2. 中国农业科学院北京畜牧兽医研究所, 北京 100193
2. Beijing Institute of Animal Science and Veterinary Medicine, Chinese Academy of Agricultural Sciences, Beijing 100193, China
大豆异黄酮(soybean isoflavone, SIF)是存在于大豆、红三叶草等豆科植物中的次级代谢产物,在自然界以游离型苷元和结合型糖苷2种形式存在[1]。SIF具有抗炎、抗氧化、增强机体免疫力、调节肠道菌群结构和类雌激素样作用[2-4],现已广泛应用于医药开发、食品保健和饲料研发等诸多领域[5-6]。相关报道表明,饲粮中添加SIF可提高动物的免疫能力和生长性能,并可调节激素分泌和改善肠道健康[7-8]。随着人们对SIF生理功能研究的不断深入,研究发现SIF组分能与肠道微生物相互作用生成高生物利用度和高生物活性的新型微生物转化产物,进而充分发挥SIF的生物活性[9],但是其在动物体内的代谢规律和对肠道的保护机制还鲜有报道。因此,本文就SIF在动物体内的代谢过程及其对肠道的保护机制进行综述,以期为SIF的深入研究与应用提供参考依据。
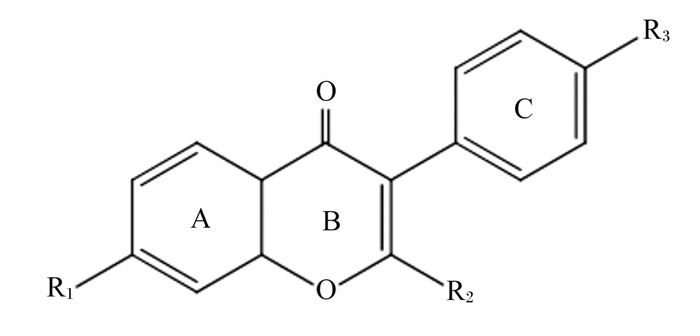
1 SIF的理化性质及提取工艺SIF母核为3-苯基苯并二氢吡喃(C15H10O2)[10],其结构通式如图 1所示,是豆科植物生长过程中产生的一种非甾族杂环多酚类化合物[11]。SIF主要在植物种子的子叶和胚轴中富集,其中子叶中的含量可占总异黄酮含量的80%~90%[2],其成分主要分为大豆苷类(daidzin groups)、染料木苷类(genistin groups)、黄豆黄素苷类(glycitin groups),以游离糖苷型、乙酰基葡萄糖苷型、葡萄糖苷型和丙二酰基葡萄糖苷型4种不同形式存在[12]。SIF糖苷在弱碱性和高温条件下可水解为乙酰基葡萄糖苷、β-葡萄糖苷,在高温低pH的条件下还可以进一步水解为SIF苷元,由于SIF苷元具有多酚羟基结构,故其还可发挥还原作用[13-14]。
|
图 1 大豆异黄酮结构通式 Fig. 1 General formula of SIF structure[15] |
目前SIF的提取方法主要有浸提法、超声辅助提取法、微波辅助提取法、超高压提取法、超临界CO2提取法和加热回流法等[16-21]。各提取方法具体优缺点的比较见表 1。
|
|
表 1 大豆异黄酮提取方法比较 Table 1 Comparison of extraction methods of SIF |
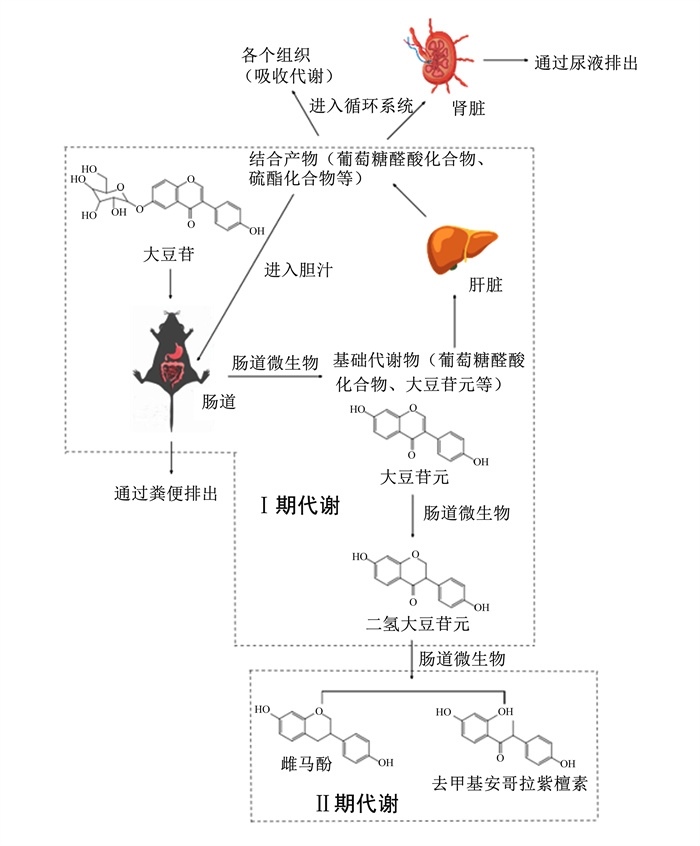
SIF的代谢分为Ⅰ期代谢和Ⅱ期代谢2个阶段,SIF在进入肠道时须被分解成相应的异黄酮苷元才能被吸收发挥功能,其分解为苷元后进入肠道细胞,部分苷元在酶的作用下转化成葡萄糖醛酸化和磺化衍生物进入肝脏,代谢后转入胆汁,随肠肝循环再次进入肠道[25-27]。在肠道中,异黄酮苷元一部分被肠道上皮吸收,另一部分由芽孢菌属、梭菌属、乳酸杆菌属等细菌分解代谢为雌马酚等活性物质供机体吸收利用[28]。
2.1 SIF的吸收与代谢SIF的代谢主要在十二指肠和空肠[29-31]。SIF中的结合型糖苷被肠腔中的β-葡萄糖苷酶水解为脂溶性苷元和水溶性苷元(染料木素、大豆苷元和黄豆黄素等苷元),其脂溶性苷元通过被动运输被小肠直接吸收,水溶性苷元则经小肠钠依赖性葡萄糖转运体运输到小肠细胞(运输顺序为染料木素>大豆苷元>黄豆黄素)。小肠细胞中有乳糖酶根皮苷水解酶(LPH)及葡萄糖脑苷酶,这些酶通过将异黄酮苷元的7-羟基部分进行葡萄糖醛酸化将其代谢为β-D-葡萄糖醛酸和磺酸酯,后扩散进入血液[32-33]。除了在肠道细胞中代谢外,十二指肠中的肠球菌、乳酸菌、拟杆菌及彼得杆菌等还可分泌β-葡萄糖苷酶和β-葡萄糖苷酸酶将其转化为葡萄糖醛酸后再扩散进入血液,代谢产物随血液进入肝脏,肝脏摄取血液中SIF的代谢物如β-甘脲类染料木素、黄豆黄素苷元和大豆苷元再进行代谢,此过程为Ⅰ期代谢;随后,代谢产物被排泄到胆汁中,随胆汁再次进入肠道进行二次水解,通过肠肝循环再次进入十二指肠[34-36]。回到十二指肠的SIF代谢物则会进入结肠在肠杆菌、梭状芽孢杆菌和类杆菌分泌的β-葡萄糖醛酸转移酶(UGT)和硫转移酶(SULT)的作用下生成雌马酚、二氢大豆苷元、邻去甲基安哥拉紫檀素等物质,这些产物最终被肠道吸收发挥其生物功能,部分产物还会被细菌直接吸收利用,此过程为Ⅱ期代谢[32-34]。简单来说,SIF首次进入肠道再进入肝脏进行的代谢为Ⅰ期代谢,离开肝脏再次进入肠道后进行的代谢为Ⅱ期代谢。此外,小肠对大豆苷元和染料木素的7-葡萄糖苷水解速度比肝脏快[37],其经UGT和SULT的硫酸化及葡萄糖醛酸化生成硫酸盐和葡萄糖醛酸结合物,经体循环运输到全身的组织进行利用,未被利用的物质经过肾脏排出或通过肠肝循环进入肠道重新代谢或排出[38-40]。其代谢过程如图 2所示。
2.2 SIF主要活性成分的代谢方式与产物SIF的成分多达12种,但其中主要发挥作用的只有染料木素和大豆苷元,在食用SIF的动物体内能够被检测到的主要是染料木素和大豆苷元以及它们的代谢产物,而主要成分之一的黄豆黄素由于功能较弱,对其代谢的研究也不够清晰[43]。
2.2.1 染料木素染料木素在Ⅰ期代谢中先被肠道微生物转化为二氢染料木素,二氢染料木素裂解C环形成6′-羟基-O-去甲基安哥拉紫檀素,之后被进一步降解为代谢终产物4-乙基苯酚[44]。部分未完全降解的染料木素被肠道吸收进入血液,在血液中生成葡萄糖醛酸和硫酸盐结合物后,随血液进入肝脏被代谢为6种羟基化染料木素代谢物,经胆汁排泄,通过肠肝循环进入肠道进行Ⅱ期代谢[45-47]。血液中的染料木素代谢物到达心血管内皮细胞(HUVEC)时会被吸收,通过UGT、SULT和邻苯二酚甲基转移酶(COMT)的作用,使其发生葡萄糖醛酸化、硫酸化和甲氧基化,在COMT的作用下染料木素的芳香羟基化产物中的邻苯二酚部分生成去甲氧基化硫酸盐以及甲氧基化葡萄糖醛酸衍生物,进而发挥其抗氧化等功能,并最终以葡萄糖醛酸和硫酸化合物的形式通过尿液和胆汁被排出体外[46]。
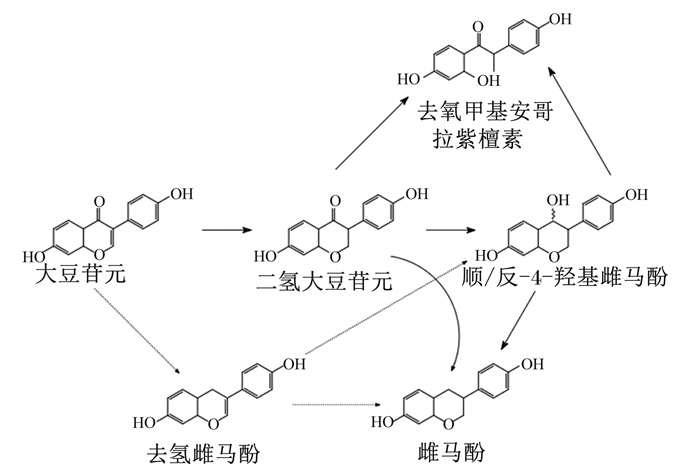
2.2.2 大豆苷元大豆苷元在肠道中被微生物分解为二氢大豆苷元后最终转化为氧去甲基安哥拉紫檀素(O-DMA)和雌马酚[48]。大豆苷元Ⅰ期代谢后产生的二氢大豆苷元在大鼠肠壁细胞内的UGT和SULT的作用下,使葡萄糖醛酸与第7位的氧原子偶联,生成大豆苷元-7-O-β-D-葡糖苷酸;硫酸盐与第4′位的氧原子偶联,得到大豆苷元-4′-O-硫酸盐,大豆苷元主要以葡萄糖醛酸和硫酸盐的形式存在于血液中并随血液到达各个器官[49]。O-DMA和雌马酚主要在结肠中生成和吸收[50],梭状芽孢杆菌和分枝真杆菌可将大豆苷元代谢为O-DMA,双歧杆菌菌株可将大豆苷元代谢为雌马酚[51]。在肝脏中,部分大豆苷元被代谢为葡萄糖醛酸和硫酸盐代谢物排泄入胆汁参与肠肝循环,进入肠道进行Ⅱ期代谢;同时,部分大豆苷元会被代谢为6, 7, 4′-三羟基异黄酮、7, 3′, 4′-三羟基异黄酮和6, 7, 3′, 4′-四羟基异黄酮后直接进入血液后经尿液排出[52]。在整个代谢过程中,大豆苷元主要以葡萄糖醛酸和硫酸盐代谢物的形式经尿液排泄,未被代谢的大豆苷元主要经粪便排出。大豆苷元的主要代谢过程如图 3所示。
