畜禽在生产过程中,不可避免遭受各种应激。应激激活下丘脑-垂体-肾上腺素(hypothalamus-pituitary-adrenergic,HPA)轴,引起血浆糖皮质激素等激素分泌增加,造成机体代谢紊乱。肌肉能量状态的改变是应激造成肌肉发育受阻的一个重要因素,因此,改善应激状态下肌肉能量代谢状态,对于提高畜禽生产水平有重要意义。糖皮质激素是肾上腺皮质束状区分泌的一种激素,在维持能量代谢的内环境平衡中起着重要作用。骨骼肌是糖皮质激素的一个重要靶器官,糖皮质激素可抑制骨骼肌葡萄糖摄取、利用以及糖原合成,并促进儿茶酚胺诱导的肌糖原分解,抑制胰岛素刺激的糖原合成,引起骨骼肌胰岛素抵抗[1]。然而,有研究表明,尽管糖原合成减少,但地塞米松(dexamethasone,DEX)处理却增加了比目鱼肌的糖原储存[2]。这些表型背后的机制尚不清楚,或许与糖皮质激素下调糖酵解相关因子有关,使得糖原分解减少。Xu等[3]研究表明,大剂量的地塞米松会下调C57BL/6小鼠糖酵解相关因子,并使葡萄糖的摄取减少。糖皮质激素进入细胞内,与糖皮质激素受体(glucocorticoid receptors,GR)结合,上调钙离子(Ca2+)释放通道,致使肌质网Ca2+流入胞浆,同时下调肌质网Ca2+-ATP酶活性,降低肌质网Ca2+摄取能力,引发胞浆内Ca2+超载[4]。Ca2+超载则会触发一系列损伤机制,包括降低线粒体呼吸链关键酶活性、抑制线粒体的氧化磷酸化、激活磷脂酶导致细胞膜结构受损、激活蛋白水解酶导致细胞凋亡等[5-8]。此外,诸多研究表明,糖皮质激素能够诱导细胞氧化应激[9-10]。氧化应激会引起线粒体损伤,活性氧(ROS)短期内急剧积累,长时间氧化应激甚至导致线粒体功能障碍[11]。细胞线粒体内呼吸链电子传递过程中偶联ATP的线粒体呼吸复合酶活性升高[12],线粒体氧化磷酸化减弱,产生的ATP供给不足,进而影响能量代谢[13]。由以上研究报道可知,短期应用糖皮质激素可引起能量代谢升高,长期应用则引起肌肉糖酵解效率降低,同时导致组织的线粒体功能下降。
饲粮营养手段能够增强畜禽机体能量代谢调节功能,有效缓解应激。肌肽(carnosine,Car)是一种由β-丙氨酸和L-组氨酸组成的内源性二肽,广泛存在于脊椎动物肌肉、大脑等组织中,具有氢离子(H+)缓冲剂、Ca2+调节剂、抗糖化、调节能量代谢、维持线粒体功能、改善肉质等作用[14-18]。β-丙氨酸(β-alanine,β-Ala)是肌肽在体内合成的重要限速前体[19],补充β-丙氨酸已被证明能够以饲粮添加剂的方式增加肌肉肌肽的合成和含量[20-22],且相比于肌肽,价格较低。β-丙氨酸被认为是竞技运动员用来提高运动成绩的较受欢迎的补充剂之一[23-25]。当β-丙氨酸与组氨酸在骨骼肌中结合时形成肌肽,组氨酸残基的咪唑环的解离常数(pKa)增加到6.83,使其成为高效的细胞内pH缓冲液,并增强细胞内缓冲能力,从而提高对持续无氧活动的耐受性[21, 26]。有研究表明,肌肽能够调控HPA轴,减少糖皮质激素诱导的机体氧化损伤和代谢紊乱[27-28]。肌肽被认为与肌肉的收缩功能和Ca2+的转运能力密切相关[17]。肌肽也被证明可以缓冲运动诱导的酸中毒[22],可以在线粒体Ca2+超载的环境中有效维持线粒体的正常功能[29-31],并防止运动诱导的氧化应激[32-33]。研究表明,肌肽能够降低拘束应激小鼠体内糖皮质激素的水平,提高葡萄糖的储备,并激活肝脏中糖原合成激酶,促进肝脏的糖原合成,有效改善应激小鼠能量代谢[34]。然而在糖皮质激素(地塞米松)诱导的小鼠骨骼肌细胞(C2C12细胞)能量代谢损伤模型中,肌肽和β-丙氨酸是否通过调节其糖酵解代谢和线粒体功能来改善能量代谢尚不清楚。因此,本研究旨在探索肌肽和β-丙氨酸对C2C12细胞能量代谢损伤的调节作用,为肌肽和β-丙氨酸在生产上应用于缓解畜禽应激提供理论依据。
