachnospiraceae:毛螺旋菌科未分类属;Bifidobacterium:双歧杆菌属;Unclassified_S24-7:S24-7菌科未分类属;Roseburia:罗氏菌属;Succinivibrio:琥珀酸弧菌属;Prevotella:普雷沃氏菌属;Clostridium1:梭菌属1;Unclassified_Peptostreptococcaceae:消化链球菌科未分类属;Dorea:多尔氏菌属;Unclassified_Rikenellaceae:理研菌科未分类属;Unclassified_Clostridiaceae:梭菌科未分类属;Treponema:密螺旋体属;Blautia:布劳特氏属;Unclassified _RF16:RF16菌群未分类属;Unclassified_RF39:RF39目未分类属;NH3-N:氨态氮;Acetate:乙酸;Propionate:丙酸;Butyrate:丁酸;Acetate/propionate:乙酸/丙酸;TVFA:总挥发性脂肪酸total volatile fatty acids。 “*”表示0.01 < P < 0.05;“* *”表示0.001 < P < 0.01;“* * *”表示P < 0.001。“*” mean 0.01 < P < 0.05; * * mean 0.001 < P < 0.01; “* * *”mean P < 0.001. 图 4 奶牛粪便菌群属水平相对丰度与粪便发酵参数的相关分析 Fig. 4 Correlation analysis between relative abundance of fecal bacterial flora on genus level and fecal fermentation parameters of dairy cows |
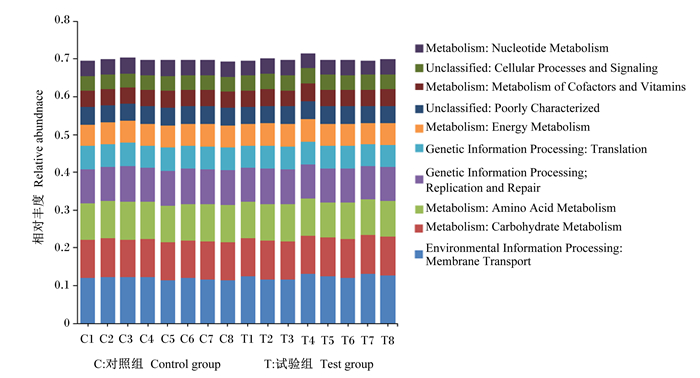
2组奶牛粪便菌群在KEGG二级代谢通路上排名前10的功能见图 5。总体上看,2组奶牛粪便菌群代谢功能相近,膜运输、碳水化合物代谢、氨基酸代谢、复制和修复、翻译等功能的相对丰度较高。由表 7可知,试验组粪便菌群的氨基酸代谢功能的相对丰度显著高于对照组(P < 0.05)。
|
Metabolism: Nucleotide Metabolism:新陈代谢:核苷酸代谢;Unclassified: Cellular Processes and Signaling:未分类:细胞过程和信号;Metabolism: Metabolism of Cofactors and Vitamins:新陈代谢:辅助因子和维生素代谢;Unclassified: Poorly Characterized:未分类:特征不明显;Metabolism: Energy Metabolism:新陈代谢;能量代谢;Genetic Information Processing: Translation:遗传信息处理:翻译;Genetic Information Processing: Replication and Repair:遗传信息处理:复制和修复;Metabolism: Amino Acid Metabolism:新陈代谢:氨基酸代谢;Metabolism: Carbohydrate Metabolism:新陈代谢:碳水化合物代谢;Environmental Information Processing: Membrane Transport:环境信息处理:膜运输。 图 5 KEGG二级代谢通路图 Fig. 5 Secondary metabolic pathway diagram of KEGG |
|
|
表 7 高淀粉高油脂饲粮对粪便菌群KEGG二级代谢通路的影响 Table 7 Effects of high starch-high oil diet on fecal bacterial flora KEGG metabolic pathway at level 2 |
DMI易受到饲粮能量水平和精粗比等多种因素的影响[18]。当饲粮中能量水平达到高限时,大量的碳水化合物在瘤胃迅速发酵,产生大量的有机酸,破坏了瘤胃内的发酵环境和营养物质消化,进一步抑制采食量[1]。粪便评分可以反映奶牛营养物质消化状况,其中2分表明可能出现了饲粮蛋白质或淀粉水平过高、酸中毒、饲料腐败变质等问题,3分为理想分数,说明饲粮平衡,过瘤胃速度适宜。本研究中试验组奶牛粪便评分较低,这主要是由于试验组奶牛饲粮的精料比例高于对照组,瘤胃中未降解的淀粉可进入小肠和大肠,引起肠道上皮结构和后肠道发酵参数发生变化,进一步造成腹泻和损害机体健康。
3.2 高淀粉高油脂饲粮对奶牛粪便发酵参数的影响淀粉在反刍动物全肠道的消化位点主要是瘤胃、小肠和后肠道。在瘤胃和小肠未被水解的淀粉会被后肠道微生物利用生成乙酸、丙酸和丁酸等VFA[19]。VFA是反刍动物能量利用过程中重要的中间产物,可经过肠壁吸收,参与代谢[20],其浓度和组成主要受饲粮组成的影响[21]。晨饲后试验组奶牛粪便中TVFA浓度呈上升趋势,这是因为试验组奶牛的后肠道可发酵碳水化合物含量较高,产生了大量的VFA,而大量的VFA会导致奶牛后肠道的酸度降低,所以试验组奶牛粪便的pH显著降低。Neubauer等[22]研究表明,在奶牛饲粮中添加淀粉会降低粪便的pH,长时间饲喂高精料比例的饲粮,会使奶牛瘤胃中大量未完全消化的食糜流入肠道,导致后肠道发酵活动加强。Mao等[23]研究也发现,奶牛饲喂高谷物饲粮后,粪便中丁酸和TVFA浓度显著升高,pH显著降低,与本试验结果相似。Møller等[24]研究发现,三叶草青贮和高脂肪浓缩饲料配制的饲粮可以增加奶牛粪便中TVFA的浓度,表明饲粮中脂肪含量也会影响后肠道的发酵。本试验中,试验组奶牛粪便中乙酸浓度较对照组有所降低,与Neubauer等[22]的研究结果相似,在其研究中用高精料饲粮饲喂奶牛后,粪便中乙酸和丙酸浓度均出现下降。Wang等[25]研究发现,高精料饲粮饲喂奶牛后瘤胃中乙酸浓度显著降低;Toral等[26]在奶牛和山羊饲粮中添加鱼油(FO组)或同时添加葵花籽油和淀粉(SOS组)后发现,瘤胃中乙酸浓度显著下降。本试验中试验组饲粮和Toral等[26]的试验中SOS组饲粮均为低NDF和高淀粉饲粮,会对纤维素分解细菌的数量产生潜在的负面影响,导致乙酸浓度降低[27],也有研究表明后肠道黏膜会优先代谢乙酸作为主要能量来源[4, 22],因此使得乙酸浓度有所降低。