|
图 3 大豆苷元在体内的代谢过程 Fig. 3 Metabolic process of daidzein in vivo[48] |
SIF主要以游离型和结合型2种形式存在,游离型主要包括染料木素、大豆苷元、黄豆黄素3种,结合型则是在游离型的基础上,以β-糖苷键分别与葡萄糖、乙酰基葡萄糖、丙二酰基葡萄糖相连而成,结合型糖苷需由肠道微生物作用生成游离型糖苷进行代谢[53]。肠道微生物通过还原、甲基化或去甲基化、羟基化或二羟基化和C环断裂等反应对SIF进行代谢[54],其在不同细菌的作用下产生多种代谢产物。目前,已分离出一些可以将SIF完全分解的肠道细菌,其分类见表 2。
|
|
表 2 肠道微生物对大豆异黄酮的代谢 Table 2 Metabolism of SIF by intestinal microorganisms |
动物肠道微生物群在不同个体和种属之间差异显著,这些细菌可分为乳酸菌类、斜门厌氧菌类、需氧菌类3类[34, 62]。目前已经分离鉴定出部分将SIF转化为雌马酚的细菌,并揭示了它们的代谢机制,其中乳酸菌20-92是一种可以将大豆苷元彻底分解的肠道细菌,其通过分泌NADP(H)依赖性大豆苷元还原酶(L-DZNR)将大豆苷元还原为二氢大豆苷元,这种酶以辅酶NADH为电子供体,通过4Fe-4S簇电子传递链传递电子发挥还原作用[57]。同时,乳酸菌20-92还可以分泌二氢大豆苷元还原酶(L-DHDR)和四氢大豆苷元还原酶(L-THDR),这2种酶分别由orf-US2和orf-US3基因编码,L-DHDR需要在NADPH存在的条件下将二氢大豆苷元还原为四氢大豆苷元,L-THDR将四氢大豆苷元还原为雌马酚时则不需要NADPH存在[56]。
3.2 SIF对肠道微生物的影响肠道微生物对SIF的分解可以使SIF更好的发挥作用,同时SIF也可以通过刺激或改善肠道微生物的结构来发挥生物学效应,SIF可直接或间接改变人肠道关键细菌群落的数量或相对比例来维持肠道内的微生物平衡[63]。研究表明,雌马酚可抑制普拉梭菌(Faecalibacterium prausnitzii)、乳酸乳球菌乳酸亚种(Lactococcus lactis subsp. lactis)和脆弱拟杆菌(Bacteroides fragilis)等肠道细菌,并且染料木素对它们的作用更加强烈,同时黏质链球菌、鼠李糖乳杆菌和黏质乳杆菌在异黄酮的存在下繁殖速度加快,主要原因是细菌可将SIF代谢产生的葡萄糖作为自身生长所需的能量来源[63]。SIF的所有成分中染料木素的抗菌活性是最强的,可以通过抑制细菌的拓扑异构酶的活性来抑制细菌DNA复制,从而起到杀灭有害细菌的作用[64]。同时,SIF对肠道微生物的作用还可增加雌马酚的产量以及预防骨质疏松,将SIF和抗性淀粉(RS)饲喂给去卵巢的小鼠,发现SIF使双歧杆菌的数量增加,双歧杆菌为雌马酚生成菌,这促进了雌马酚的合成,雌马酚通过降低骨髓中炎症相关基因的表达抑制卵巢切除诱导的大鼠骨质流失,说明SIF和抗性淀粉可调节肠道微生物群,通过免疫系统调节骨骼健康[65]。SIF不仅对肠道微生物具有一定的调节作用,其对牛瘤胃微生物也具有调节作用,在奶牛基础饲粮中添加12.5 g的40%的SIF,每天饲喂2次,奶牛瘤胃中的拟杆菌门、变形菌门、厚壁菌门和扁平菌门等细菌的数量均发生变化,并使雌马酚生成菌的数量增多,进而使奶牛乳汁中含有更多的雌马酚[66]。总之,SIF对肠道微生物的调节主要通过促进或抑制某些肠道微生物的生长,一些肠道微生物(如乳酸杆菌、双歧杆菌)可以对SIF进行初步分解,同时也可将代谢产生的苷元用于自身生长使菌群数量增加,实现SIF与肠道微生物之间的相互促进[67]。SIF中主要是染料木素发挥了抑菌作用,对于肠道中的一些机会致病菌,染料木素可以通过抑制细菌的拓扑异构酶Ⅳ使细菌的DNA复制受到抑制,起到抑制有害菌生长的作用,发挥对肠道微生物的调节作用[68]。
4 SIF调控肠道功能 4.1 SIF对肠道运动的影响肠道运动依赖于平滑肌细胞、Cajal间质细胞(ICC)和肠神经元间的协同作用,三者形成的网络对调节肠道运动起到重要作用[69]。ICC是肠道肌肉内的漫波活动和收缩的关键细胞,存在于小肠起搏区,通常与静脉曲张的神经末梢紧密相连产生漫波电位,通过缝隙连接将漫波电位传递给平滑肌细胞产生兴奋-收缩耦联来发挥作用[70]。早期研究发现,染料木素可干扰平滑肌的胆碱能激活,芹菜素和染料木素干扰肌肉兴奋或兴奋-收缩耦联来发挥抑制作用[71],但其具体机制尚未见报道。随着研究的深入,人们发现丝裂原活化蛋白(MAP)和酪氨酸激酶可参与结肠ICC起搏电位的产生,通过通道蛋白的直接磷酸化、细胞内第二信使的间接改变或其他蛋白激酶的反式激活来调节HCN通道(一种非选择性阳离子通道,维持或决定自律细胞的细胞兴奋性),染料木素则通过抑制HCN通道发挥其抑制功能[72]。此外,染料木素使胃舒张的作用在很大程度上受分子结构的影响,其基础结构为苯并-γ-吡喃酮,具有松弛剂的功能,如果苯并-γ-吡喃酮的C-3上存在羟基,C-2、C-3之间的双键的饱和度降低或C-4羰基缺乏发生糖基化,胃的舒张功能则会逐渐减弱[73]。Lord等[74]发现,染料木素通过抑制钙离子的吸收降低结肠起搏器活性,同时染料木素还具有润肠通便的作用,其对雌性小鼠的作用显著,可增加囊性纤维病雌性小鼠的存活率。总之,SIF中主要是染料木素发挥对肠道运动的抑制作用,染料木素可通过增加细胞膜上的K+通道的通透性加速K+的外流,同时阻止Ca2+内流,使肠道平滑肌收缩受到抑制[75]。染料木素还可以抑制HCN通道来抑制结肠ICC起搏电位的产生,并且其本身的结构就具有松弛剂的功能,促使其更好地发挥对肠道运动的抑制作用。
4.2 SIF对肠道屏障的影响肠道屏障指的是相邻的肠上皮细胞形成紧密连接调节细胞旁交通,又通过跨膜蛋白的细胞外延伸部分相互连接,使紧密连接与相邻肠细胞的细胞骨架完全整合形成肠道屏障[76]。肠道屏障功能受损与某些病理学和衰老有关,可能是细菌感染、肝脏炎症、食物中毒和自动免疫失调等原因造成的[77]。一些细菌侵入动物机体后分泌脂多糖(LPS)与Toll样受体4(TLR4),两者结合上调TLR4-髓样分化因子88(MyD88)-白细胞介素-1受体相关激酶(IRAK)信号通路的表达,通过肿瘤坏死因子受体相关因子-6激活核因子-κB(NF-κB)信号通路,诱导白细胞介素-1(IL-1)、白细胞介素-8(IL-8)等炎性因子分泌,引发一系列炎症反应和氧化应激,从而破坏紧密连接使肠道屏障受损[78]。研究表明,染料木素对TLR4具有拮抗作用,可抑制LPS诱导的TLR4和MyD88的表达,通过抑制NF-κB的激活来发挥其抗炎作用,从而保护肠道屏障[79]。SIF也可通过抑制p38 MAP激酶(p38)的磷酸化及TLR4与LPS的结合,削弱LPS诱导的炎症的发生,增强断奶仔猪的生长性能和肠道屏障功能[80]。此外,染料木素具有较强的杀菌作用,可阻止细菌对肠道屏障进行破坏,其可诱导CD4+T细胞分泌白细胞介素-17(IL-17)减轻炎症反应,抑制肠紧密连接蛋白的酪氨酸磷酸化,缓解氧化应激和炎症因子诱导的肠屏障功能障碍[81]。