1 材料与方法 1.1 试验材料肌肽购自上海希格玛高技术有限公司,商品编号:C9635-5G,纯度≥99.9%。β-丙氨酸购自北京索莱宝科技有限公司,商品编号:SA8090,纯度≥99.9%。测定ATP、糖原和乳酸含量,测定磷酸果糖激酶(phosphofructokinase,PFK)、己糖激酶(hexokinase,HK)、丙酮酸激酶(pyruvate kinase,PK)、乳酸脱氢酶(lactate dehydrogenase,LDH)、柠檬酸合酶(citrate synthase,CS)、线粒体呼吸链复合酶Ⅱ和Ⅳ以及Ca2+-ATP酶活性的相关试剂盒购自南京建成生物工程研究所。测定葡萄糖含量的试剂盒购自上海荣盛生物药业有限公司。
试验细胞C2C12小鼠成肌细胞由江西农业大学曾庆杰老师课题组馈赠。
1.2 试验方法 1.2.1 C2C12小鼠成肌细胞培养将C2C12小鼠成肌细胞接种于T25细胞培养瓶中,加入3 mL基础培养基(高糖DMEM溶液中加入10%胎牛血清,1% 100 U/L青霉素+链霉素),置于37 ℃、5% CO2条件下的培养箱中培养。在镜下观察细胞汇合率达到80%~90%时,更换培养基(高糖DMEM溶液中加入2%马血清,1% 100 U/L青霉素+链霉素),继续培养,诱导分化。每2 d更换培养液,待细胞分化5~6 d形成可收缩的肌管后,收集细胞待用。
1.2.2 试验分组将分化后的C2C12骨骼肌细胞接种于6孔培养板中,其中一组加入正常分化基持续孵育36 h作为对照组,记为Control组;一组加入正常分化基孵育24 h后再加入10 μmol/L地塞米松处理细胞12 h(地塞米松添加量参考Chai等[4]),记为DEX组;一组加入10 mmol/L肌肽孵育24 h后再加入10 μmol/L地塞米松处理细胞12 h(肌肽添加量参考Cripps等[35]),记为Car组;一组加入10 mmol/L β-丙氨酸孵育24 h后再加入10 μmol/L地塞米松处理细胞12 h(β-丙氨酸添加量参考Schnuck等[36]),记为β-Ala组。试验结束后,即刻收样。
1.2.3 C2C12细胞内Ca2+含量测定参照游敏芳等[37]的方法,将培养后的C2C12细胞按照钙离子荧光探针Fluo-3/AM检测试剂盒说明书对Ca2+进行荧光负载,倒置荧光显微镜观察负载效果,并利用荧光酶标仪检测其荧光值,通过公式计算出C2C12细胞内Ca2+浓度
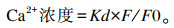
|
式中:F为表达载体的荧光值;F0为水对照组的荧光值;Kd为Ca2+的解离常数(224)。
1.2.4 ATP含量、糖酵解及相关酶活性、线粒体相关酶活性测定各组C2C12细胞上清液弃去,将细胞刮下,用PBS冲洗并离心得细胞沉淀,按照相应说明书加入相应提取液,混匀后在冰上使用超声波细胞破碎仪经行破碎。破碎的C2C12细胞悬液经离心后取相应上清液,按照厂家说明书在96孔板上进行ATP、葡萄糖、糖原(表示每管收集到的所有细胞总糖原的含量,细胞数量在106个左右)、乳酸含量检测和PFK、HK、PK、LDH、CS、线粒体呼吸链复合酶Ⅱ和Ⅳ以及Ca2+-ATP酶活性检测。
1.3 统计分析采用SPSS 25.0软件对数据进行分析。数据以平均值和标准误(SE)表示,各组数据之间的比较采用单因素方差分析,并采用Dunnett t法检验进行组间比较,P < 0.05为差异显著,P < 0.01为差异极显著。
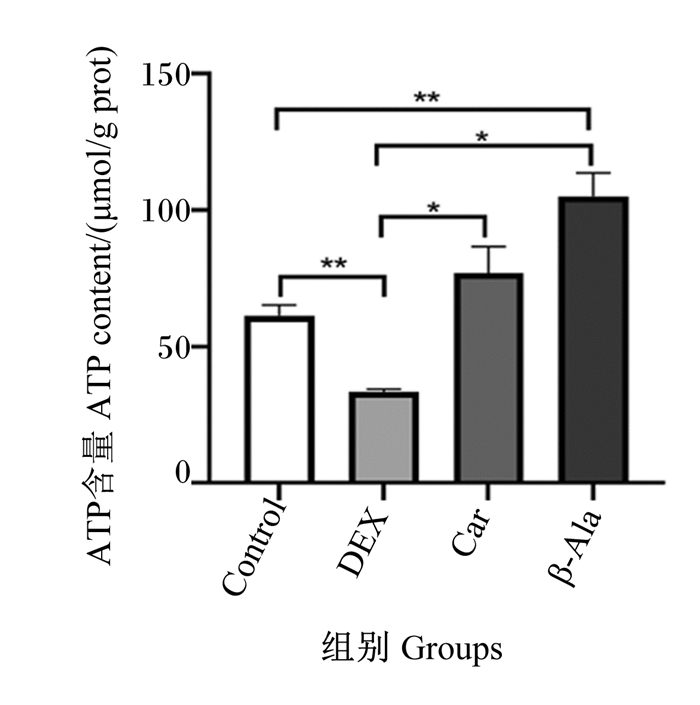
2 结果 2.1 地塞米松、肌肽及β-丙氨酸对C2C12细胞内ATP含量的影响由图 1可知,与Control组相比,DEX组细胞内ATP含量显著降低(P < 0.05),β-Ala组细胞内ATP含量极显著升高(P < 0.01)。与DEX组相比,Car组和β-Ala组细胞内ATP含量显著升高(P < 0.05)。
|