饲粮碳水化合物含量可影响肠道微生物多样性、群落功能和代谢产物,如短链脂肪酸、胆汁酸、维生素等,进而调节机体营养物质代谢和免疫水平[28]。晨饲后,试验组和对照组粪便的pH和各VFA浓度呈一定的变化规律,表明饲粮处理会影响后肠道中可发酵碳水化合物的发酵速度及规律,可能是由2组饲粮经过瘤胃和小肠到达后肠道中可发酵的碳水化合物数量不同引起的,因此微生物发酵产物的数量也存在不一致。后肠道的内环境会随着可发酵底物和采食时间出现动态变化,因此晨饲后粪便pH和各VFA浓度也相应出现波动。
3.3 高淀粉高油脂饲粮对奶牛粪便菌群多样性和组成的影响ACE指数和Chao指数可反映微生物的丰度,Simpion指数和Shannon指数可反映微生物的多样性[29]。本研究发现,高淀粉高油脂饲粮显著降低了粪便菌群的ACE指数、Chao指数和Shannon指数,表明高淀粉高油脂饲粮会使奶牛粪便菌群的丰富度和多样性显著降低。研究发现,Neubauer等[22]在饲粮中添加27.7%的淀粉后,奶牛粪便菌群丰富度和多样性会降低;Tao等[30]的研究也表明,短期或长期饲喂高精料饲粮后,山羊后肠道菌群丰富度和多样性均显著降低。因而,饲粮中精料比例过高使反刍动物后肠道出现失调,可对微生态环境的生物多样性产生不利影响。
碳水化合物是反刍动物的主要能量来源,同时也是肠道微生物的发酵底物,因此是影响肠道微生物组成的重要因素[31-32]。本研究中,2组奶牛粪便菌群在门水平上的优势菌均为厚壁菌门、拟杆菌门、放线菌门和变形菌门,与其他反刍动物后肠道和粪便微生物的研究结果[33-34]一致。试验组奶牛后肠道微生态环境受饲粮处理的显著影响,但其优势菌门中种类没有发生改变,表明哺乳动物肠道中优势菌群在门水平上受饲粮因素影响较小。放线菌门是天然抗生素的来源,其代谢物具有抗病毒、抗肿瘤和抗菌等活性,可以起到保护宿主的作用[35];疣微菌门具有调节性免疫的潜力,肠道微生物可能通过干预它来达到调节免疫[36]。试验组奶牛粪便中疣微菌门和放线菌门的相对丰度较对照组显著下降,表明高淀粉高油脂饲粮可能会降低有益菌的繁殖,影响后肠道的健康和免疫调节。
在属水平上,2组奶牛粪便中未分类的菌属中优势菌属为瘤胃菌科未分类属、拟杆菌目未分类属、梭菌目未分类属和毛螺旋菌科未分类属。瘤胃球菌科属于厚壁菌门,可以分解纤维和淀粉而产生乙酸、甲酸和琥珀酸等[37]。Russell等[38]研究表明,瘤胃球菌科对酸度较敏感,在低pH下不会消化纤维,瘤胃球菌科未分类属相对丰度显著降低;Neubaue等[22]研究表明,高精料饲粮导致奶牛粪便中瘤胃球菌科相对丰度降低,与本试验结果一致。拟杆菌目属于拟杆菌门,为革兰氏阴性菌,在植物结构多糖的分解和发酵中发挥重要作用。本研究中,试验组拟杆菌目未分类属的相对丰度较对照组显著降低,表明高淀粉高油脂饲粮会影响后肠道中植物多糖的分解和发酵。Liu等[5]研究发现,高谷物饲喂山羊后其盲肠腔内pH和拟杆菌属相对丰度降低,与本研究结果相似。毛螺菌科属于厚壁菌门,为牛、羊胃肠道和粪便中的常见菌,可以水解淀粉和其他糖以产生丁酸及其他短链脂肪酸,当饲粮中精料比例突然增加时,菌群具有旺盛的代谢潜力。琥珀酸弧菌属可发酵多种糖,其代谢最终产物为乙酸、琥珀酸及少量的甲酸和乳酸。本试验中,试验组奶牛粪便中毛螺菌科未分类属和琥珀酸弧菌属的相对丰度均较对照组显著增加。Mao等[23]研究也表明,饲喂高精料饲粮可导致奶牛粪便中毛螺菌科和琥珀酸弧菌属的相对丰度增加。双歧杆菌属可以消化肠道的复杂多糖或低聚糖,具有抗炎作用[39]。本研究中,试验组奶牛粪便中双歧杆菌属的相对丰度与对照组相比呈下降趋势,说明发生炎症的风险增加。
3.4 奶牛粪便菌群属水平相对丰度和粪便发酵参数的相关性及功能预测分析采用高精料饲粮饲喂时,奶牛后肠道细菌会利用淀粉等营养素,引起密螺旋体属多糖降解菌数量的增加[40]。本研究从相关性分析表明,密螺旋体属的相对丰度与乙酸浓度和乙酸/丙酸呈显著负相关,说明了密螺旋体属参与碳水化合物降解和VFA代谢。罗氏菌属属于厚壁菌门,可以分解不可消化的碳水化合物产生短链脂肪酸,主要产物为丁酸,作为一种高产丁酸菌,可能对控制肠道炎症过程具有重要作用[41],与本研究中试验组奶牛粪便中丁酸摩尔比显著增加的结果相对应。利用粪便菌群挖掘一些与重要营养生理功能密切相关的后肠道菌群功能是非常必要的。本试验中,2组奶牛粪便菌群的代谢功能相近,高淀粉高油脂饲粮降低了粪便菌群氨基酸代谢功能的相对丰度,且试验组粪便的NH3-N浓度显著降低,表明高淀粉高油脂饲粮影响了奶牛胃肠道的微生态环境。
4 结论高淀粉高油脂饲粮可降低奶牛的DMI和粪便评分,影响后肠道的发酵参数,降低粪便菌群的丰富度和多样性,存在降低奶牛肠道免疫性能和诱发炎症的风险。因此,生产中采用高精料饲粮饲喂奶牛时,不仅要关注瘤胃健康,同时也要关注粪便状态、后肠道发酵参数及后肠道健康。
| [1] |
