2. 云南农业大学食品科学技术学院, 昆明 650201
2. College of Food Science and Technology, Yunnan Agricultural University, Kunming 650201, China
哺乳动物肠道内定居着细菌、真菌和病毒等众多微生物,数量达1014个[1-2]。这些微生物群具有助消化、供给营养、促进肠上皮细胞成熟和抵御病原体的功能[3],它们与宿主形成共生关系,共同参与宿主的生长发育、消化代谢和免疫调节[4-5]。在畜禽养殖中,可以通过添加益生元、益生菌或进行粪菌移植(fecal microbiota transplantation, FMT)等方式干预肠道菌群组成,从而达到调节代谢、促进生长发育、治疗疾病和维护机体健康的目的[6]。
FMT是指将健康供体的肠道微生物群移植至受体,以重建肠道菌群,从而达到治疗肠道和肠道外疾病、促进受体生长发育等目的[7]。FMT技术早期的研究热点主要集中于疾病治疗,并在肠道疾病和其他疾病治疗中取得诸多成效。近些年来,许多学者在畜牧领域逐渐开展了有关FMT对以猪为代表的动物模型生长、发育、肠道菌群组成和肠道健康等影响的相关研究,研究结果表明,通过FMT引入外源菌群能够改变受体猪的肠道菌群结构,改善生长性能[8],降低腹泻率[9],对受体的生长、肠道发育[10]以及免疫调节[11]等产生积极影响。
云南省特殊的地理位置、复杂的地形地貌、独特多样的气候环境和多样的民族农耕习俗孕育了丰富的地方猪资源[12]。撒坝猪作为云南地方猪品种,具有耐粗饲、适应性强和抗病力强等优点[13],其消化道中蕴含着丰富的微生物资源,亟待开发。本试验以散养和圈养撒坝猪为供体,分别将其粪菌液移植至昆明小鼠,探究其对小鼠生长性能、养分表观消化率和肠道形态结构的影响,以期为FMT技术在畜牧生产中的应用奠定理论基础,同时为饲用益生菌的开发利用提供参考。
1 材料与方法 1.1 试验设计选择云南楚雄当地3月龄内健康、无病史以及无抗生素服用记录的纯种农户散养撒坝猪和猪场圈养撒坝猪各10头作为FMT供体,分组采集新鲜粪便,根据Hu等[14]的方法,取新鲜粪便置于无菌器皿,按照1 g粪便∶ 5 mL无菌生理盐水的比例加入无菌生理盐水,搅拌均匀,依次过2.0、1.0、0.5和0.25 mm的实验室用筛,滤液在4 ℃条件下、6 000×g离心15 min,弃上清,用无菌生理盐水重悬沉淀,加入等体积30%甘油置于-80 ℃冰箱低温保存[15-16]。正式试验前,将冷冻保存的菌液37 ℃水浴溶解后,4 ℃、6 000×g离心15 min,弃上清,用无菌生理盐水重悬沉淀得到原菌液,再用1/2原菌液体积的无菌生理盐水重悬沉淀即得高剂量FMT菌液,高剂量FMT菌液稀释2倍得到中剂量FMT菌液、稀释4倍得到低剂量FMT菌液。
选取6周龄体重18~22 g的健康雄性昆明小鼠(购自昆明医科大学实验动物中心)30只作为FMT受体,预饲1周后,随机分为对照组(CON组)、散养撒坝猪低剂量组(SY-L组)、散养撒坝猪中剂量组(SY-M组)、散养撒坝猪高剂量组(SY-H组)和圈养撒坝猪中剂量组(JY-M组),每组6只。各组小鼠分别灌胃生理盐水、散养撒坝猪低剂量FMT菌液、散养撒坝猪中剂量FMT菌液、散养撒坝猪高剂量FMT菌液和圈养撒坝猪中剂量FMT菌液,连续灌胃14 d,每天1次,每次200 μL。试验期28 d。
饲养试验在云南省动物营养与饲料重点实验室进行,饲养环境温度维持在23~26 ℃,相对湿度为50%~70%,昼夜循环设定为各12 h。整个饲养阶段小鼠自由采食、饮水,饮用水为经高温高压灭菌处理的自来水,饲粮为无抗颗粒饲料,饲粮组成及营养水平同文献[17]。
1.2 样品采集和指标测定 1.2.1 生长性能每周以个体为单位称量体重,记录采食量,计算平均日增重(ADG)、平均日采食量(ADFI)和料重比(F/G)。
1.2.2 脏器指数FMT试验结束后,根据夏介英等[18]的方法,采用颈椎脱臼法处死小鼠,称重后,迅速取出心脏、肝脏、脾脏、胃和肾脏称重,计算脏器指数。脏器指数(%)=100×[脏器鲜重(g)/活体重(g)]。
1.2.3 养分表观消化率参照李月明等[19]的方法,采用全收粪法测定小鼠饲粮养分表观消化率。试验最后1周收集每组小鼠粪便样品,按10 g/mL滴加10%盐酸固氮,经过65 ℃、8~12 h烘干处理为风干样品,然后放置空气中回潮,用粉碎机粉碎通过40目网筛,于封口袋中置干燥器保存。参照现行国标测定水分、粗蛋白质(CP)、粗脂肪(EE)、粗灰分(Ash)、钙(Ca)和磷(P)含量,然后计算养分表观消化率。某养分表观消化率(%)=100×[(食入饲粮该养分含量-粪中该养分含量)/食入饲粮该养分含量]。
1.2.4 血脂代谢指标试验结束后,使用摘眼球法采集血液,静置20~30 min后,3 000 r/min、离心10 min,获得血清样品,于-80 ℃冷冻保存待用。