*表示差异显著(P < 0.05),* *表示差异极显著(P < 0.01)。下图同。 * mean significantly difference (P < 0.05), and * * mean extremely significantly difference (P < 0.05). The same as below. 图 1 地塞米松、肌肽及β-丙氨酸对C2C12细胞ATP含量的影响 Fig. 1 Effects of dexamethasone, carnosine and β-alanine on content of ATP in C2C12 cells |
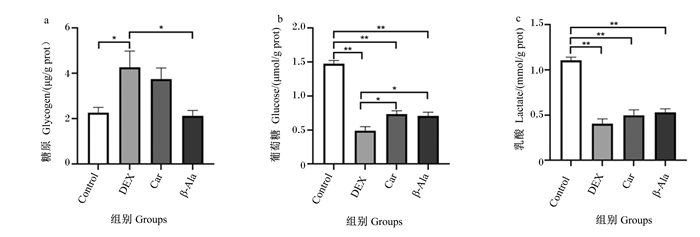
由图 2可知,与Control组相比,DEX组细胞内糖原含量显著升高(P < 0.05),细胞内葡萄糖和乳酸含量极显著降低(P < 0.01)。与DEX组相比,Car组和β-Ala组细胞内葡萄糖含量显著升高(P < 0.05),β-Ala组细胞内糖原含量显著降低(P < 0.05),Car组和β-Ala组细胞内乳酸含量无显著差异(P>0.05)。
|
图 2 地塞米松、肌肽及β-丙氨酸对C2C12细胞内糖酵解的影响 Fig. 2 Effects of dexamethasone, carnosine and β-alanine on glycolysis in C2C12 cells |
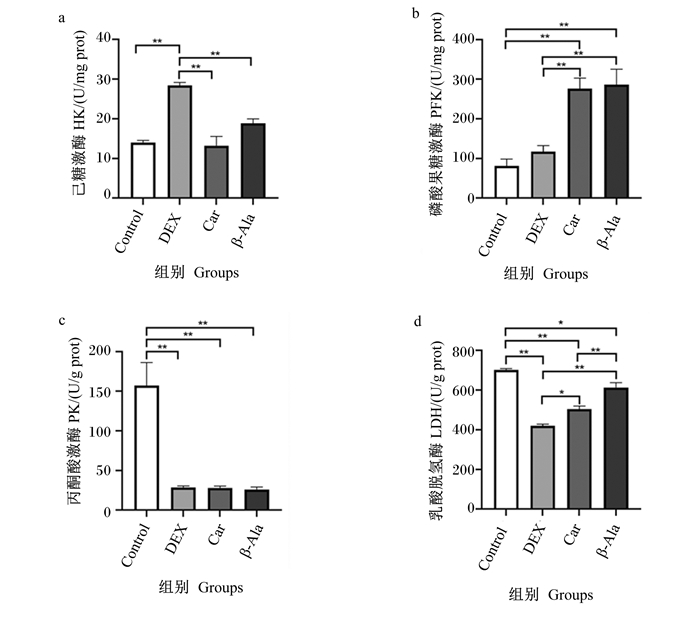
由图 3可知,与Control组相比,DEX组细胞内HK活性极显著升高(P < 0.01),细胞内PK和LDH活性极显著降低(P < 0.01)。与DEX组相比,Car组和β-Ala组细胞内HK活性极显著降低(P < 0.01),细胞内PFK活性极显著升高(P < 0.01);β-Ala组细胞内LDH活性极显著升高(P < 0.01),Car组细胞内LDH活性显著升高(P < 0.05);Car组和β-Ala组细胞内PK活性无显著差异(P>0.05)。
|
图 3 地塞米松、肌肽及β-丙氨酸对C2C12细胞内糖酵解相关酶活性的影响 Fig. 3 Effects of dexamethasone, carnosine and β-alanine on activities of glycolytic related enzyme in C2C12 cells |
由图 4可知,与Control组相比,DEX组细胞内Ca2+含量显著升高(P < 0.05)。与DEX组相比,Car组和β-Ala组细胞内Ca2+含量显著降低(P < 0.05)。
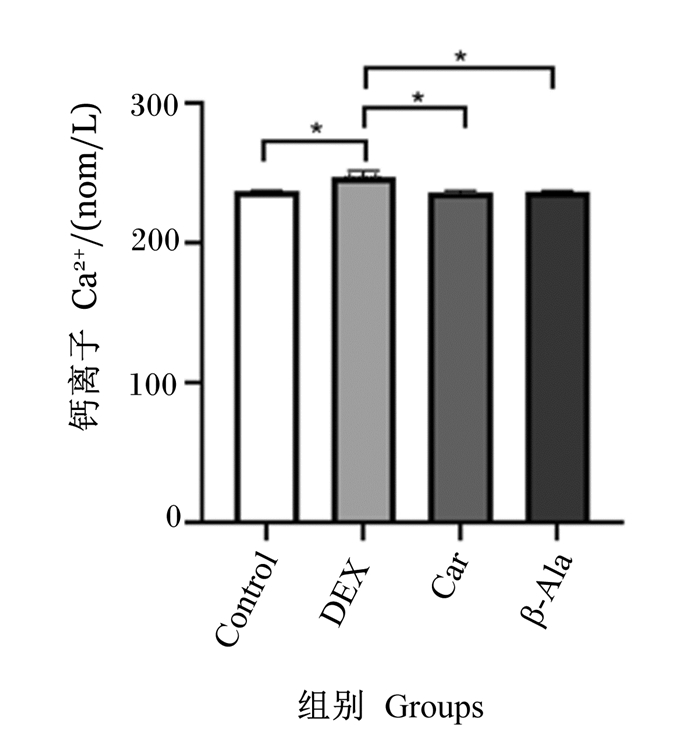
|
图 4 地塞米松、肌肽及β-丙氨酸对C2C12细胞Ca2+含量的影响 Fig. 4 Effects of dexamethasone, carnosine and β-alanine on content of Ca2+ in C2C12 cells |
由图 5可知,与Control组相比,DEX组、Car组和β-Ala组细胞内Ca2+-ATP酶活性极显著降低(P < 0.01),线粒体呼吸链复合酶Ⅳ活性极显著升高(P < 0.01)。各组之间细胞内CS、线粒体呼吸链复合酶Ⅱ活性无显著差异(P>0.05)。
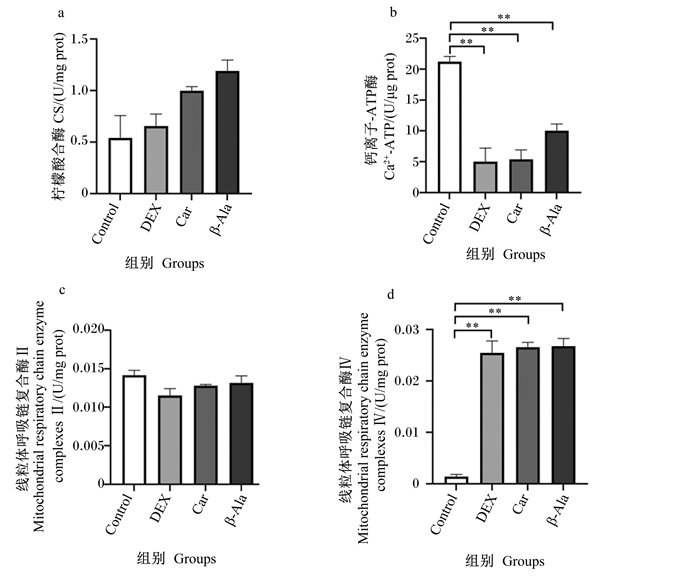
|
图 5 肌肽、地塞米松及β-丙氨酸对C2C12细胞线粒体相关酶活性的影响 Fig. 5 Effects of dexamethasone, carnosine and β-alanine on activities of mitochondrial related enzyme in C2C12 cells |