耿亚楠. 日粮精粗比对奶山羊泌乳性能、血液生化指标和瘤胃微生物区系的影响[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2020. GENG Y N. Effects of dietary forage to concentrate ratio on milk performance, blood biochemical indexes and rumen microflora in dairy goats[D]. Master's Thesis. Yangling: Northwest A & F University, 2020. (in Chinese) |
| [2] |
OETZEL G R. Diagnosis and management of subacute ruminal acidosis in dairy herds[J]. Veterinary Clinics of North America: Food Animal Practice, 2017, 33(3): 463-480. DOI:10.1016/j.cvfa.2017.06.004 |
| [3] |
GRESSLEY T F, HALL M B, ARMENTANO L E. Ruminant nutrition symposium: productivity, digestion, and health responses to hindgut acidosis in ruminants[J]. Journal of Animal Science, 2011, 89(4): 1120-1130. DOI:10.2527/jas.2010-3460 |
| [4] |
陶诗煜. 高精料日粮对泌乳期奶山羊后段肠道上皮屏障的影响及其机制[D]. 博士学位论文. 南京: 南京农业大学, 2018. TAO S Y. Effects of high-concentrate diet on hindgut epithelial barrier in lactating dairy goats and the mechanisms involved[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2018. (in Chinese) |
| [5] |
LIU J H, XU T T, ZHU W Y, et al. High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats[J]. British Journal of Nutrition, 2014, 112(3): 416-427. DOI:10.1017/S0007114514000993 |
| [6] |
ALTENHOFER C, SPORNRAFT M, KIENBERGER H, et al. Effects of rapeseed and soybean oil dietary supplementation on bovine fat metabolism, fatty acid composition and cholesterol levels in milk[J]. Journal of Dairy Research, 2014, 81(1): 120-128. DOI:10.1017/S002202991300071X |
| [7] |
DE SOUZA J, BATISTEL F, SANTOS F A P. Effect of sources of calcium salts of fatty acids on production, nutrient digestibility, energy balance, and carryover effects of early lactation grazing dairy cows[J]. Journal of Dairy Science, 2017, 100(2): 1072-1085. DOI:10.3168/jds.2016-11636 |
| [8] |
DOOLATABAD S S, SARI M, GHORBANI G R. Effect of partial replacement of dietary starch with fiber and fat on performance, feeding behavior, ruminal fermentation and some blood metabolites of Holstein calves[J]. Animal Feed Science and Technology, 2020, 270: 114691. DOI:10.1016/j.anifeedsci.2020.114691 |
| [9] |
张海波. 日粮补充大豆油对泌乳早期奶牛泌乳性能、血液生化指标和激素浓度的影响[J]. 中国畜牧杂志, 2018, 54(9): 89-93. ZHANG H B. Effects of dietary supplement soybean oil on lactation performance, blood biochemical index and hormone concentration in early lactation dairy cows[J]. Chinese Journal of Animal Science, 2018, 54(9): 89-93 (in Chinese). |
| [10] |
朱航榉, 王锋, 杨贤, 等. 不同油脂对小鼠肠道菌群的影响[J]. 环境与职业医学, 2017, 34(11): 995-998. ZHU H J, WANG F, YANG X, et al. Effects of different oils on intestinal microbiota of mice[J]. Journal of Environmental & Occupational Medicine, 2017, 34(11): 995-998 (in Chinese). |