用全自动动物血液生化分析仪及试剂盒(深圳迈瑞生物医疗电子股份有限公司)测定血清甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)、高密度脂蛋白胆固醇(high density lipoprotein cholesterol, HDL-C)和低密度脂蛋白胆固醇(low density lipoprotein cholesterol, LDL-C)含量。
1.2.5 回肠形态和杯状细胞数试验结束后,采用颈椎脱臼法处死小鼠,剪取试验小鼠中段回肠组织置于含4%多聚甲醛的磷酸盐缓冲溶液(PBS)中,用于苏木精-伊红(HE)染色和高碘酸雪夫氏(PAS)染色分析,分别检测绒毛高度、隐窝深度和杯状细胞数。
1.3 数据处理与分析采用Excel 2019对试验数据进行初步整理,然后用SPSS 25.0进行单因素方差分析(one-way ANOVA),并用Duncan氏法进行多重比较,试验结果以“平均值±标准差”表示,P < 0.05表示差异显著。
2 结果与分析 2.1 FMT对小鼠生长性能的影响FMT对小鼠生长性能的影响结果见文献[17],结果显示,与CON组相比,各FMT组小鼠ADG提高、F/G降低,其中SY-L组和SY-M组差异显著(P < 0.05);与JY-M组相比,SY-M组小鼠ADG显著提高(P < 0.05),F/G显著降低(P < 0.05)。
2.2 FMT对小鼠养分表观消化率的影响由表 1可知,与CON组相比,各FMT散养组粗蛋白质、粗脂肪和磷的表观消化率均显著提高(P < 0.05),JY-M组以上指标差异不显著(P>0.05);各FMT组粗灰分表观消化率均显著提高(P < 0.05);SY-M和SY-H组钙表观消化率显著提高(P < 0.05),其他FMT组钙表观消化率差异不显著(P>0.05)。与JY-M组相比,SY-M组粗蛋白质、粗脂肪、粗灰分、钙和磷的表观消化率分别提高了10.32%(P < 0.05)、0.56%(P>0.05)、28.39%(P < 0.05)、33.32%(P < 0.05)和2.50%(P < 0.05)。
|
|
表 1 FMT对小鼠养分表观消化率的影响 Table 1 Effects of FMT on nutrient apparent digestibility in mice |
由表 2可知,与CON组相比,各FMT组小鼠心脏、肝脏、脾脏和肾脏指数均无显著差异(P>0.05),SY-M组与JY-M组上述指标之间也均无显著差异(P>0.05)。上述结果表明,FMT对小鼠内脏器官整体无毒副作用。
|
|
表 2 FMT对小鼠脏器指数的影响 Table 2 Effects of FMT on organ indices in mice |
由表 3可知,与CON组相比,SY-M组和JY-M组小鼠血清TG含量降低,其中SY-M组差异显著(P < 0.05);各FMT组小鼠血清TC和HDL-C含量均降低,其中SY-L组、SY-M组和JY-M组差异显著(P < 0.05);各FMT组小鼠血清LDL-C含量降低,其中SY-M组差异显著(P < 0.05)。SY-M组与JY-M组上述指标之间均无显著差异(P>0.05)。
|
|
表 3 FMT对小鼠血脂代谢指标的影响 Table 3 Effects of FMT on blood lipid metabolism indices in mice |
由表 4、图 1和图 2可知,与CON组相比,各FMT组小鼠回肠绒毛高度无显著变化(P>0.05);各FMT组小鼠回肠隐窝深度降低,其中JY-M组差异显著(P < 0.05);各FMT组绒毛高度/隐窝深度值提高,其中SY-M组差异显著(P < 0.05);SY-L组、SY-M组、SY-H组和JY-M组小鼠回肠杯状细胞数分别增加了1.79%、28.51%、27.47%和15.02%(P>0.05)。与JY-M组相比,SY-M组小鼠回肠绒毛高度和隐窝深度无显著差异(P>0.05),绒毛高度/隐窝深度值有升高趋势(P>0.05),回肠杯状细胞数增加了11.72%(P>0.05)。
|
|
表 4 FMT对小鼠回肠形态和杯状细胞数的影响 Table 4 Effects of FMT on ileum morphology and goblet cell number in mice |
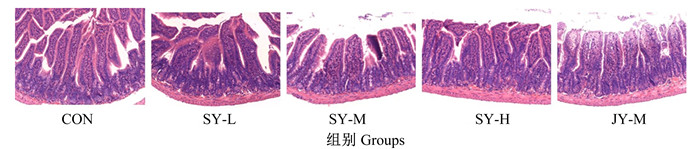
|
图 1 小鼠回肠绒毛高度和隐窝深度HE染色图 Fig. 1 HE staining figure of villus height and crypt depth of ileum in mice (5×) |
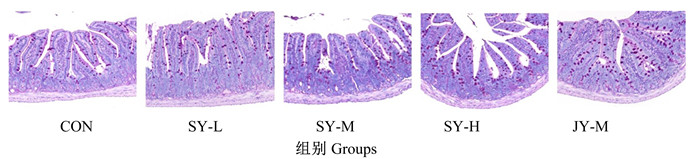
|
图 2 小鼠回肠杯状细胞PAS染色图 Fig. 2 PAS staining figure of ileum goblet cells in mice (5×) |