地塞米松被广泛用于治疗炎症性疾病、神经系统疾病和自身免疫性疾病等[37-39]。然而,地塞米松可以通过增加蛋白质分解率来降低骨骼肌蛋白质合成速率,并大量产生ROS,引发胞浆内Ca2+超载,诱导骨骼肌细胞受损[4, 40]。地塞米松能够通过降低静息状态下骨骼肌的氧化磷酸化,减少ATP的生成,并通过降低葡萄糖的摄取影响能量代谢[41-42]。
本试验采用10 μmol/L地塞米松作用于小鼠C2C12细胞,模拟畜禽遭受应激时糖皮质激素对于骨骼肌能量代谢的影响,结果显示,与Control组相比,DEX组C2C12细胞内ATP含量显著下降,表明地塞米松刺激下C2C12细胞能量代谢严重受损;进一步试验表明,DEX组细胞内糖原含量显著升高,葡萄糖和乳酸含量显著下降,提示地塞米松诱导C2C12细胞糖酵解能力下降。据报道,单独使用地塞米松会导致雄性SD大鼠骨骼肌中糖原含量升高[43]。长时间地塞米松刺激,细胞葡萄糖的摄取和糖酵解途径的关键调节因子都会下调[2, 44-45],肌质网Ca2+释放率降低,导致肌糖原的利用率随之下降,肌糖原的分解受到抑制。所以本试验结果呈现为DEX组细胞内糖原含量升高,葡萄糖含量下降。虽然添加地塞米松提高了细胞内HK活性,促进骨骼肌进行糖酵解的第一步,即加速葡萄糖向葡萄糖-6-磷酸(glucose-6-phosphate,G6P)的转化,但由于细胞内PK与LDH活性降低,使转化而成的G6P不能更快地转化为乳酸并提供ATP。而添加肌肽和β-丙氨酸后,通过调节细胞内PFK和LDH活性,提高C2C12细胞糖酵解能力,并且肌肽能够在线粒体Ca2+超载的环境中有效维持Ca2+对肌糖原的转运能力[31-32],调节C2C12细胞对肌糖原的利用能力[46],所以肌肽与β-丙氨酸组细胞内糖原含量降低,乳酸含量升高。Hipkiss等[27]研究报道,肌肽在糖酵解前体1, 6-二磷酸果糖形成之前抑制糖酵解,即对HK活性一定抑制作用,但整体促进糖酵解,与本研究结果一致。Tsoi等[47]研究表明,肌肽不仅能逆转应激所致的糖耐量和肌肉中糖原含量的下降,而且还能降低血液中皮质酮含量。肌肽还可能通过抑制糖原异生,促进糖原合成,促进糖酵解,并通过调节葡萄糖代谢酶促进葡萄糖从血液到肝脏的转运,从而部分改善机体能量代谢[47]。
添加肌肽和β-丙氨酸提高了细胞内ATP含量,这可能不仅由于肌肽和β-丙氨酸可以刺激糖酵解供能,还与能够调节ROS诱导的线粒体功能障碍有关[48-49]。线粒体是细胞的能量供应中心,提供细胞代谢所需的95%以上的ATP[50],当ROS大量产生,肌肽也能通过调控线粒体呼吸链维持能量供应[51]。因此,本试验又对线粒体相关酶活性进行了测定,以探究肌肽和β-丙氨酸对地塞米松影响的C2C12细胞线粒体功能的调控作用。添加肌肽和β-丙氨酸对细胞内CS活性有一定的提高作用,这提示肌肽和β-丙氨酸不仅可能刺激糖酵解供能,还可能减弱ROS对骨骼肌细胞线粒体的损伤,加强应激状态下线粒体的氧化磷酸化。Macedo等[49]报道也发现急性肌肽给药提高了幼鼠大脑CS活性。骨骼肌产生的能量与特定酶的活性有关。在细胞中,一些酶代表无氧代谢,即柠檬酸合成酶(Krebs循环酶之一)、细胞色素C氧化酶(线粒体呼吸链复合酶Ⅳ),以及与无氧代谢相关的酶,如LDH[52]。研究表明,在运动性疲劳后,骨骼肌的有氧呼吸酶和厌氧呼吸酶活性都没有变化,但会刺激线粒体的生物生成,并促进肌肉组织重塑为糖酵解更少的纤维型成分[53]。呼吸链主要负责进行生物氧化并且能够与ATP酶结合完成氧化磷酸化为细胞提供能量。因此,线粒体呼吸链复合体的活性高低直接影响线粒体呼吸链传递电子的速率和细胞正常的能量代谢活动。地塞米松导致线粒体呼吸功能下降,表现为线粒体呼吸控制率降低、线粒体膜电位降低、ATP合成减少、线粒体通透性转换孔开放、线粒体DNA损伤、ROS积累[44]。地塞米松诱导肌肉受损的另一个机制是导致肌肉抑制素基因表达上调,这也与地塞米松导致线粒体肿胀或空泡化有关[54]。本研究中,与Control组相比,DEX组、Car组和β-Ala组细胞内线粒体呼吸链复合酶Ⅳ活性显著上升,线粒体呼吸链复合酶Ⅱ活性无显著差异。