程东静[82]研究表明,SIF可通过增加高脂肥胖大鼠结肠中杯状细胞和淋巴细胞的数量来增强肠道的免疫功能,抑制肿瘤坏死因子-α(TNF-α)、白细胞介素-4(IL-4)、白细胞介素-6(IL-6)等促炎因子的表达及NF-κB的激活,促进抗炎因子白细胞介素-10(IL-10)的表达,增强超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)等抗氧化酶的活性提升抗氧化能力,改善肠道菌群,从而使肥胖大鼠体重减轻,肠道屏障功能得到改善。磷酸肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(PI3K/Akt/mTOR)信号通路、5-AMP活化蛋白激酶(AMPK)和雌激素受体β(ERβ)可促进肠道紧密连接的形成,而激活丝裂原活化蛋白激酶(MAPK)、c-Jun N末端激酶(JNK)、p38的炎症环境往往会促进肠道紧密连接的分解[77]。而SIF可抑制MAPK和p38的产生来保护肠道紧密连接,SIF还可通过降低TNF-α激发的小肠隐窝上皮细胞(IEC-6)中白细胞介素-1β(IL-1β)和IL-6及其他促炎因子基因的表达,增强肠道屏障功能[83]。
5 小结与展望随着黄酮类活性物质对动物代谢调控和改善肠道健康作用的逐步确认,SIF及其代谢物在动物健康调控方面发挥的积极作用受到了国内外学者的广泛关注。目前,肠道微生物对SIF进行复杂代谢转化过程时的作用条件、转化时的代谢通路以及具体代谢作用机理等虽然已有报道,但其研究尚浅。因此,阐明SIF在动物体内的代谢规律,明确SIF在肠道微生物作用下的代谢通路,解决SIF与肠道微生物的协同、拮抗关系,寻找调控作用中的关键因子将有利于推动动物健康养殖的核心发展理念,促进SIF在畜牧业中的开发与应用。
| [1] |
陈嘉序, 陈如扬, 连媛, 等. 大豆异黄酮的生物转化及功能活性研究进展[J]. 食品研究与开发, 2021, 42(9): 176-182. CHEN J X, CHEN R Y, LIAN Y, et al. Progress in microbial conversion and functional activity of soy isoflavones[J]. Food Research and Development, 2021, 42(9): 176-182 (in Chinese). |
| [2] |
付雪梅, 王之盛, 代天飞. 大豆异黄酮的营养生理功能及其在动物生产中的应用[J]. 畜禽业, 2006(18): 10-13. FU X M, WANG Z S, DAI T F. Nutritional and physiological functions of soybean isoflavone and its application in animal production[J]. Livestock and Poultry Industry, 2006(18): 10-13 (in Chinese). DOI:10.3969/j.issn.1008-0414.2006.18.003 |
| [3] |
任维利. 大豆异黄酮的作用研究进展[J]. 食品安全导刊, 2021(14): 74-75. REN W L. Research progress on the effect of soybean isoflavone[J]. China Food Safety Magazine, 2021(14): 74-75 (in Chinese). |
| [4] |
郭静, 周凯, 蔡冬青. 大豆异黄酮对大鼠的体液免疫调节作用[J]. 中西医结合心血管病电子杂志, 2020, 8(29): 84-85. GUO J, ZHOU K, CAI D Q. Effects of soybean isoflavone on humoral immunity in rats[J]. Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine, 2020, 8(29): 84-85 (in Chinese). |
| [5] |
XIAO Y Q, ZHANG S, TONG H B, et al. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades[J]. Phytotherapy Research, 2018, 32(3): 384-394. DOI:10.1002/ptr.5966 |
| [6] |
姜淑妍. 大豆异黄酮对高温下体外培养奶牛乳腺上皮细胞增殖和抗氧化能力的影响[J]. 中国饲料, 2020(15): 29-32. JIANG S Y. Effects of isoflavones on proliferation and antioxidation of bovine mammary epithelial cells cultures in vitro under high temperature[J]. China Feed, 2020(15): 29-32 (in Chinese). |
| [7] |
TSUGAMI Y, SUZUKI N, SUZUKI T, et al. Regulatory effects of soy isoflavones and their metabolites in milk production via different ways in mice[J]. Journal of Agricultural and Food Chemistry, 2020, 68(21): 5847-5853. DOI:10.1021/acs.jafc.0c01288 |
| [8] |
LI Y P, JIANG X R, WEI Z X, et al. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs[J]. Animal, 2020, 14(11): 2262-2270. DOI:10.1017/S1751731120001123 |
| [9] |
常化松, 袁雯雯, 玄红专, 等. 黄酮类化合物吸收代谢及其对胃肠道功能影响的研究进展[J]. 食品工业科技, 2019, 40(18): 340-347. CHANG H S, YUAN W W, XUAN H Z, et al. Research progress on absorption and metabolism of flavonoids and their effects on the gastrointestinal tract function[J]. Science and Technology of Food Industry, 2019, 40(18): 340-347 (in Chinese). |
| [10] |
施洋, 候宝林, 吴胜利, 等. 植物雌激素的研究进展[J]. 亚太传统医药, 2019, 15(11): 172-176. SHI Y, HOU B L, WU S L, et al. Research advancement of phytoestrogen[J]. Asia-Pacific Traditional Medicine, 2019, 15(11): 172-176 (in Chinese). |