肠道微生物是影响动物体生长发育的重要因素之一,哺乳动物肠道微生物能够促进动物对养分的消化吸收及代谢,将动物肠道中不能被消化吸收的碳水化合物等物质转化为能被动物机体消化吸收的物质,进而促进动物生长[20]。大量研究证实,由FMT介导的外源性微生物群的植入能够提高动物ADG,促进动物生长。Hu等[8]将健康成年金华猪的粪菌液移植至“杜长大”新生仔猪,结果显示仔猪的ADG显著提高。张卓[21]将健康成年“杜长大”公猪粪菌液移植至新生长荣二杂仔猪,结果表明,FMT组仔猪的ADG比对照组提高了36.1%。黄金华等[22]研究表明,在断奶仔猪基础饲粮中添加7%的母体粪菌液,能够显著提高其ADG,降低F/G。本试验将散养和圈养撒坝猪粪菌液移植至小鼠,结果发现,各FMT组小鼠的ADG提高、F/G降低,且SY-M组ADG最高、F/G最低,这与黄金华等[23]的研究结果相似,表明不同剂量以及不同来源的粪菌液移植均能提高小鼠的生长性能,且在本试验条件下,SY-M组的效果最佳。在本试验中,SY-M组的ADG显著高于JY-M组,F/G显著低于JY-M组,这可能是因为散养撒坝猪所处环境和采食的复杂性,其肠道菌群多样性较高,将其粪菌液移植给小鼠后,小鼠肠道菌群多样性增加,对养分的作用机制增强,从而更能促进机体生长。
3.2 FMT对小鼠养分表观消化率的影响养分表观消化率是衡量动物消化能力的重要指标。肠道内的益生菌能够促进饲粮在动物体内的消化吸收,提高养分表观消化率。Hou等[24]研究表明,在断奶仔猪基础饲粮中添加复合益生菌制剂能够显著提高仔猪对粗蛋白质的表观消化率。宋献艺等[25]在断奶仔猪基础饲粮中添加0.1%的复合益生菌制剂,结果表明,益生菌组仔猪粗蛋白质表观消化率显著提高。王志成等[26]研究表明,在断奶仔猪基础饲粮中添加植物乳杆菌和枯草芽孢杆菌复合益生菌制剂能够显著提高仔猪粗蛋白质和粗脂肪表观消化率。由此可知,肠道微生态环境的改变可直接影响动物对养分的表观消化率。本试验结果显示,各FMT散养组粗蛋白质和粗脂肪表观消化率均显著提高,表明FMT可通过增加小鼠肠道菌群多样性和有益菌数量,提高小鼠对养分的消化,从而促进小鼠生长。
3.3 FMT对小鼠血脂代谢的影响血清TG、TC、LDL-C和HDL-C含量是反映机体脂代谢水平的重要指标。TG和TC含量在一定程度上可以反映机体的脂肪代谢水平,TG是体脂的主要成分,血液中TG含量升高会导致机体的脂肪沉积[27];TC是机体脂类代谢的重要指标,它参与机体的代谢和物质的合成,其含量升高会引起心血管病等疾病[28]。
FMT能够重塑肠道菌群结构,直接或间接影响机体代谢[29]。郭上齐等[30]用高脂高能饲粮诱导产生肥胖大鼠模型,将常规饮食大鼠粪菌液移植至模型鼠,结果表明FMT可显著降低模型鼠血清TG和TC含量。李月芹[31]将正常大鼠粪菌液移植至高脂饲粮模型大鼠,结果表明,FMT组大鼠血清TG含量显著低于模型组。Zhang等[32]将哈萨克族人的粪菌液移植至db/db小鼠,结果发现小鼠血清TG、TC和LDL-C含量显著降低。本试验将散养和圈养撒坝猪粪菌液移植至小鼠后,各FMT组小鼠粗脂肪表观消化率提高,血清TC、LDL-C和HDL-C含量降低,且SY-M组差异显著。此外,本团队前期研究结果表明,与CON组相比,各FMT组小鼠总脂肪指数均显著降低[17],而总脂肪指数是反映机体脂肪总量的指标,一定程度上可以反映机体脂肪沉积能力。以上结果表明,散养和圈养撒坝猪粪菌液移植均能增强小鼠的脂肪代谢能力,减少脂肪沉积,且综合各指标可知SY-M组小鼠脂代谢能力最强。有研究指出,脂肪沉积与肠道微生物组成的改变高度相关,肥胖个体肠道中厚壁菌门丰度增加,厚壁菌门/拟杆菌门值升高[33]。本试验前期研究结果表明,门水平上物种丰度结果显示,与CON组相比,各FMT组小鼠肠道中厚壁菌门丰度降低,拟杆菌门丰度升高,厚壁菌门/拟杆菌门值降低[17]。这与Ley等[34]的研究结果一致,表明FMT能够通过改变肠道微生物的组成,减少脂肪沉积。
以上研究结果表明,FMT可通过改善肠道微生物组成、降低厚壁菌门丰度、提高拟杆菌门丰度、降低厚壁菌门/拟杆菌门值以及提高粗脂肪表观消化率增强小鼠脂代谢能力,从而减少脂肪沉积,降低总脂肪指数,降低血清中TC、LDL-C和HDL-C含量。
3.4 FMT对小鼠回肠形态和杯状细胞数的影响小肠是动物养分消化吸收的主要部位,小肠绒毛高度和隐窝深度是衡量小肠消化吸收能力的重要指标[35]。肠绒毛长短决定其含有成熟绒毛细胞的数量,从而反映其对养分的吸收能力;隐窝深度主要反映肠上皮细胞的生成率,上皮细胞不断从隐窝基部向绒毛端部迁移、分化,形成具有吸收能力的绒毛细胞,从而补充绒毛细胞的正常脱落。隐窝变浅,表明细胞成熟率上升,分泌功能增强[36];绒毛高度/隐窝深度值综合反映小肠消化吸收功能,其值减小表明黏膜受损、消化吸收功能下降[37]。杯状细胞源于肠道隐窝基底部的多能干细胞[38],广泛分布于消化道的上皮细胞中,小肠杯状细胞是由其前身低黏液细胞分化而来[39],能够合成黏蛋白防止肠道过度炎性反应,对维持黏膜稳态起重要作用[40]。