由于细胞种类的不同,肌肽对线粒体功能调控的能力有差异。据报道,在以糖酵解为主提供能量的SGC7901细胞、宫颈癌细胞、胃癌细胞中,肌肽显著降低线粒体呼吸链复合酶Ⅰ~Ⅳ的活性和线粒体ATP的生成,并降低三羧酸循环中的异柠檬酸脱氢酶和苹果酸脱氢酶的活性,抑制细胞糖酵解供能[55-57]。而在以线粒体氧化磷酸化为主供能的公羊精子细胞与肌细胞中,肌肽不仅可以刺激细胞糖酵解供能,还可以促进线粒体氧化磷酸化[58-59]。我们推测肌肽保护与促进应激状态下的线粒体功能是由于肌肽的抗氧化功能[60-61],肌肽通过清除ROS并直接与超氧阴离子和过氧基反应,减少线粒体因ROS大量堆积带来的线粒体肿胀与空泡化[62]。
细胞内钙稳态的平衡对于维持细胞正常机能非常重要,钙稳态的破坏会给机体带来许多危害。在肌肉疲劳收缩期间,肌浆网的Ca2+释放、肌纤维对Ca2+的敏感性和对Ca2+的重新吸收都会减少[63]。这些因素与代谢副产物H+的堆积协同作用,导致肌肉在剧烈收缩活动期间失去功能。地塞米松刺激骨骼肌细胞中Ca2+-ATP酶活性显著下降,胞浆中游离多余钙摄入肌质网腔,导致Ca2+转运功能改变。肌肽和β-丙氨酸的添加对Ca2+-ATP酶有一定的提高作用,但效果不显著,但对细胞内因地塞米松引起的Ca2+超载有显著的调节作用。肌肽在促进肌质网的Ca2+释放和增加肌纤维对Ca2+的敏感性方面发挥了作用[64-65]。肌肉的pH和Ca2+的转运能力是密不可分的,在应激情况下,负责Ca2+摄取的肌浆/内质网-ATP酶泵率在pH从7.1下降到6.6的过程中下降了近2倍[53]。而肌肽中的咪唑环缓冲pH来源于肌肽结合肌肉H+的能力,间接地维持肌肉细胞对Ca2+的转运能力[32, 66]。
本研究发现,作为肌肽合成前体的β-丙氨酸对C2C12细胞的能量代谢与线粒体代谢的调控表现出更佳的效果,具体表现为细胞内ATP含量及HK、LDH、CS活性的上升。肌肽进入肠道后,空肠黏膜中的组织肌肽酶2(CN2)会将肌肽水解为其组成氨基酸β-丙氨酸和L-组氨酸,在此过程中需要消耗部分ATP[68]。最终只有约14%的肌肽可以进入血液被机体直接使用,而β-丙氨酸却可以直接进入血液通过质子辅助的氨基酸转运蛋白1和牛磺酸转运蛋白进入骨骼肌,并在肌肽合成酶的催化下合成肌肽[67-68]。此外,由于肌肽在肠道中的生物利用度有限,补充β-丙氨酸通过肌肽合成酶1(Carns1)介导的反应比补充肌肽更大程度地增加肌肉中肌肽的合成[40]。而在Schnuck等[36]的研究中,β-丙氨酸对线粒体代谢的影响可能更多来源于对氧化代谢改善的标志物增加,包括细胞内过氧化体增殖物激活受体β/δ(PPARβ/δ)、线粒体转录因子A(TFAM)和葡萄糖转运蛋白4含量增加,这导致线粒体含量增加与葡萄糖摄取的增加[40]。在今后的研究中,应当设置α-丙氨酸、组氨酸作对照,以便更加明确地区别在细胞能量代谢调控中,β-丙氨酸是作为底物促进肌肽合成还是作为功能物质发挥的作用。
4 结论① 地塞米松处理导致C2C12细胞内PK、PFK、LDH活性降低,Ca2+含量升高,ATP生成减少,影响细胞正常的能量代谢。
② 添加肌肽能够促进C2C12细胞在应激状态下的葡萄糖含量,并促进PFK、LDH的糖酵解进程,为细胞供能,对Ca2+超载有一定缓解作用,但并未见对细胞线粒体功能相关酶活信有显著作用。
③ 添加β-丙氨酸能够调节C2C12细胞在应激状态下的能量代谢,通过促进糖酵解相关活PFK、LDH活性促进细胞ATP含量提升,并对Ca2+超载有一定缓解作用。
| [1] |
KUO T Y, MCQUEEN A, CHEN T C, et al. Regulation of glucose homeostasis by glucocorticoids[M]//WANG J C, HARRIS C. Glucocorticoid signaling. New York: Springer, 2015: 99-126.