| [11] |
ELDRIDGE A C, KWOLEK W F. Soybean isoflavones: effect of environment and variety on composition[J]. Journal of Agricultural and Food Chemistry, 1983, 31(2): 394-396. DOI:10.1021/jf00116a052 |
| [12] |
殷丽君, 李里特, 李再贵, 等. 大豆异黄酮的研究近况与展望[J]. 食品科学, 2002, 23(4): 152-154. YIN L J, LI L D, LI Z G, et al. Development and expectation of research on soybean isoflavone[J]. Food Science, 2002, 23(4): 152-154 (in Chinese). DOI:10.3321/j.issn:1002-6630.2002.04.040 |
| [13] |
王继峰. 大豆异黄酮的保健功效及高异黄酮大豆的遗传育种研究[J]. 黑龙江科学, 2019, 10(14): 42-43. WANG J F. Study on health function of soybean isoflavones and genetics and breeding of soybean with high isoflavones[J]. Heilongjiang Science, 2019, 10(14): 42-43 (in Chinese). DOI:10.3969/j.issn.1674-8646.2019.14.014 |
| [14] |
黄金昌. 大豆异黄酮对种鸭生理机能调控的研究进展[J]. 湖南饲料, 2015(5): 20-23. HUANG J C. Research progress on physiological function regulation of soybean isoflavones in breeding ducks[J]. Hunan Feed, 2015(5): 20-23 (in Chinese). DOI:10.3969/j.issn.1673-7539.2015.05.010 |
| [15] |
石群, 李波. 大豆异黄酮研究进展及前景展望[J]. 大豆科技, 2018(5): 37-39. SHI Q, LI B. Research progress and prospect of soybean isoflavones[J]. Soybean Science & Technology, 2018(5): 37-39 (in Chinese). DOI:10.3969/j.issn.1674-3547.2018.05.011 |
| [16] |
林执绚. 超高压提取技术对葛根异黄酮提取率的影响[J]. 化学工程与装备, 2009(2): 36-37. LIN Z X. Effects of ultra-high pressure extraction technology on extraction rate of isoflavone from Pueraria root[J]. Fujian Chemical Industry, 2009(2): 36-37 (in Chinese). |
| [17] |
潘利华, 罗建平. 大豆异黄酮超临界流体萃取工艺与动力学模型[J]. 农业机械学报, 2010, 41(6): 137-141. PAN L H, LUO J P. Process and kinetics of supercritical fluid extraction for soybean isoflavones[J]. Transactions of the Chinese Society for Agricultural Machinery, 2010, 41(6): 137-141 (in Chinese). DOI:10.3969/j.issn.1000-1298.2010.06.027 |
| [18] |
阮洪生, 毛越, 王海燕, 等. 豆粕中大豆异黄酮的醇提工艺[J]. 粮油食品科技, 2007, 15(3): 35-36. RUAN H S, MAO Y, WANG H Y, et al. The study on alcohol extraction of soybean isoflavones from soybean residue[J]. Science and Technology of Cereals, Oils and Foods, 2007, 15(3): 35-36 (in Chinese). DOI:10.3969/j.issn.1007-7561.2007.03.014 |
| [19] |
郭婕, 刘中华, 袁淑培, 等. 黑豆中大豆异黄酮微波提取工艺的优化[J]. 食品工业科技, 2015, 36(5): 255-257, 305. GUO J, LIU Z H, YUAN S P, et al. Optimization of microwave-assisted extraction technology of soybean isoflavones from black soybean[J]. Science and Technology of Food Industry, 2015, 36(5): 255-257, 305 (in Chinese). |
| [20] |
陈燕. 黑豆中提取大豆异黄酮研究[J]. 当代化工, 2019, 48(1): 74-76, 91. CHEN Y. Extraction of soybean isoflavones from black soybeans[J]. Contemporary Chemical Industry, 2019, 48(1): 74-76, 91 (in Chinese). |
| [21] |
王丹. 脱脂豆粕中大豆异黄酮提取工艺的研究[J]. 食品研究与开发, 2017, 38(6): 52-55. WANG D. Study on the extraction technology of isoflavones from defatted soybean meal[J]. Food Research and Development, 2017, 38(6): 52-55 (in Chinese). DOI:10.3969/j.issn.1005-6521.2017.06.012 |
| [22] |
张月洁, 兰韬, 初侨, 等. 大豆异黄酮的制备技术与功能活性进展研究[J]. 食品安全质量检测学报, 2020, 11(17): 5964-5970. ZHANG Y J, LAN T, CHU Q, et al. Progress in preparation technology and functional activity of soybean isoflavones[J]. Journal of Food Safety & Quality, 2020, 11(17): 5964-5970 (in Chinese). |