| [11] |
MAIA M R G, CHAUDHARY L C, FIGUERES L, et al. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen[J]. Antonie van Leeuwenhoek, 2007, 91(4): 303-314. DOI:10.1007/s10482-006-9118-2 |
| [12] |
SHANG X K, WANG C, ZHANG G W, et al. Effects of soybean oil and dietary copper levels on nutrient digestion, ruminal fermentation, enzyme activity, microflora and microbial protein synthesis in dairy bulls[J]. Archives of Animal Nutrition, 2020, 74(4): 257-270. DOI:10.1080/1745039X.2019.1679562 |
| [13] |
JENKINS T C, FOTOUHI N. Effects of lecithin and corn oil on site of digestion, ruminal fermentation and microbial protein synthesis in sheep[J]. Journal of Animal Science, 1990, 68(2): 460-466. DOI:10.2527/1990.682460x |
| [14] |
BAL M A, SHAVER R D, JIROVEC A G, et al. Crop processing and chop length of corn silage: effects on intake, digestion, and milk production by dairy cows[J]. Journal of Dairy Science, 2000, 83(6): 1264-1273. DOI:10.3168/jds.S0022-0302(00)74993-9 |
| [15] |
LAWRENCE M, POLUKIS S, BARNARD A M, et al. Evaluating the effects of Lactobacillus animalis and Propionibacterium freudenreichii on performance and rumen and fecal measures in lactating dairy cows[J]. Journal of Dairy Science, 2021, 104(4): 4119-4133. DOI:10.3168/jds.2020-19291 |
| [16] |
冯宗慈, 高民. 通过比色测定瘤胃液氨氮含量方法的改进[J]. 畜牧与饲料科学, 2010(6): 37. FENG Z C, GAO M. An improved method for determination of ammonia nitrogen content in gastric juice of tumor by colorimetry[J]. Animal Husbandry and Feed Science, 2010(6): 37 (in Chinese). DOI:10.3969/j.issn.1672-5190.2010.06.015 |
| [17] |
CAO Y C, YANG H J. Ruminal digestibility and fermentation characteristics in vitro of fenugreek and alfalfa hay combination with or without the inoculation of Neocallimastix sp. YAK11[J]. Animal Feed Science and Technology, 2011, 169(1/2): 53-60. |
| [18] |
霍路曼, 曹玉凤, 高艳霞, 等. 饲粮能量水平对荷斯坦育成牛生长性能和瘤胃发酵的影响[J]. 畜牧兽医学报, 2019, 50(2): 332-342. HUO L M, CAO Y F, GAO Y X, et al. The effect of dietary energy levels on growth performance and rumen fermentation in Chinese Holstein heifers[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(2): 332-342 (in Chinese). |
| [19] |
ZHANG R Y, LIU J H, JIANG L S, et al. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows[J]. Animal Feed Science and Technology, 2020, 269: 114619. DOI:10.1016/j.anifeedsci.2020.114619 |
| [20] |