|
| [2] |
RUZZIN J, WAGMAN A S, JENSEN J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor[J]. Diabetologia, 2005, 48(10): 2119-2130. DOI:10.1007/s00125-005-1886-0 |
| [3] |
XU L, XIA H, NI D S, et al. High-dose dexamethasone manipulates the tumor microenvironment and internal metabolic pathways in anti-tumor progression[J]. International Journal of Molecular Sciences, 2020, 21(5): 1846. DOI:10.3390/ijms21051846 |
| [4] |
CHAI J, XIONG Q, ZHANG P P, et al. Induction of Ca2+ signal mediated apoptosis and alteration of IP3R1 and SERCA1 expression levels by stress hormone in differentiating C2C12 myoblasts[J]. General and Comparative Endocrinology, 2010, 166(2): 241-249. DOI:10.1016/j.ygcen.2009.08.011 |
| [5] |
梁晚益, 陶惠, 杨宗城, 等. Ca2+介导严重烧伤早期心肌线粒体呼吸功能损害[J]. 西北国防医学杂志, 2002, 23(1): 11-14. LIANG W Y, TAO H, YANG Z C, et al. Calcium induced the damage of myocardial mitochondrial respiratory function in the early stage after severe burns[J]. Medical Journal of National Defending Forces in Northwest China, 2002, 23(1): 11-14 (in Chinese). DOI:10.3969/j.issn.1007-8622.2002.01.004 |
| [6] |
陈存芳, 赵凤琴. 能量代谢障碍与心肌缺血再灌注损伤[J]. 临床误诊误治, 2009, 22(2): 77-79. CHEN C F, ZHAO F Q. Energy dysmetabolism and myocardial ischemical reperfusion injury[J]. Clinical Misdiagnosis & Mistherapy, 2009, 22(2): 77-79 (in Chinese). DOI:10.3969/j.issn.1002-3429.2009.02.053 |
| [7] |
夏杨, 张惠军, 聂亚莉. 丹酚酸B预处理对心肌缺血/再灌注损伤能量代谢的影响[J]. 药物评价研究, 2018, 41(12): 2210-2213. XIA Y, ZHANG H J, NIE Y L. Salvianolic acid B pretreatment protects myocardial ischemia/reperfusion injury through improvement of energy metabolism[J]. Drug Evaluation Research, 2018, 41(12): 2210-2213 (in Chinese). |
| [8] |
LIOU Y M, HSIEH S R, WU T J, et al. Green tea extract given before regional myocardial ischemia-reperfusion in rats improves myocardial contractility by attenuating calcium overload[J]. Pflügers Archiv-European Journal of Physiology, 2010, 460(6): 1003-1014. DOI:10.1007/s00424-010-0881-6 |
| [9] |
SPIERS J G, CHEN H J C, SERNIA C, et al. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress[J]. Frontiers in Neuroscience, 2015, 8: 456. |
| [10] |
COSTANTINI D, MARASCO V, MØLLER A P. A Meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates[J]. Journal of Comparative Physiology B, 2011, 181(4): 447-456. |
| [11] |
龚文翰. 不同形式的运动预适应对力竭运动大鼠骨骼肌线粒体自噬和功能的影响[D]. 硕士学位论文. 大连: 辽宁师范大学, 2021. GONG W H. Effects of different forms of exercise preconditioning on autophagy and function of skeletal muscle mitochondria in rats after exhaustive exercise[D]. Master's Thesis. Dalian: Liaoning Normal University, 2021. (in Chinese) |
| [12] |
徐梦, 马青, 范春兰, 等. STAT3与线粒体电子传递链[J]. 生理科学进展, 2019, 50(5): 366-370. XU M, MA Q, FAN C L, et al. STAT3 and mitochondrial electron transport chain[J]. Progress in Physiological Sciences, 2019, 50(5): 366-370 (in Chinese). DOI:10.3969/j.issn.0559-7765.2019.05.011 |
| [13] |
NAIK P P, BIRBRAIR A, BHUTIA S K. Mitophagy-driven metabolic switch reprograms stem cell fate[J]. Cellular and Molecular Life Sciences, 2019, 76(1): 27-43. DOI:10.1007/s00018-018-2922-9 |
| [14] |
MA X Y, JIANG Z Y, LIN Y C, et al. Dietary supplementation with carnosine improves antioxidant capacity and meat quality of finishing pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2010, 94(6): e286-e295. DOI:10.1111/j.1439-0396.2010.01009.x |
| [15] |
HIPKISS A R, BAYE E, DE COURTEN B. Carnosine and the processes of ageing[J]. Maturitas, 2016, 93: 28-33. DOI:10.1016/j.maturitas.2016.06.002 |
| [16] |
MATTHEWS J J, ARTIOLI G G, TURNER M D, et al. The physiological roles of carnosine and β-alanine in exercising human skeletal muscle[J]. Medicine & Science in Sports & Exercise, 2019, 51(10): 2098-2108. |
| [17] |
DERAVE W, DE COURTEN B, BABA S P. An update on carnosine and anserine research[J]. Amino Acids, 2019, 51(1): 1-4. DOI:10.1007/s00726-018-02689-9 |
| [18] |
GHODSI R, KHEIROURI S. Carnosine and advanced glycation end products: a systematic review[J]. Amino Acids, 2018, 50(9): 1177-1186. DOI:10.1007/s00726-018-2592-9 |
| [19] |
BLANCQUAERT L, EVERAERT I, MISSINNE M, et al. Effects of histidine and β-alanine supplementation on human muscle carnosine storage[J]. Medicine and Science in Sports and Exercise, 2017, 49(3): 602-609. DOI:10.1249/MSS.0000000000001213 |
| [20] |
HARRIS R C, TALLON M J, DUNNETT M, et al. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis[J]. Amino Acids, 2006, 30(3): 279-289. DOI:10.1007/s00726-006-0299-9 |
| [21] |
GAUNITZ F, HIPKISS A R. Carnosine and cancer: a perspective[J]. Amino Acids, 2012, 43(1): 135-142. DOI:10.1007/s00726-012-1271-5 |
| [22] |
DERAVE W, EVERAERT I, BEECKMAN S, et al. Muscle carnosine metabolism and β-alanine supplementation in relation to exercise and training[J]. Sports Medicine, 2010, 40(3): 247-263. DOI:10.2165/11530310-000000000-00000 |
| [23] |
ARTIOLI G G, GUALANO B, SMITH A, et al. Role of beta-alanine supplementation on muscle carnosine and exercise performance[J]. Medicine and Science in Sports and Exercise, 2010, 42(6): 1162-1173. DOI:10.1249/MSS.0b013e3181c74e38 |
| [24] |
BLANCQUAERT L, EVERAERT I, DERAVE W. Beta-alanine supplementation, muscle carnosine and exercise performance[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2015, 18(1): 63-70. DOI:10.1097/MCO.0000000000000127 |
| [25] |
MAUGHAN R J, GREENHAFF P L, HESPEL P. Dietary supplements for athletes: emerging trends and recurring themes[M]//MAUGHAN R J, SHIRREFFS S M. Food, nutrition and sports performance Ⅲ. London: Routledge, 2013: 65-74.