| [23] |
王双侠, 苏适, 柴宝丽. 黑豆中大豆异黄酮超声波法提取工艺优化[J]. 齐齐哈尔大学学报(自然科学版), 2021, 37(2): 70-72. WANG S X, SU S, CHAI B L. Optimization of ultrasonic extraction on soy isoflavones from back beans[J]. Journal of Qiqihar University (Natural Science Edition), 2021, 37(2): 70-72 (in Chinese). DOI:10.3969/j.issn.1007-984X.2021.02.014 |
| [24] |
周文红, 郭咪咪, 李秀娟, 等. 大豆异黄酮提取及其生物转化的研究进展[J]. 粮油食品科技, 2019, 27(5): 37-42. ZHOU W H, GUO M M, LI X J, et al. Research progress of extraction and biotransformation of soybean isoflavones[J]. Science and Technology of Cereals, Oils and Foods, 2019, 27(5): 37-42 (in Chinese). |
| [25] |
SOUKUP S T, HELPPI J, MVLLER D R, et al. Phase Ⅱ metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: a cross-species and sex comparison[J]. Archives of Toxicology, 2016, 90(6): 1335-1347. DOI:10.1007/s00204-016-1663-5 |
| [26] |
KULLING S E, LEHMANN L, METZLER M. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2002, 777(1/2): 211-218. |
| [27] |
FRANKE A A, LAI J F, HALM B M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake[J]. Archives of Biochemistry and Biophysics, 2014, 559: 24-28. DOI:10.1016/j.abb.2014.06.007 |
| [28] |
SÁNCHEZ-CALVO J M, RODRÍGUEZ-IGLESIAS M A, MOLINILLO J M, et al. Soy isoflavones and their relationship with microflora: beneficial effects on human health in equol producers[J]. Phytochemistry Reviews, 2013, 12(4): 979-1000. DOI:10.1007/s11101-013-9329-x |
| [29] |
LIU Y, HU M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perused rat intestinal model[J]. Drug Metabolism and Disposition, 2002, 30(4): 370-377. DOI:10.1124/dmd.30.4.370 |
| [30] |
WALSH K R, HAAK S J, BOHN T, et al. Isoflavonoid glucosides are deconjugated and absorbed in the small intestine of human subjects with ileostomies[J]. American Journal of Clinical Nutrition, 2007, 85(4): 1050-1056. DOI:10.1093/ajcn/85.4.1050 |
| [31] |
DAY A J, DUPONT M S, RIDLEY S, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity[J]. FEBS Letters, 1998, 436(1): 71-75. DOI:10.1016/S0014-5793(98)01101-6 |
| [32] |
ISLAM M A, PUNT A, SPENKELINK B, et al. Conversion of major soy isoflavone glucosides and aglycones in in vitro intestinal models[J]. Molecular Nutrition & Food Research, 2014, 58(3): 503-515. |
| [33] |
BARNES S, PRASAIN J, D'ALESSANDRO T, et al. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems[J]. Food & Function, 2011, 2(5): 235-244. |
| [34] |
TURNER N J, THOMSON B M, SHAW I C. Bioactive isoflavones in functional foods: the importance of gut microflora on bioavailability[J]. Nutrition Reviews, 2003, 61(6): 204-213. DOI:10.1301/nr.2003.jun.204-213 |
| [35] |
VITALE D C, PIAZZA C, MELILLI B, et al. Isoflavones: estrogenic activity, biological effect and bioavailability[J]. European Journal of Drug Metabolism and Pharmacokinetics, 2013, 38(1): 15-25. DOI:10.1007/s13318-012-0112-y |
| [36] |
YUAN J P, WANG J H, LIU X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora-implications for health[J]. Molecular Nutrition & Food Research, 2007, 51(7): 765-781. |
| [37] |
DAY A J, CAÑADA F J, DÍAZ J C, et al. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase[J]. FEBS Letters, 2000, 468(2/3): 166-170. |