张卓等[10]将成年猪粪菌液移植至新生长荣二杂仔猪,结果表明,FMT能够极显著降低十二指肠绒毛高度。岳晓敬[41]将金华猪粪菌液灌喂“杜长大”三元杂交新生仔猪,结果表明,FMT可提高小肠绒毛高度/隐窝深度值,增加回肠和结肠杯状细胞数,改善肠道黏膜形态,促进肠黏膜发育。以上研究表明FMT可以促进肠道发育。本试验中,各FMT组小鼠回肠隐窝深度降低,绒毛高度/隐窝深度值升高,杯状细胞数增加,与耿世杰等[42]的研究结果相似,表明散养和圈养撒坝猪粪菌液移植能够降低肠道隐窝深度,提高绒毛高度/隐窝深度值,增加杯状细胞数,从而改善小鼠肠黏膜发育、维持肠黏膜屏障并增强肠道的消化能力。
4 结论散养和圈养撒坝猪粪菌液移植可促进小鼠肠道黏膜发育,提高养分表观消化率,增强脂代谢能力,降低F/G,从而提高生长性能;其中,散养撒坝猪中剂量粪菌液移植效果较优。
| [1] |
SONNENBURG J L, ANGENENT L T, GORDON J I. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine?[J]. Nature Immunology, 2004, 5(6): 569-573. DOI:10.1038/ni1079 |
| [2] |
田亚针, 张晨曦, 杨涛, 等. 益生菌和粪菌移植调节炎症性肠病的研究进展[J]. 食品科学, 2021, 42(19): 250-259. TIAN Y Z, ZHANG C X, YANG T, et al. Progress in understanding the role of probiotics and fecal microbiota transplantation in regulating inflammatory bowel disease[J]. Food Science, 2021, 42(19): 250-259 (in Chinese). |
| [3] |
WANG J W, KUO C H, KUO F C, et al. Fecal microbiota transplantation: review and update[J]. Journal of the Formosan Medical Association, 2019, 118(Suppl 1): S23-S31. |
| [4] |
SHEN J, OBIN M S, ZHAO L P. The gut microbiota, obesity and insulin resistance[J]. Molecular Aspects of Medicine, 2013, 34(1): 39-58. DOI:10.1016/j.mam.2012.11.001 |
| [5] |
陈秀琴, 黄梅清, 郑敏, 等. 动物肠道菌群与病原微生物感染关系的研究进展[J]. 中国畜牧兽医, 2019, 46(2): 628-634. CHEN X Q, HUANG M Q, ZHENG M, et al. Research progress on the relationship between gut microbiota of animals and pathogenic microorganism infection[J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(2): 628-634 (in Chinese). DOI:10.16431/j.cnki.1671-7236.2019.02.035 |
| [6] |
程冉冉. 粪菌移植对鸡生长发育及肠道菌群的影响[D]. 硕士学位论文. 武汉: 华中农业大学, 2020. CHENG R R. The effect of fecal microbiota transplantaion on growth performance and gut microbiota in chickens[D]. Master's Thesis. Wuhan: Huazhong Agricultural University, 2020. (in Chinese) |
| [7] |
张发明, 范志宁, 季国忠. 粪菌移植的概念、历史、现状和未来[J]. 中国内镜杂志, 2012, 18(9): 930-934. ZHANG F M, FAN Z N, JI G Z. Fecal microbiota transplantation: definition, past, current and future[J]. China Journal of Endoscopy, 2012, 18(9): 930-934 (in Chinese). |
| [8] |