任豪. 亮氨酸对荷斯坦青年牛小肠淀粉消化利用的影响及机制[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2020. REN H. Leucine regulates the small intestinal starch digestion and utilization in Holstein heifer: responses and potential mechanisms[D]. Ph. D. Thesis. Yangling: Northwest A & F University, 2020. (in Chinese) |
| [21] |
李洋. 亚急性瘤胃酸中毒对奶山羊瘤胃上皮挥发性脂肪酸吸收的影响及其机制研究[D]. 博士学位论文. 呼和浩特: 内蒙古农业大学, 2019. LI Y. Effects of subacute rumen acidosis on the absorption of volatile fatty acids in rumen epithelium of dairy goats and its mechanism research[D]. Ph. D. Thesis. Hohhot: Inner Mongolia Agricultural University, 2019. (in Chinese) |
| [22] |
NEUBAUER V, PETRI R M, HUMER E, et al. Starch-rich diet induced rumen acidosis and hindgut dysbiosis in dairy cows of different lactations[J]. Animals, 2020, 10(10): 1727. DOI:10.3390/ani10101727 |
| [23] |
MAO S Y, ZHANG R Y, WANG D S, et al. The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows[J]. BMC Veterinary Research, 2012, 8: 237. DOI:10.1186/1746-6148-8-237 |
| [24] |
MØLLER H B, MOSET V, BRASK M, et al. Feces composition and manure derived methane yield from dairy cows: influence of diet with focus on fat supplement and roughage type[J]. Atmospheric Environment, 2014, 94: 36-43. DOI:10.1016/j.atmosenv.2014.05.009 |
| [25] |
WANG L J, ZHANG G N, LI Y, et al. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen[J]. Animals, 2020, 10(2): 223. DOI:10.3390/ani10020223 |
| [26] |
TORAL P G, BERNARD L, BELENGUER A, et al. Comparison of ruminal lipid metabolism in dairy cows and goats fed diets supplemented with starch, plant oil, or fish oil[J]. Journal of Dairy Science, 2016, 99(1): 301-316. DOI:10.3168/jds.2015-10292 |
| [27] |
BEAUCHEMIN K A, KREUZER M, O'MARA F, et al. Nutritional management for enteric methane abatement: a review[J]. Australian Journal of Experimental Agriculture, 2008, 48(2): 21-27. DOI:10.1071/EA07199 |
| [28] |
WANG L Z, JIN L, XUE B, et al. Characterizing the bacterial community across the gastrointestinal tract of goats: composition and potential function[J]. Microbiology Open, 2019, 8(9): e00820. |
| [29] |
张振宇, 梁春年, 姚喜喜, 等. 饲养方式和饲粮能量水平对牦牛生长性能、瘤胃发酵参数和瘤胃菌群的影响[J]. 动物营养学报, 2021, 33(6): 3343-3355. ZHANG Z Y, LIANG C N, YAO X X, et al. Effects of feeding model and dietary energy level on growth performance, rumen fermentation parameters and rumen bacterial community of yaks[J]. Chinese Journal of Animal Nutrition, 2021, 33(6): 3343-3355 (in Chinese). |
| [30] |