| [38] |
XU X, WANG H J, MURPHY P A, et al. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women[J]. The Journal of Nutrition, 1994, 124(6): 825-832. DOI:10.1093/jn/124.6.825 |
| [39] |
SETCHELL K D, BROWN N M, DESAI P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements[J]. The Journal of Nutrition, 2001, 131(Suppl.4): 1362S-1375S. |
| [40] |
XU X, HARRIS K S, WANG H J, et al. Bioavailability of soybean isoflavones depends upon gut microflora in women[J]. The Journal of Nutrition, 1995, 125(9): 2307-2315. DOI:10.1093/jn/125.9.2307 |
| [41] |
LARKIN T, PRICE W E, ASTHEIMER L. The key importance of soy isoflavone bioavailability to understanding health benefits[J]. Critical Reviews in Food Science and Nutrition, 2008, 48(6): 538-552. DOI:10.1080/10408390701542716 |
| [42] |
RAFII F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol[J]. Metabolites, 2015, 5(1): 56-73. DOI:10.3390/metabo5010056 |
| [43] |
PABICH M, MATERSKA M. Biological effect of soy isoflavones in the prevention of civilization diseases[J]. Nutrients, 2019, 11(7): 1660. DOI:10.3390/nu11071660 |
| [44] |
HVSER S, GUTH S, JOOST H G, et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: a comprehensive safety evaluation[J]. Archives of Toxicology, 2018, 92(9): 2703-2748. DOI:10.1007/s00204-018-2279-8 |
| [45] |
YUAN B, WANG L L, JIN Y, et al. Role of metabolism in the effects of genistein and its phase Ⅱ conjugates on the growth of human breast cell lines[J]. The AAPS Journal, 2012, 14(2): 329-344. DOI:10.1208/s12248-012-9338-5 |
| [46] |
TORO-FUNES N, MORALES-GUTIÉRREZ F J, VECIANA-NOGUÉS M T, et al. The intracellular metabolism of isoflavones in endothelial cells[J]. Food & Function, 2015, 6(1): 98-108. |
| [47] |
KULLING S E, HONIG D M, METZLER M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo[J]. Journal of Agricultural and Food Chemistry, 2001, 49(6): 3024-3033. DOI:10.1021/jf0012695 |
| [48] |
ROWLAND I, FAUGHNAN M, HOEY L, et al. Bioavailability of phyto-oestrogens[J]. British Journal of Nutrition, 2003, 89(Suppl.1): S45-S58. |
| [49] |
YASUDA T, KANO Y, SAITO K, et al. Urinary and biliary metabolites of daidzin and daidzein in rats[J]. Biological & Pharmaceutical Bulletin, 1994, 17(10): 1369-1374. |
| [50] |
黄娜, 尤春玲. 大豆异黄酮代谢产物——雌马酚的功能作用研究[J]. 农产品加工学刊, 2008(3): 50-53. HUANG N, YOU C L. Progress in research of equol: a metabolite of the isoflavone daidzein[J]. Academic Periodical of Farm Products Processing, 2008(3): 50-53 (in Chinese). |
| [51] |
ATKINSON C, FRANKENFELD C L, LAMPE J W. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health[J]. Experimental Biology and Medicine, 2005, 230(3): 155-170. DOI:10.1177/153537020523000302 |
| [52] |
KULLING S E, HONIG D M, SIMAT T J, et al. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein[J]. Journal of Agricultural and Food Chemistry, 2000, 48(10): 4963-4972. DOI:10.1021/jf000524i |
| [53] |
葛蔚, 张忠远, 刘树欣. 饲料添加剂大豆异黄酮研究[J]. 中国饲料, 2004(7): 24-25, 28. GE W, ZHANG Z Y, LIU S X. Study on soybean isoflavone as feed additive[J]. China Feed, 2004(7): 24-25, 28 (in Chinese). DOI:10.3969/j.issn.1004-3314.2004.07.012 |