|
| [26] |
BAUER K, SCHULZ M. Biosynthesis of carnosine and related peptides by skeletal muscle cells in primary culture[J]. European Journal of Biochemistry, 1994, 219(1/2): 43-47. |
| [27] |
HIPKISS A R, CARTWRIGHT S P, BROMLEY C, et al. Carnosine: can understanding its actions on energy metabolism and protein homeostasis inform its therapeutic potential?[J]. Chemistry Central Journal, 2013, 7(1): 38. DOI:10.1186/1752-153X-7-38 |
| [28] |
CALABRESE V, SCUTO M, SALINARO A T, et al. Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis[J]. Antioxidants, 2020, 9(12): 1303. DOI:10.3390/antiox9121303 |
| [29] |
BROOKES P S, YOON Y, ROBOTHAM J L, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle[J]. American Journal of Physiology: Cell Physiology, 2004, 287(4): C817-C833. DOI:10.1152/ajpcell.00139.2004 |
| [30] |
BOLDYREV A A. Carnosine and oxidative stress in cells and tissues[M]. New York: Nova Publishers, 2007.
|
| [31] |
HEIDARI R, GHANBARINEJAD V, OMMATI M M, et al. Regulation of mitochondrial function and energy metabolism: a primary mechanism of cytoprotection provided by carnosine[J]. Trends in Pharmaceutical Sciences, 2018, 4(1): 43-52. |
| [32] |
DAWSON R, Jr, BIASETTI M, MESSINA S, et al. The cytoprotective role of taurine in exercise-induced muscle injury[J]. Amino Acids, 2002, 22(4): 309-324. DOI:10.1007/s007260200017 |
| [33] |
PALIN M F, LAPOINTE J, GARIÉPY C, et al. Characterisation of intracellular molecular mechanisms modulated by carnosine in porcine myoblasts under basal and oxidative stress conditions[J]. PLoS One, 2020, 15(9): e0239496. DOI:10.1371/journal.pone.0239496 |
| [34] |
阳冬辉, 李怡芳, 姚楠, 等. 肌肽对应激负荷小鼠糖代谢的影响[J]. 广东药学院学报, 2010, 26(1): 95-98. YANG D H, LI Y F, YAO N, et al. Effect of carnosine on glycometabolism in restraint-stressed mice[J]. Journal of Guangdong College of Pharmacy, 2010, 26(1): 95-98 (in Chinese). |
| [35] |
CRIPPS M J, HANNA K, LAVILLA C, Jr, et al. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake[J]. Scientific Reports, 2017, 7(1): 13313. DOI:10.1038/s41598-017-13649-w |
| [36] |
SCHNUCK J K, SUNDERLAND K L, KUENNEN M R, et al. Characterization of the metabolic effect of β-alanine on markers of oxidative metabolism and mitochondrial biogenesis in skeletal muscle[J]. Journal of Exercise Nutrition & Biochemistry, 2016, 20(2): 34-41. |
| [37] |
游敏芳, 覃媛钰, 张依裕, 等. ITPRⅠ基因过表达对鸭子宫上皮细胞Ca2+浓度、脂质含量及钙转运相关基因的调控效应[J]. 生物工程学报, 2021, 37(7): 2443-2452. YOU M F, QIN Y Y, ZHANG Y Y, et al. Effects of the ITPR1 gene overexpression on Ca2+ concentration, lipid content and calcium transport-related genes in duck uterine epithelial cells[J]. Chinese Journal of Biotechnology, 2021, 37(7): 2443-2452 (in Chinese). |
| [38] |
CRUZ-TOPETE D, CIDLOWSKI J A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids[J]. Neuroimmunomodulation, 2015, 22(1/2): 20-32. |
| [39] |
RHEN T, CIDLOWSKI J A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs[J]. New England Journal of Medicine, 2005, 353(16): 1711-1723. DOI:10.1056/NEJMra050541 |
| [40] |
SCHAKMAN O, KALISTA S, BARBÉ C, et al. Glucocorticoid-induced skeletal muscle atrophy[J]. The International Journal of Biochemistry & Cell Biology, 2013, 45(10): 2163-2172. |
| [41] |
DUMAS J F, BIELICKI G, RENOU J P, et al. Dexamethasone impairs muscle energetics, studied by 31P NMR, in rats[J]. Diabetologia, 2005, 48(2): 328-335. |
| [42] |
DIMITRIADIS G, LEIGHTON B, PARRY-BILLINGS M, et al. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle[J]. Biochemical Journal, 1997, 321(Pt.3): 707-712. |
| [43] |
CODERRE L, VALLEGA G A, PILCH P F, et al. Regulation of glycogen concentration and glycogen synthase activity in skeletal muscle of insulin-resistant rats[J]. Archives of Biochemistry and Biophysics, 2007, 464(1): 144-150. |
| [44] |
LUAN G X, LI G, MA X, et al. Dexamethasone-induced mitochondrial dysfunction and insulin resistance-study in 3T3-L1 adipocytes and mitochondria isolated from mouse liver[J]. Molecules, 2019, 24(10): 1982. |
| [45] |
POSA D K, BABA S P. Intracellular pH regulation of skeletal muscle in the milieu of insulin signaling[J]. Nutrients, 2020, 12(10): 2910. |
| [46] |
