肠道菌群是肠道功能的重要组成部分,其数量庞大,与宿主相互依存,可影响肠道内蛋白质、脂肪等营养物质的消化吸收,还与机体免疫机能、疾病预防[1]、肠道健康密切相关[2]。毛皮动物的饲喂以动物源性的鲜饲料为主,鲜饲料在炎热的夏季容易滋生细菌,引发肠道疾病[3],因此保持肠道健康对于提高毛皮动物生产水平、减少经济损失尤为重要。抗菌肽(antimicrobial peptides,ABPs)是生物细胞特定基因编码产生的一类小分子多肽,是由多种生物细胞特定基因编码经外界条件诱导产生的一类具有广谱抗细菌、真菌、病毒、原虫,抑杀肿瘤细胞,具有免疫活性[4-6]等作用的多肽,是机体对抗微生物、病毒和真菌的先天防御系统[7]。天蚕素抗菌肽是被发现最早、研究最彻底、效果最明显的一类抗菌肽[8],在畜禽养殖中广泛应用。已有研究表明,添加天蚕素抗菌肽可以改善动物肠道黏膜形态[9],修复肠道炎症损伤[10-11],改善肠道菌群[12],降低发病率,提高动物的生长性能[13]。肠道健康与营养调控关系的研究越来越备受关注。因此,本试验以育成期母貂为研究对象,在饲粮中添加不同水平的抗菌肽,探究其对育成期母貂生长性能、养分表观消化率和肠道菌群的影响,以确定其在水貂养殖过程中的作用效果及适宜添加水平。
1 材料与方法 1.1 试验设计和饲养管理选择60只65日龄体重相近的健康短毛黑母貂,随机分为6组,每组10个重复,每个重复1只。各组饲粮中分别添加0(对照组)、100、200、300、400、500 mg/kg的抗菌肽(天蚕素活性多肽≥100万单位/g,纯度≥98%)。基础饲粮参照NRC(1982)和文献[14-16]配制,其组成及营养水平见表 1。试验于2020年7月4日至2020年9月6日在山东省海阳市某水貂养殖场进行,预试期1周,正试期8周。试验分为1~5周和6~8周2个阶段。试验貂已完成犬瘟热和细小病毒疫苗接种。水貂采用棚舍饲养,自然光照,每天05:00和17:00各饲喂1次,自由饮水。
|
|
表 1 基础饲粮组成及营养水平 Table 1 Composition and nutrient levels of basal diets |
试验开始时和试验结束后对水貂逐只进行空腹称重,记录每天采食量,计算平均日增重(g/d)、平均日采食量(g/d)、料重比(平均日采食量/平均日增重)。试验结束时每组取6只水貂放于水平地面上,测量鼻尖至尾根的距离,记为活体长。
1.2.2 养分表观消化率分别在正式试验后第3周和第7周,每组选6个重复进行消化试验。消化试验采用内源指示剂法收集新鲜的貂粪,用10%的盐酸固氮,同时采集每组饲料样本。将3 d收集的粪便、饲料样品分别混合均匀后于65 ℃烘至恒重,制成风干样。样品粉碎过40目筛,参照相应标准测定粪样和饲料样品中的盐酸不溶灰分(GB/T 23742—2009)、粗蛋白质(GB/T 6432—2018)、粗脂肪(GB/T 6433—2006)、水分(GB/T 6435—2014)、钙(GB/T 6436—2018)、磷(GB/T 6437—2018)含量,并计算养分表观消化率:
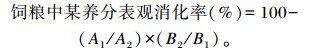
|
式中:A1为饲粮中盐酸不溶灰分含量;A2为粪样中盐酸不溶灰分含量;B1为饲粮中该养分的含量;B2为粪样中该养分的含量。
1.2.3 肠道菌群饲养试验结束后,每组取6个重复进行屠宰,收集水貂直肠内容物于冻存管中,先置于液氮中,后迅速转移至-80 ℃冰箱保存。用Fast DNA Spin Kit for Soil试剂盒(MP公司,美国)对直肠内容物进行DNA抽提,完成基因组DNA抽提后,利用1%琼脂糖凝胶电泳检测抽提的基因组DNA。采用通用引物338F(5′-ACTCCTACGGGAGGCAGCAG-3′)和806R(5′-GGACTACHVGGGTWTCTAAT-3′)对细菌16S rRNA V3~V4可变区进行PCR扩增。