| [54] |
MAYO B, VÁZQUEZ L, FLÓREZ A B. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects[J]. Nutrients, 2019, 11(9): 2231. DOI:10.3390/nu11092231 |
| [55] |
TAMURA M, TSUSHIDA T, SHINOHARA K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces[J]. Anaerobe, 2007, 13(1): 32-35. DOI:10.1016/j.anaerobe.2006.10.001 |
| [56] |
SHIMADA Y, TAKAHASHI M, MIYAZAWA N, et al. Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20-92[J]. Journal of Molecular Microbiology and Biotechnology, 2011, 21(3/4): 160-172. |
| [57] |
SHIMADA Y, YASUDA S, TAKAHASHI M, et al. Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20-92[J]. Applied and Environmental Microbiology, 2010, 76(17): 5892-5901. DOI:10.1128/AEM.01101-10 |
| [58] |
SCHOEFER L, MOHAN R, BRAUNE A, et al. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus[J]. FEMS Microbiology Letters, 2002, 208(2): 197-202. DOI:10.1111/j.1574-6968.2002.tb11081.x |
| [59] |
GAYA P, PEIROTÉN Á, MEDINA M, et al. Bifidobacterium adolescentis INIA P784:the first probiotic bacterium capable of producing enterodiol from lignan extracts[J]. Journal of Functional Foods, 2017, 29: 269-274. DOI:10.1016/j.jff.2016.12.044 |
| [60] |
ELGHALI S, MUSTAFA S, AMID M, et al. Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536[J]. Journal of Functional Foods, 2012, 4(4): 736-745. DOI:10.1016/j.jff.2012.04.013 |
| [61] |
MATTHIES A, BLAUT M, BRAUNE A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion[J]. Applied and Environmental Microbiology, 2009, 75(6): 1740-1744. DOI:10.1128/AEM.01795-08 |
| [62] |
YURIST-DOUTSCH S, ARRIETA M C, VOGT S L, et al. Gastrointestinal microbiota-mediated control of enteric pathogens[J]. Annual Review of Genetics, 2014, 48: 361-382. DOI:10.1146/annurev-genet-120213-092421 |
| [63] |
VÁZQUEZ L, FLÓREZ A B, GUADAMURO L, et al. Effect of soy isoflavones on growth of representative bacterial species from the human gut[J]. Nutrients, 2017, 9(7): 727. DOI:10.3390/nu9070727 |
| [64] |
王海涛. 大豆异黄酮的抑菌活性及其机制的研究[D]. 硕士学位论文. 大连: 辽宁师范大学, 2009. WANG H T. Anti-microoganism activity and anti-microbial mechanism of soybean isoflavone[D]. Master's Thesis. Dalian: Liaoning Normal University, 2009. (in Chinese) |
| [65] |
TOUSEN Y, MATSUMOTO Y, MATSUMOTO C, et al. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice[J]. British Journal of Nutrition, 2016, 116(2): 247-257. DOI:10.1017/S0007114516001537 |
| [66] |
KASPAROVSKA J, PECINKOVA M, DADAKOVA K, et al. Effects of isoflavone-enriched feed on the rumen microbiota in dairy cows[J]. PLoS One, 2016, 11(4): e0154642. DOI:10.1371/journal.pone.0154642 |
| [67] |
GAYA P, PEIROTÉN Á, MEDINA M, et al. Isoflavone metabolism by a collection of lactic acid bacteria and Bifidobacteria with biotechnological interest[J]. International Journal of Food Sciences and Nutrition, 2016, 67(2): 117-124. DOI:10.3109/09637486.2016.1144724 |
| [68] |
HONG H, LANDAUER M R, FORISKA M A, et al. Antibacterial activity of the soy isoflavone genistein[J]. Journal of Basic Microbiology, 2006, 46(4): 329-335. DOI:10.1002/jobm.200510073 |
| [69] |