HU L S, GENG S J, LI Y, et al. Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets[J]. Frontiers in Microbiology, 2018, 8: 2663. DOI:10.3389/fmicb.2017.02663 |
| [9] |
CHENG S S, MA X, GENG S J, et al. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury[J]. mSystems, 2018, 3(5): e00137-18. |
| [10] |
张卓, 黄金秀, 杨飞云, 等. 早期粪菌移植对仔猪肠道发育、肠道菌群组成和肠道激素分泌的影响[J]. 动物营养学报, 2021, 33(7): 3745-3758. ZHANG Z, HUANG J X, YANG F Y, et al. Effects of early fecal microbiota transplantation on intestinal development, intestinal microbiota composition and intestinal hormone secretion of piglets[J]. Chinese Journal of Animal Nutrition, 2021, 33(7): 3745-3758 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.07.017 |
| [11] |
CHENG C S, WEI H K, WANG P, et al. Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets[J]. Animal, 2019, 13(3): 533-541. DOI:10.1017/S1751731118001611 |
| [12] |
鲁绍雄, 李明丽, 严达伟, 等. 云南地方猪种质特性及其保种与多样化利用[J]. 云南农业大学学报(自然科学), 2020, 35(6): 1096-1105. LU S X, LI M L, YAN D W, et al. Germplasm characteristics, conservation and various utilization of Yunnan local pig breeds[J]. Journal of Yunnan Agricultural University (Natural Science), 2020, 35(6): 1096-1105 (in Chinese). |
| [13] |
邹星炳, 尚美聪, 刘祥学, 等. 撒坝猪遗传资源调查报告[J]. 中国畜禽种业, 2010, 6(1): 41-44. ZOU X B, SHANG M C, LIU X X, et al. Investigation report on genetic resources of Saba pig[J]. The Chinese Livestock and Poultry Breeding, 2010, 6(1): 41-44 (in Chinese). |
| [14] |
HU J, CHEN L L, TANG Y M, et al. Standardized preparation for fecal microbiota transplantation in pigs[J]. Frontiers in Microbiology, 2018, 9: 1328. DOI:10.3389/fmicb.2018.01328 |
| [15] |
LEE C H, STEINER T, PETROF E O, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial[J]. JAMA, 2016, 315(2): 142-149. DOI:10.1001/jama.2015.18098 |
| [16] |
SATOKARI R, MATTILA E, KAINULAINEN V, et al. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection-an observational cohort study[J]. Alimentary Pharmacology & Therapeutics, 2015, 41(1): 46-53. |
| [17] |