在ABI GeneAmp ® 9700型PCR仪器上进行程序设置:95 ℃预变性3 min,27个循环(95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s),最后72 ℃延伸10 min。使用2%琼脂糖凝胶回收PCR产物,用AxyPrep DNA(Axygen公司,美国)试剂盒进行纯化,2%琼脂糖凝胶电泳检测。利用QuantiFluorTM-ST蓝色荧光定量系统(Promega公司,美国)进行定量检测。根据Illumina MiSeq平台进行对纯化后的扩增片段构建PE 300文库,利用Illumina MiSeq PE 300平台进行测序。
1.3 数据统计分析试验数据用Excel 2019整理,采用SPSS 24.0进行单因素方差分析(one-way ANOVA),采用Duncan氏法进行多重比较,结果均以平均值±标准差表示,P<0.05表示差异显著。菌群原始测序序列使用Trimmomatic 1.2.11软件质控,FLASH软件进行拼接。对优化后的序列根据97%的相似度进行操作分类单元(OTU)聚类,并且对每个样本在各个水平的菌群组成进行统计,试验结果在Ⅰ-Sanger云平台进行分析。
2 结果 2.1 抗菌肽对育成期母貂生长性能的影响由表 2可知,抗菌肽显著影响育成期母貂末重、平均日增重和体长(P<0.05)。与对照组相比,200 mg/kg抗菌肽添加组末重、平均日增重显著升高(P<0.05),100、200、400、500 mg/kg抗菌肽添加组体长显著升高(P<0.05)。
|
|
表 2 抗菌肽对育成期母貂生长性能的影响 Table 2 Effects of antimicrobial peptide on growth performance of growing female minks |
由表 3可知,抗菌肽显著影响育成期母貂1~5周粗蛋白质表观消化率和6~8周粗脂肪表观消化率(P<0.05),对干物质、钙、磷表观消化率无显著影响(P>0.05)。与对照组相比,200、300、400 mg/kg抗菌肽添加组1~5周粗蛋白质表观消化率显著升高(P<0.05),100、200、300、400 mg/kg抗菌肽添加组6~8周粗脂肪表观消化率显著升高(P<0.05)。
|
|
表 3 抗菌肽对育成期母貂养分表观消化率的影响 Table 3 Effects of antimicrobial peptide on nutrient apparent digestibilities of growing female minks |
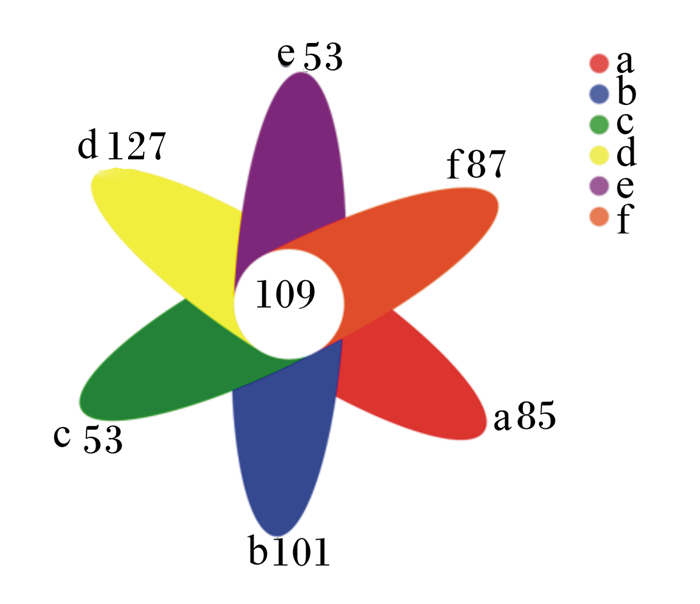
由图 1可知,各组直肠内容物中共有的OTU数量为109个,300 mg/kg抗菌肽添加组特有的OTU数量最多,为127个。
|
a:对照组control group;b:100 mg/kg抗菌肽添加组100 mg/kg antimicrobial peptide addition group;c:200 mg/kg抗菌肽添加组200 mg/kg antimicrobial peptide addition group;d:300 mg/kg抗菌肽添加组300 mg/kg antimicrobial peptide addition group;e:400 mg/kg抗菌肽添加组400 mg/kg antimicrobial peptide addition group;f:500 mg/kg抗菌肽添加组500 mg/kg antimicrobial peptide addition group。下图同the same as below。 图 1 基于OTU水平的肠道菌群Venn图 Fig. 1 Venn diagram of intestinal microbial flora at OTU level |
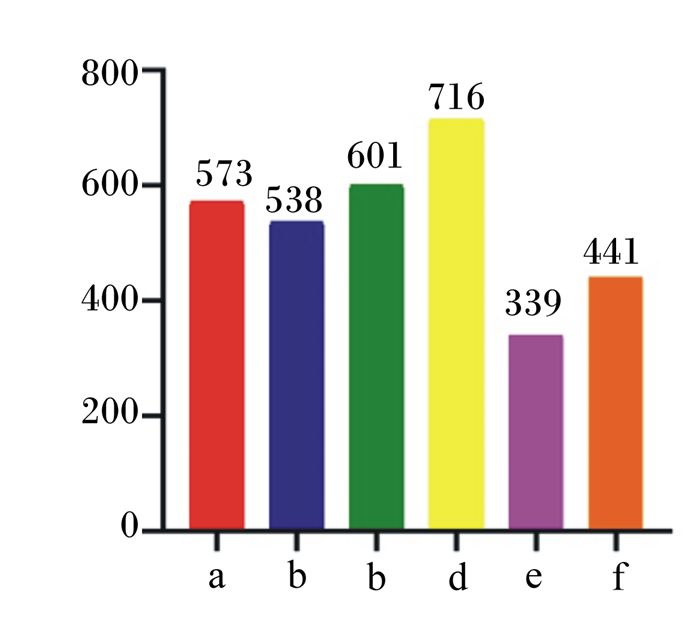
由图 2可知,300 mg/kg抗菌肽添加组总物种数最多,400 mg/kg抗菌肽添加组总物种数最少。