王亚梅, 贾漪涛, 李中信. 肠神经胶质细胞在肠道功能与肠道疾病中的作用[J]. 世界华人消化杂志, 2020, 28(19): 979-985. WANG Y M, JIA Y T, LI Z X. Role of enteric glial cells in intestinal function and intestinal diseases[J]. World Chinese Journal of Digestology, 2020, 28(19): 979-985 (in Chinese). |
| [70] |
FOONG D, ZHOU J, ZARROUK A, et al. Understanding the biology of human interstitial cells of Cajal in gastrointestinal motility[J]. International Journal of Molecular Sciences, 2020, 21(12): 4540. DOI:10.3390/ijms21124540 |
| [71] |
GHARZOULI K, HOLZER P. Inhibition of guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein[J]. Pharmacology, 2004, 70(1): 5-14. DOI:10.1159/000074237 |
| [72] |
SHIN D H, LEE M J, JIAO H Y, et al. Regulatory roles of endogenous mitogen-activated protein kinases and tyrosine kinases in the pacemaker activity of colonic interstitial cells of Cajal[J]. Pharmacology, 2015, 96(1/2): 16-24. |
| [73] |
AMIRA S, ROTONDO A, MULō F. Relaxant effects of flavonoids on the mouse isolated stomach: structure-activity relationships[J]. European Journal of Pharmacology, 2008, 599(1/3): 126-130. |
| [74] |
LORD R, FAIRBOURN N, MYLAVARAPU C, et al. Consuming genistein improves survival rates in the absence of laxative in ΔF508-CF female mice[J]. Nutrients, 2018, 10(10): 1418. DOI:10.3390/nu10101418 |
| [75] |
SANTOS-FAGUNDES D, GRASA L, GONZALO S, et al. Different mechanisms of actions of genistein, quercetin on spontaneous contractions of rabbit duodenum[J]. Revista Espanola de Enfermedades Digestivas, 2015, 107(7): 413-416. |
| [76] |
SUZUKI T. Regulation of the intestinal barrier by nutrients: the role of tight junctions[J]. Animal Science Journal, 2020, 91(1): e13357. |
| [77] |
MCCARTY M F, LERNER A. Perspective: prospects for nutraceutical support of intestinal barrier function[J]. Advances in Nutrition, 2021, 12(2): 316-324. DOI:10.1093/advances/nmaa139 |
| [78] |
LV Z P, DAI H J, WEI Q W, et al. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile[J]. Poultry Science, 2020, 99(7): 3411-3427. DOI:10.1016/j.psj.2020.03.020 |
| [79] |
JEONG J W, LEE H H, HAN M H, et al. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia[J]. Chemico-Biological Interactions, 2014, 212: 30-39. DOI:10.1016/j.cbi.2014.01.012 |
| [80] |
CHACKO B K, CHANDLER R T, MUNDHEKAR A, et al. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: modulation of leukocyte-endothelial cell interactions[J]. American Journal of Physiology: Heart and Circulatory Physiology, 2005, 289(2): H908-H915. DOI:10.1152/ajpheart.00781.2004 |
| [81] |
ZHU C, WU Y P, JIANG Z Y, et al. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide[J]. International Immunopharmacology, 2015, 28(1): 288-294. DOI:10.1016/j.intimp.2015.04.054 |
| [82] |
程东静. 大豆异黄酮对高脂肥胖大鼠结肠屏障功能的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2019. CHENG D J. Improvement of colonic barrier function with soy isoflavones in high-fat diet induced obese in rats[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2019. (in Chinese) |
| [83] |
LI S S, ZHENG M, ZHANG Z T, et al. Galli gigeriae endothelium corneum: its intestinal barrier protective activity in vitro and chemical composition[J]. Chinese Medicine, 2021, 16(1): 22. DOI:10.1186/s13020-021-00432-3 |