胡天念, 程燕东, 殷桦娟, 等. 散养和圈养撒坝猪粪菌移植对小鼠脂肪沉积和粪便微生物区系的影响[J]. 动物营养学报, 2022, 34(9): 6097-6109. HU T N, CHENG Y D, YIN H J, et al. Effects of fecal microbiota transplantation of free range and captive Saba pigs on fat deposition and fecal microflora in mice[J]. Chinese Journal of Animal Nutrition, 2022, 34(9): 6097-6109 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.09.062 |
| [18] |
夏介英, 雷培琪, 曾晓兰, 等. SPF级KM小鼠主要脏器重量和血液生化值的测定[J]. 四川生理科学杂志, 2009, 31(3): 104-107. XIA J Y, LEI P Q, ZENG X L, et al. Determinations to the weight of main organs and biochemical indexes of SPF KM Mice[J]. Sichuan Journal of Physiological Sciences, 2009, 31(3): 104-107 (in Chinese). |
| [19] |
李月明, 高凯, 郭建强, 等. 活性酿酒酵母对小鼠生长性能及养分消化率的影响[J]. 吉林畜牧兽医, 2017, 38(1): 10-12. LI Y M, GAO K, GUO J Q, et al. Effects of active Saccharomyces cerevisiae on growth performance and nutrient digestibility of mice[J]. Jilin Animal Husbandry and Veterinary Medicine, 2017, 38(1): 10-12 (in Chinese). |
| [20] |
MARTENS E C, LOWE E C, CHIANG H, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts[J]. PLoS Biology, 2011, 9(12): e1001221. |
| [21] |
张卓. 粪菌移植和结肠菌移植对新生仔猪生长、代谢及肠道健康的差异影响[D]. 硕士学位论文. 重庆: 西南大学, 2021. ZHANG Z. Differential effects of fecal microbiota transplantation and colonic microbiota transplantation on growth, metabolism and intestinal health of neonatal piglets[D]. Master's Theses. Chongqing: Southwest University, 2021. (in Chinese) |
| [22] |
黄金华, 李泰佑, 宁国信, 等. 粪菌移植技术对断奶仔猪生长性能和下痢率的影响[J]. 黑龙江畜牧兽医, 2018(10): 60-62. HUANG J H, LI T Y, NING G X, et al. Effects of fecal bacteria transplantation on growth performance and dysentery rate of weaned piglets[J]. Heilongjiang Animal Science and Veterinary Medicine, 2018(10): 60-62 (in Chinese). |
| [23] |
黄金华, 宁国信, 梁珠民, 等. 粪菌液来源及添加量对断奶仔猪生长性能与下痢率的影响[J]. 中国畜牧杂志, 2018, 54(6): 107-111. HUANG J H, NING G X, LIANG Z M, et al. Effect of source and amount of fecal suspension on growth performance and diarrhea rate of weaned pigs[J]. Chinese Journal of Animal Science, 2018, 54(6): 107-111 (in Chinese). |
| [24] |
HOU G F, PENG W, WEI L K, et al. Probiotics and achyranthes bidentata polysaccharides improve growth performance via promoting intestinal nutrient utilization and enhancing immune function of weaned pigs[J]. Animals, 2021, 11(9): 2617. |
| [25] |