|
图 2 基于OTU水平各组总物种数柱形图 Fig. 2 Column diagram of total species in each group at OTU level |
由表 4可知,抗菌肽显著影响育成期母貂肠道菌群Ace指数和Chao1指数(P<0.05)。与对照组相比,200 mg/kg抗菌肽添加组Ace指数显著升高(P<0.05),300 mg/kg抗菌肽添加组Ace和Chao1指数显著升高(P<0.05)。
|
|
表 4 抗菌肽对育成期母貂alpha多样性指数的影响 Table 4 Effects of antimicrobial peptide on alpha diversity index of growing female minks |
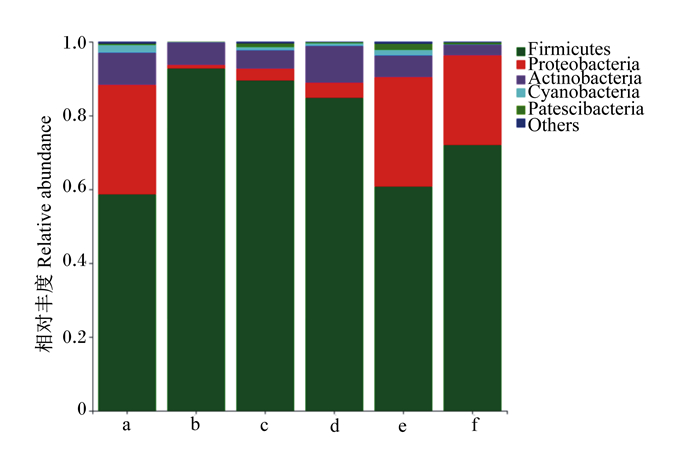
由图 3可知,在门水平上,各组盲肠菌群中相对丰度大于1%的菌门有厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、蓝藻菌门(Cyanobacteria)、拟杆菌门(Patescibacteria)。由表 5可知,与对照组相比,100、200 mg/kg抗菌肽添加组厚壁菌门相对丰度显著升高(P<0.05),100、200、300 mg/kg抗菌肽添加组变形菌门相对丰度显著降低(P<0.05)。
|
Firmicutes:厚壁菌门;Proteobacteria:变形菌门;Actinobacteria:放线菌门;Cyanobacteria:蓝藻菌门;Patescibacteria:拟杆菌门;Others:其他。 图 3 育成期母貂肠道菌群门水平相对丰度图 Fig. 3 Relative abundance figure of intestinal flora at phylum level of growing female minks |
|
|
表 5 抗菌肽对育成期母貂肠道菌群在门水平、属水平上相对丰度的影响 Table 5 Effects of antimicrobial peptide on relative abundance of intestinal flora at phylum level and genus level of growing female minks |
由图 4可知,在属水平上,各盲肠菌群中相对丰度大于1%的菌属有伯克霍尔德菌属和其他菌属。由表 5可知,与对照组相比,200、300 mg/kg抗菌肽添加组乳酸菌属相对丰度显著升高(P<0.05),400 mg/kg抗菌肽添加组伯克霍尔德菌属相对丰度显著升高(P<0.05),100、200、300、400和500 mg/kg抗菌肽添加组梭菌属相对丰度显著降低(P<0.05)。
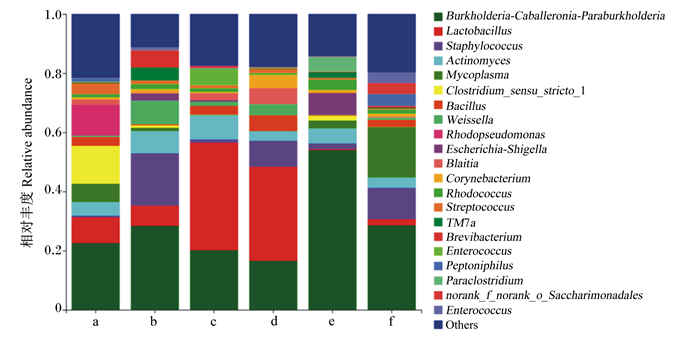
|
Burkholderia:伯克霍尔德菌属;Lactobacillu:乳酸菌属;Staphylococcus:葡萄球菌属;Actinomyces:放线菌属;Mycoplasma:支原体属;Clostridium:梭菌属;Bacillus:芽孢杆菌属;Weissella:魏斯氏菌属;Rhodopseudomonas:红假单胞菌属;Escherichia-Shigella:大肠杆菌-志贺氏菌;Corynebacterium:棒状杆菌属;Streptococcus:链球菌属;Brevibacterium:短杆菌属;Paraclostridium:副梭菌属;Saccharimonadales:糖单胞菌;Enterococcus:肠球菌属;Others:其他。 图 4 育成期母貂肠道菌群属水平相对丰度图 Fig. 4 Relative abundance figure of intestinal flora at genus level of growing female minks |