TAO S Y, TIAN P, LUO Y W, et al. Microbiome-metabolome responses to a high-grain diet associated with the hind-gut health of goats[J]. Frontiers in Microbiology, 2017, 8: 1764. DOI:10.3389/fmicb.2017.01764 |
| [31] |
韩笑瑛. 日粮瘤胃可降解淀粉调控奶山羊肠道微生物的机制[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2021. HAN X Y. Effects of dietary rumen degradable starch on intestinal microbiota in dairy goats[D]. Ph. D. Thesis. Yangling: Northwest A & F University, 2021. (in Chinese) |
| [32] |
TANG W H W, LI D Y, HAZEN S L. Dietary metabolism, the gut microbiome, and heart failure[J]. Nature Reviews Cardiology, 2019, 16(3): 137-154. DOI:10.1038/s41569-018-0108-7 |
| [33] |
ROMERO-PÉREZ G A, OMINSKI K H, MCALLISTER T A, et al. Effect of environmental factors and influence of rumen and hindgut biogeography on bacterial communities in steers[J]. Applied and Environmental Microbiology, 2011, 77(1): 258-268. DOI:10.1128/AEM.01289-09 |
| [34] |
GODOY-VITORINO F, GOLDFARB K C, KARAOZ U, et al. Comparative analyses of foregut and hindgut bacterial communities in hoatzins and cows[J]. The ISME Journal, 2012, 6(3): 531-541. DOI:10.1038/ismej.2011.131 |
| [35] |
郭婷婷. 甘露寡糖对高精料诱导的低乳脂奶牛瘤胃发酵及乳脂调控的研究[D]. 硕士学位论文. 银川: 宁夏大学, 2019. GUO T T. Effects of mannan oligosaccharides on rumen fermentation and milk fat regulation of low milk fat dairy cows induced by high concentrate[D]. Master's Thesis. Yinchuan: Ningxia University, 2019. (in Chinese) |
| [36] |
LINDENBERG F, KRYCH L, FIELDEN J, et al. Expression of immune regulatory genes correlate with the abundance of specific Clostridiales and Verrucomicrobia species in the equine ileum and cecum[J]. Scientific Reports, 2019, 9(1): 12674. DOI:10.1038/s41598-019-49081-5 |
| [37] |
LI F Y, LI C X, CHEN Y H, et al. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle[J]. Microbiome, 2019, 7(1): 92. |
| [38] |
RUSSELL J B, WILSON D B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH?[J]. Journal of Dairy Science, 1996, 79(8): 1503-1509. DOI:10.3168/jds.S0022-0302(96)76510-4 |
| [39] |
LUO J M, LI Y T, XIE J L, et al. The primary biological network of Bifidobacterium in the gut[J]. FEMS Microbiology Letters, 2018, 365(8): fny057. |
| [40] |
TAJIMA K, AMINOV R I, NAGAMINE T, et al. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR[J]. Applied and Environmental Microbiology, 2001, 67(6): 2766-2774. |
| [41] |
NIE K, MA K J, LUO W W, et al. Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and species[J]. Frontiers in Cellular and Infection Microbiology, 2021, 11: 757718. |