宋献艺, 刘洋, 周胜花. 复合益生菌制剂对断奶仔猪生长性能、腹泻率和营养物质消化率的影响[J]. 饲料工业, 2018, 39(15): 27-31. SONG X Y, LIU Y, ZHOU S H. Effects of compound probiotics on growth performance, diarrhea rate and nutrient digestibility of weaned piglets[J]. Feed Industry, 2018, 39(15): 27-31 (in Chinese). |
| [26] |
王志成, 赵春芳, 赵俊国. 益生菌对断奶仔猪生长性能、养分表观消化率和肠道菌群的影响[J]. 中国饲料, 2018(4): 40-44. WANG Z C, ZHAO C F, ZHAO J G. Effect of probiotics on growth performance, digestibility of main nutrients and intestinal flora of weaned piglets[J]. China Feed, 2018(4): 40-44 (in Chinese). |
| [27] |
徐晓娟. 绿茶粉在肉鸡养殖中的应用研究[D]. 硕士学位论文. 合肥: 安徽农业大学, 2011. XU X J. Application research on green tea powder in broilers breeding[D]. Master's Thesis. Hefei: Anhui Agricultural University, 2011. (in Chinese) |
| [28] |
张召兴. 复方中草药超微粉防治鸡致病性大肠杆菌病的研究[D]. 硕士学位论文. 秦皇岛: 河北科技师范学院, 2017. ZHANG Z X. Application of Chinese herbal medicine ultrafine powder in prevention and treatment of pathogenic E. coli from chicken[J]. Master's Thesis. Qinhuangdao: Hebei Normal University of Science & Technology, 2017. (in Chinese) |
| [29] |
张娴, 陈容平, 陈宏. 基于高通量测序技术分析肠道菌群与肥胖的研究进展[J]. 医学综述, 2017, 23(23): 4577-4583. ZHANG X, CHEN R P, CHEN H. Research advances in the relationship between gut microbiota and obesity by the application of high-throughput sequencing technologies[J]. Medical Recapitulate, 2017, 23(23): 4577-4583 (in Chinese). |
| [30] |
郭上齐, 武华, 翟春宝, 等. 粪菌移植对营养性肥胖大鼠血脂及肠道屏障的影响[J]. 中国现代医生, 2016, 54(4): 25-27, 33, 169. GUO S Q, WU H, ZHAI C B, et al. Effects of fecal microbiota transplantation on lipidemia and gut barrier in diet-induced obesity rats[J]. China Modern Doctor, 2016, 54(4): 25-27, 33, 169 (in Chinese). |
| [31] |
李月芹. 粪菌移植对非酒精性脂肪性肝病大鼠肝脏及肠黏膜屏障保护作用的研究[D]. 硕士学位论文. 石家庄: 河北医科大学, 2016. LI Y Q. The protective effect of FMT on the liver and intestinal mucosal barrier in NAFLD rats[D]. Master's Thesis. Shijiazhuang: Hebei Medical University, 2016. (in Chinese) |
| [32] |
ZHANG P P, LI L L, HAN X, et al. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice[J]. Acta Pharmacologica Sinica, 2020, 41(5): 678-685. |
| [33] |