本试验结果表明,抗菌肽可以提高育成期母貂末重、平均日增重,与徐博成等[17]在仔猪上,王棚等[18]在蛋鸡上,Bao等[19]、刘洹兵[20]和李波等[21]在肉鸡上的应用效果一致。有研究发现,抗菌肽可以通过改善肠道健康、增强机体免疫能力来促进动物生长[22]。肠道健康的改善有利于营养物质的消化吸收,本研究的消化试验已证实,抗菌肽能提高粗蛋白质和粗脂肪表观消化率,而养分表观消化率的提高与改善肠道菌群有关,这说明抗菌肽对于水貂的促生长作用可能是通过改善肠道健康,提高养分消化率来实现的。
3.2 抗菌肽对育成期母貂养分表观消化率的影响本试验结果表明,抗菌肽可提高水貂粗蛋白质和粗脂肪表观消化率。这与Yoon等[23]和李平等[24]在断奶仔猪上的研究结果一致。都海明[25]和白建勇等[26]研究表明,在肉鸡和仔猪饲粮中添加抗菌肽能够显著提高其肠道内容物中脂肪酶、蛋白酶等活性,从而提高养分表观消化率。水貂养分的消化吸收与免疫力及肠道健康状态有着密切联系[27]。由于抗菌肽具有广泛的抗菌、耐热、耐酸等优点[28],使其更容易到达水貂肠道从而发挥生物学作用,改善肠道菌群和形态,进而提高养分表观消化率。
3.3 抗菌肽对育成期母貂肠道菌群的影响动物肠道菌群是按一定的比例组合,菌群间互相制约、互相依存,从而构建的一个复杂的微生物生态系统[29]。肠道菌群的多样性可以调节肠道内环境[30],提高动物的消化吸收能力,有利于保持动物机体的健康[31]。本试验在alpha多样性分析中发现,随着在饲粮中抗菌肽添加水平增加,试验组水貂直肠微生物的多样性有先升高后下降的趋势,这与谭凤霞等[32]研究结果一致,这可能是由于抗菌肽具有广谱杀菌效果,对不同菌种抑制浓度不同。在饲粮中适量添加抗菌肽能提高肠道菌群的多样性,改善肠道的微环境。
范忠原[33]研究发现,水貂肠道细菌主要归类为厚壁菌门(59.99%)、拟杆菌门(16.20%)、梭杆菌门(11.54%)、变形菌门(3.55%)、放线菌门(3.51%),其中厚壁菌门在水貂远端肠道中所占的比例最高。本试验结果表明,厚壁菌门和拟杆菌门是水貂肠道内相对丰度较高的菌群,具体相对丰度偏差可能与水貂遗传、生长环境等因素有关。研究发现,厚壁菌门相对丰度与动物肠道中脂肪、蛋白质的消化有关,具有促进饲粮营养消化吸收的作用[34-37]。这与本试验发现抗菌肽可以提高水貂不同时期粗蛋白质和粗脂肪表观消化率的结果一致,可能是与添加适量抗菌肽能提高育成期母貂肠道中厚壁菌门、乳酸菌属相对丰度,降低变形菌门、梭菌属相对丰度,从而改善肠道内环境有关。有研究发现,当抗菌添加水平过高时,肠道条件致病菌的组成比例有所增加,有益菌的组成比例下降,菌群多样性水平下降[32],可能是由于不同菌种对抗菌肽敏感度不同,抗菌肽是否存在剂量限制仍需更多试验进一步验证。
4 结论在本试验条件下,饲粮中添加100、200 mg/kg抗菌肽可提高育成期母貂末重、平均日增重、体长,改善粗蛋白质和粗脂肪表观消化率,调节肠道菌群多样性,提高厚壁菌门、乳酸菌属相对丰度,降低变形菌门、梭菌属相对丰度。
| [1] |
张静, 王肖枭, 周怡, 等. 肠道菌群与疾病相关性的研究进展[J]. 基础医学与临床, 2020, 40(2): 243-247. ZHANG J, WANG X X, ZHOU Y, et al. Advances in research on the relationship between intestinal flora and diseases[J]. Basic & Clinical Medicine, 2020, 40(2): 243-247 (in Chinese). DOI:10.3969/j.issn.1001-6325.2020.02.022 |
| [2] |
DUCATELLE R, GOOSSENS E, DE MEYER F, et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives[J]. Veterinary Research, 2018, 49(1): 43. DOI:10.1186/s13567-018-0538-6 |