BROWN C E. Interactions among carnosine, anserine, ophidine and copper in biochemical adaptation[J]. Journal of Theoretical Biology, 1981, 88(2): 245-256. |
| [47] |
TSOI B, HE R R, YANG D H, et al. Carnosine ameliorates stress-induced glucose metabolism disorder in restrained mice[J]. Journal of Pharmacological Sciences, 2011, 117(4): 223-229. |
| [48] |
MACARINI J R, MARAVAI S G, CARARO J H, et al. Impairment of electron transfer chain induced by acute carnosine administration in skeletal muscle of young rats[J]. BioMed Research International, 2014, 2014: 632986. |
| [49] |
MACEDO L W, CARARO J H, MARAVAI S G, et al. Acute carnosine administration increases respiratory chain complexes and citric acid cycle enzyme activities in cerebral cortex of young rats[J]. Molecular Neurobiology, 2016, 53(8): 5582-5590. |
| [50] |
KAAMAN M, SPARKS L M, VAN HARMELEN V, et al. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue[J]. Diabetologia, 2007, 50(12): 2526-2533. |
| [51] |
CALABRESE V, COLOMBRITA C, GUAGLIANO E, et al. Protective effect of carnosine during nitrosative stress in astroglial cell cultures[J]. Neurochemical Research, 2005, 30(6): 797-807. |
| [52] |
ITOH K, NAKAMURA K, ⅡJIMA M, et al. Mitochondrial dynamics in neurodegeneration[J]. Trends in Cell Biology, 2013, 23(2): 64-71. |
| [53] |
KENT-BRAUN J A, FITTS R H, CHRISTIE A. Skeletal muscle fatigue[J]. Comprehensive Physiology, 2012, 2(2): 997-1044. |
| [54] |
QIN J, DU R, YANG Y Q, et al. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity[J]. Research in Veterinary Science, 2013, 94(1): 84-89. |
| [55] |
CHENG J Y, YANG J B, LIU Y, et al. Profiling and targeting of cellular mitochondrial bioenergetics: inhibition of human gastric cancer cell growth by carnosine[J]. Acta Pharmacologica Sinica, 2019, 40(7): 938-948. |
| [56] |
BAO Y, DING S D, CHENG J Y, et al. Carnosine inhibits the proliferation of human cervical gland carcinoma cells through inhibiting both mitochondrial bioenergetics and glycolysis pathways and retarding cell cycle progression[J]. Integrative Cancer Therapies, 2018, 17(1): 80-91. |
| [57] |
CORONA C, FRAZZINI V, SILVESTRI E, et al. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice[J]. PLoS One, 2011, 6(3): e17971. |
| [58] |
谭竹钧, 韩雅莉. 肌肽棉酚对绵羊精子运动及超微结构的影响[J]. 动物学研究, 1995, 16(2): 119-125. TAN Z J, HAN Y L. Effects of carnosine and gossypol on the motility and ultrastructure of ram spermatozoa[J]. Zoological Research, 1995, 16(2): 119-125 (in Chinese). |
| [59] |
QURESHI Y, WOOD T. The effect of carnosine on glycolysis[J]. Biochimica et Biophysica Acta, 1962, 60: 190-192. |
| [60] |
BOLDYREV A, BULYGINA E, LEINSOO T, et al. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds[J]. Comparative Biochemistry and Physiology.Part B: Biochemistry & Molecular Biology, 2004, 137(1): 81-88. |
| [61] |
HIPKISS A R. Carnosine, a protective, anti-ageing peptide?[J]. The International Journal of Biochemistry & Cell Biology, 1998, 30(8): 863-868. |
| [62] |
BOLDYREV A A, ALDINI G, DERAVE W. Physiology and pathophysiology of carnosine[J]. Physiological Reviews, 2013, 93(4): 1803-1845. |
| [63] |
ALLEN D G, LAMB G D, WESTERBLAD H. Skeletal muscle fatigue: cellular mechanisms[J]. Physiological Reviews, 2008, 88(1): 287-332. |
| [64] |
DUTKA T L, LAMBOLEY C R, MCKENNA M J, et al. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers[J]. Journal of Applied Physiology, 2012, 112(5): 728-736. |
| [65] |
BATRUKOVA M A, RUBTSOV A M. Histidine-containing dipeptides as endogenous regulators of the activity of sarcoplasmic reticulum Ca-release channels[J]. Biochimica et Biophysica Acta, 1997, 1324(1): 142-150. |
| [66] |
HOFFMAN J R, VARANOSKE A, STOUT J R. Effects of β-alanine supplementation on carnosine elevation and physiological performance[J]. Advances in Food and Nutrition Research, 2018, 84: 183-206. |
| [67] |
PERRY T L, HANSEN S, LOVE D L. Serum-carnosinase deficiency in carnosinaemia[J]. The Lancet, 1968, 1(7554): 1229-1230. |
| [68] |
EVERAERT I, DE NAEYER H, TAES Y, et al. Gene expression of carnosine-related enzymes and transporters in skeletal muscle[J]. European Journal of Applied Physiology, 2013, 113(5): 1169-1179. |