RAHAT-ROZENBLOOM S, FERNANDES J, GLOOR G B, et al. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans[J]. International Journal of Obesity (2005), 2014, 38(12): 1525-1531. |
| [34] |
LEY R E, BÄCKHED F, TURNBAUGH P, et al. Obesity alters gut microbial ecology[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31): 11070-11075. |
| [35] |
沈霞芬. 家畜组织学与胚胎学[M]. 4版. 北京: 中国农业出版社, 2009. SHEN X F. Histology and embryology of livestock[M]. 4th ed. Beijing: China Agriculture Press, 2009 (in Chinese). |
| [36] |
卫旭彪, 张璐璐, 马广, 等. 酵母菌对猪肠道绒毛、隐窝及菌群的影响[J]. 饲料工业, 2016, 37(4): 61-64. WEI X B, ZHANG L L, MA G, et al. Effects of yeasts on intestinal villus, crypt and flora in pigs[J]. Feed Industry, 2016, 37(4): 61-64 (in Chinese). |
| [37] |
杨凤娟, 曾祥芳, 谯仕彦. 罗伊氏乳杆菌I5007对新生仔猪肠道形态、二糖酶活性和紧密连接蛋白表达的影响[J]. 中国农业科学, 2014, 47(22): 4506-4515. YANG F J, ZENG X F, QIAO S Y. Effect of Lactobacillus reuteri I5007 on intestinal morphology, disaccharidase activity and tight junction protein expression in newborn piglets[J]. Scientia Agricultura Sinica, 2014, 47(22): 4506-4515 (in Chinese). |
| [38] |
VAN DER FLIER L G, CLEVERS H. Stem cells, self-renewal, and differentiation in the intestinal epithelium[J]. Annual Review of Physiology, 2009, 71: 241-260. |
| [39] |
史玉兰, 段相林. 杯状细胞的研究进展[J]. 解剖科学进展, 2001, 7(4): 358-361. SHI Y L, DUAN X L. Progress on the study of the goblet cell[J]. Progress of Anatomical Sciences, 2001, 7(4): 358-361 (in Chinese). |
| [40] |
AUDIE J P, JANIN A, PORCHET N, et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization[J]. Journal of Histochemistry & Cytochemistry, 1993, 41(10): 1479-1485. |
| [41] |
岳晓敬. 粪菌移植对仔猪生产性能、肠道菌群和肠粘膜发育的影响[D]. 硕士学位论文. 杭州: 浙江大学, 2017. YUE X J. Effect of fecal microbiota transplantation on growth performance, intestinal microbiota and intestinal mucosa development of piglets[D]. Master's Thesis. Hangzhou: Zhejiang University, 2017. (in Chinese) |
| [42] |
耿世杰, 李媛, 程赛赛, 等. 外源粪菌干预对受体猪肠道屏障功能的影响研究[J]. 中国畜牧杂志, 2018, 54(3): 92-98. GENG S J, LI Y, CHENG S S, et al. Effect of exogenous fecal microbiota intervention on intestinal barrier function in recipient pigs[J]. Chinese Journal of Animal Science, 2018, 54(3): 92-98 (in Chinese). |