| [3] |
葛平萍, 葛向平, 宋晓飞, 等. 水貂病毒性肠炎的诊断与预防[J]. 特种经济动植物, 2021, 24(5): 25-26. GE P P, GE X P, SONG X F, et al. Diagnosis and prevention of viral enteritis in mink[J]. Special Economic Animal and Plant, 2021, 24(5): 25-26 (in Chinese). DOI:10.3969/j.issn.1001-4713.2021.05.011 |
| [4] |
SONG D G, ZONG X, ZHANG H W, et al. Antimicrobial peptide cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia[J]. International Immunopharmacology, 2015, 25(1): 141-147. DOI:10.1016/j.intimp.2015.01.017 |
| [5] |
NARAYANA J L, HUANG H N, WU C J, et al. Epinecidin-1 antimicrobial activity: in vitro membrane lysis and in vivo efficacy against Helicobacter pylori infection in a mouse model[J]. Biomaterials, 2015, 61: 41-51. DOI:10.1016/j.biomaterials.2015.05.014 |
| [6] |
MORAVEJ H, MORAVEJ Z, YAZDANPARAST M, et al. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria[J]. Microbial Drug Resistance, 2018, 24(6): 747-767. DOI:10.1089/mdr.2017.0392 |
| [7] |
卞璐. 昆虫抗菌肽天蚕素的研究进展[J]. 中国动物保健, 2021, 23(4): 116, 123. BIAN L. Research progress of insect antibacterial peptide cecropin[J]. China Animal Health, 2021, 23(4): 116, 123 (in Chinese). |
| [8] |
HULTMARK D, STEINER H, RASMUSON T, et al. Insect immunity.Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia[J]. European Journal of Biochemistry, 1980, 106(1): 7-16. DOI:10.1111/j.1432-1033.1980.tb05991.x |
| [9] |
张耀文, 马文峰, 张志丹, 等. 天蚕素抗菌肽对产蛋后期蛋鸡生产性能、蛋品质及肠道黏膜形态的影响[J]. 家畜生态学报, 2020, 41(5): 30-35. ZHANG Y W, MA W F, ZHANG Z D, et al. Effects of antimicrobial peptides on laying performance, egg quality and intestinal morphology of hens during the late laying period[J]. Acta Ecologae Animalis Domastici, 2020, 41(5): 30-35 (in Chinese). DOI:10.3969/j.issn.1673-1182.2020.05.006 |
| [10] |
DAI J H, OU W H, YU G J, et al. The antimicrobial peptide cecropin AD supplement alleviated soybean meal-induced intestinal inflammation, barrier damage, and microbial dysbiosis in juvenile Turbot, Scophthalmus maximus[J]. Frontiers in Marine Science, 2020, 7: 584482. DOI:10.3389/fmars.2020.584482 |
| [11] |
徐慧心, 王宝维, 葛文华, 等. 鹅油甘油二酯与天蚕素抗菌肽合用对葡聚糖硫酸钠诱导的小鼠溃疡性结肠炎损伤的修复作用[J]. 动物营养学报, 2020, 32(8): 3877-3886. XU H X, WANG B W, GE W H, et al. Repairing effects of goose oil diglyceride combined with cecropin antimicrobial peptide on dextran sodium sulfate-induced ulcerative colitis injury in mice[J]. Chinese Journal of Animal Nutrition, 2020, 32(8): 3877-3886 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.08.046 |
| [12] |
姚远, 匡伟, 黄忠阳, 等. 抗菌肽天蚕素对鸡生长性能、肠道黏膜形态、盲肠菌群及免疫功能的影响[J]. 江苏农业学报, 2014, 30(2): 331-338. YAO Y, KUANG W, HUANG Z Y, et al. Effect of antibacterial peptide cecropin on growth performance, intestinal mucosal morphology, caecal microflora and immune function of chickens[J]. Jiangsu Journal of Agricultural Sciences, 2014, 30(2): 331-338 (in Chinese). DOI:10.3969/j.issn.1000-4440.2014.02.017 |
| [13] |
许君茹, 胡晓丹, 沈精精, 等. 天蚕素抗菌肽体外抑菌及肉鸡饲喂效果观察[J]. 上海畜牧兽医通讯, 2019(1): 54-55, 57. XU J R, HU X D, SHEN J J, et al. In vitro antibacterial activity of cecropin antimicrobial peptide and the effect of broiler feeding[J]. Shanghai Journal of Animal Husbandry and Veterinary Medicine, 2019(1): 54-55, 57 (in Chinese). |
| [14] |
Subcommittee on Furbearer Nutrition, Committee on Animal Nutrition, Board on Agriculture and Renewable Resources, et al. Nutrient requirements of mink and foxes[M]. 2nd ed. Washington, D.C.: National Academies Press, 1982.
|
| [15] |
张铁涛, 张志强, 任二军, 等. 不同蛋白质水平日粮对不同日龄育成期公貂生长性能与消化代谢规律的影响[J]. 畜牧兽医学报, 2011, 42(10): 1387-1395. ZHANG T T, ZHANG Z Q, REN E J, et al. Effect of dietary protein on the growth performance and the regularity of digestibility and metabolism in mink at the period of development[J]. Acta Veterinaria Et Zootechnica Sinica, 2011, 42(10): 1387-1395 (in Chinese). |
| [16] |
吴学壮, 杨颖, 刘汇涛, 等. 饲粮铜水平对育成期雄性水貂铜元素代谢和血清生化指标的影响[J]. 畜牧兽医学报, 2018, 49(4): 746-753. WU X Z, YANG Y, LIU H T, et al. Effects of dietary copper levels on copper metabolism and serum biochemical indices in late growing male mink[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(4): 746-753 (in Chinese). |
| [17] |
徐博成, 李智, 汪以真, 等. 抗菌肽对仔猪生长性能、腹泻率和免疫球蛋白水平影响的Meta分析[J]. 动物营养学报, 2020, 32(8): 3584-3593. XU B C, LI Z, WANG Y Z, et al. Effects of antimicrobial peptides on growth performance, diarrhea rate and immunoglobulin levels of piglets: a Meta-analysis[J]. Chinese Journal of Animal Nutrition, 2020, 32(8): 3584-3593 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.08.017 |
| [18] |
王棚, 曹原, 铁鲲源, 等. 抗菌肽粗提物对产蛋后期鸡产蛋性能、蛋品质、脏器指数、血清生化指标及免疫功能的影响[J]. 中国兽医学报, 2018, 38(4): 819-823. WANG P, CAO Y, TIE K Y, et al. Effect of crude extract of antimicrobial peptide on production performance, egg quality, viscera index, serum biochemical indexes and immune function of late laying hens[J]. Chinese Journal of Veterinary Science, 2018, 38(4): 819-823 (in Chinese). |
| [19] |
BAO H, SHE R, LIU T, et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens[J]. Poultry Science, 2009, 88(2): 291-297. DOI:10.3382/ps.2008-00330 |
| [20] |
刘洹兵, 刘毅. 抗菌肽在肉鸡日粮中添加效果研究[J]. 湖南畜牧兽医, 2015(4): 6-9. LIU H B, LIU Y. Study on the effect of adding antimicrobial peptides in broiler diet[J]. Hunan Journal of Animal Science & Veterinary Medicine, 2015(4): 6-9 (in Chinese). |
| [21] |
李波, 郭强, 杨利, 等. 饲粮中添加天蚕素抗菌肽对肉鸡生长性能的影响[J]. 中国家禽, 2010, 32(20): 55-56. LI B, GUO Q, YANG L, et al. Effect of dietary cecropin on growth performance of broilers[J]. China Poultry, 2010, 32(20): 55-56 (in Chinese). |
| [22] |
WANG J J, DOU X J, SONG J, et al. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era[J]. Medicinal Research Reviews, 2019, 39(3): 831-859. DOI:10.1002/med.21542 |
| [23] |
YOON J H, INGALE S L, KIM J S, et al. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs[J]. Journal of the Science of Food and Agriculture, 2013, 93(3): 587-592. DOI:10.1002/jsfa.5840 |
| [24] |
李平, 孙玉龙, 廖吉林. 抗菌肽对断奶仔猪生长性能、养分消化率、肠道微生物含量及绒毛结构的影响[J]. 中国饲料, 2019(8): 68-72. LI P, SUN Y L, LIAO J L. Effects of antimicrobial peptide on growth performance, nutrient digestibility, intestinal microflora and intestinal morphology of weaning pigs[J]. China Feed, 2019(8): 68-72 (in Chinese). |
| [25] |
都海明. 抗菌脂肽对AA肉鸡生产性能、养分代谢及抗氧化机能的影响[D]. 硕士学位论文. 南京: 南京农业大学, 2009. DOU H M. Effects of antimicrobial lipopeptides on performance, nutrient metabolism and antioxidant capacity in AA broilers[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2009. (in Chinese) |
| [26] |
白建勇, 宦海琳, 闫俊书, 等. 抗菌肽对发酵床饲养仔猪生长性能、消化酶及垫料理化性质的影响[J]. 家畜生态学报, 2014, 35(12): 27-32. BAI J Y, HUAN H L, YAN J S, et al. Effect of antimicrobial peptides on growth performance, digestive enzymes and physicochemical property of litters in piglets raised in fermentation bed[J]. Acta Ecologae Animalis Domastici, 2014, 35(12): 27-32 (in Chinese). |
| [27] |
PENG Z X, WANG A R, XIE L Q, et al. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets[J]. Scientific Reports, 2016, 6: 26790. DOI:10.1038/srep26790 |
| [28] |
卢军霞, 张宁, 王娟, 等. 抗菌肽的作用机制及在畜禽生产中的应用[J]. 中国饲料, 2020(4): 20-22. LU J X, ZHANG N, WANG J, et al. Action mechanism of antibacterial peptide and its application in livestock and poultry production[J]. China Feed, 2020(4): 20-22 (in Chinese). |
| [29] |
SIVIERI K, BASSAN J, PEIXOTO G, et al. Gut microbiota and antimicrobial peptides[J]. Current Opinion in Food Science, 2017, 13: 56-62. |
| [30] |
GAO Z H, WU H H, SHI L, et al. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens[J]. Animal Nutrition, 2017, 3(2): 109-113. |
| [31] |
张雪, 张珊, 钟光, 等. 枯草芽孢杆菌对肉鸡生长性能、肠道组织形态和盲肠微生物组成的影响[J]. 动物营养学报, 2020, 32(11): 5195-5208. ZHANG X, ZHANG S, ZHONG G, et al. Effects of Bacillus subtilis on growth performance, intestinal morphology and cecal microbial composition of broilers[J]. Chinese Journal of Animal Nutrition, 2020, 32(11): 5195-5208 (in Chinese). |
| [32] |
谭凤霞, 柴毅, 彭梦, 等. 饲料中添加抗菌肽对黄鳝肠道菌群的影响[J]. 淡水渔业, 2020, 50(4): 76-82. TAN F X, CHAI Y, PENG M, et al. Effects of dietary antimicrobial peptides supplementation on intestinal microflora of Monopterus albus[J]. Freshwater Fisheries, 2020, 50(4): 76-82 (in Chinese). |
| [33] |
范忠原. 家养水貂肠道微生物多样性分析[D]. 硕士学位论文. 北京: 中国农业科学院, 2015. FAN Z Y. The analysis of microorganism diversity in the intestinal tract of mink[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2015. (in Chinese) |
| [34] |
LEY R E, BÄCKHED F, TURNBAUGH P, et al. Obesity alters gut microbial ecology[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31): 11070-11075. |
| [35] |
MOSCHEN A R, WIESER V, TILG H. Dietary factors: major regulators of the gut's microbiota[J]. Gut and Liver, 2012, 6(4): 411-416. |
| [36] |
DE FILIPPO C, CAVALIERI D, DI PAOLA M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(33): 14691-14696. |
| [37] |
TURNBAUGH P J, LEY R E, MAHOWALD M A, et al. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature, 2006, 444(7122): 1027-1031. |




