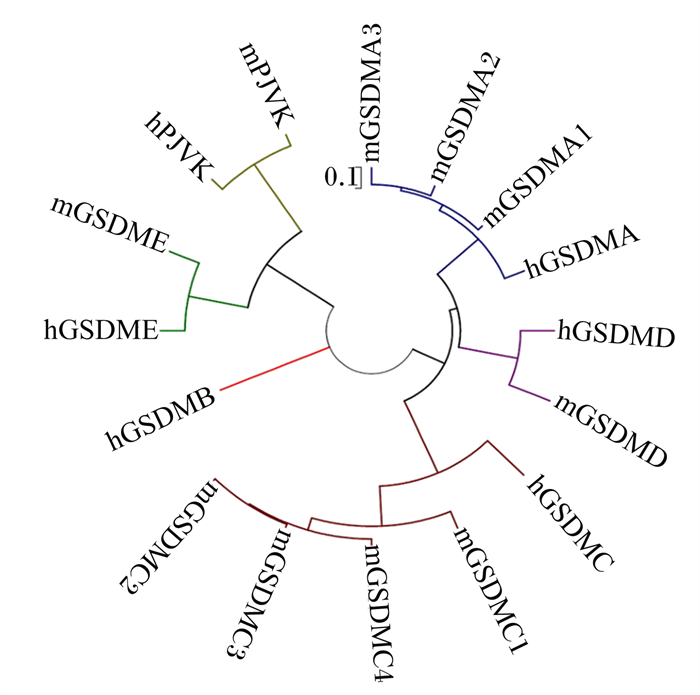
gasdermin(GSDM)家族是近年来发现的成孔效应蛋白家族,GSDM家族由gasdermin A(GSDMA)、gasdermin B(GSDMB)、gasdermin C(GSDMC)、gasdermin D(GSDMD)、gasdermin E(GSDME,也称DFNA5)和pejvakin(PJVK,也称DFNB59)组成[1-3](图 1)。GSDM家族成员在不同组织细胞中表达不同,除PJVK外,其余GSDM家族均含有1个N端结构域和1个C端结构域,当GSDM的C端结构域被水解切割后,GSDM的N端结构域便能插入细胞膜并形成大的孔隙,从而影响离子稳态并诱导细胞死亡[1, 4-6]。
|
人类编码6种GSDM蛋白,分别为hGSDMA、hGSDMB、hGSDMC、hGSDMD、hGSDME和hPJVK;小鼠编码10种GSDM蛋白,分别为mGSDMA1、mGSDMA2、mGSDMA3、mGSDMC1、mGSDMC2、mGSDMC3、mGSDMC4、mGSDMD、mGSDME和mPJVK。 Humans encoded 6 GSDM proteins, which were hGSDMA, hGSDMB, hGSDMC, hGSDMD, hGSDME and hPJVK, respectively; mice encoded 10 GSDM proteins, which were mGSDMA1, mGSDMA2, mGSDMA3, mGSDMC1, mGSDMC2, mGSDMC3, mGSDMC4, mGSDMD, mGSDME and mPJVK, respectively. 图 1 GSDM家族基因 Fig. 1 GSDM family genes[3] |
细胞焦亡是GSDM家族介导的细胞程序性死亡[7-8],细胞焦亡的显著特征是细胞肿胀、细胞膜穿孔、细胞破裂、细胞促炎因子白细胞介素-1β(IL-1β)和白细胞介素-18(IL-18)等的释放[1, 9-11]。细胞焦亡不仅在抗微生物感染中发挥重要作用,而且与非病原性感染有关,与细胞凋亡和坏死不同,细胞焦亡是一种免疫防御[12-15]。细胞焦亡发生过程中,半胱天冬酶(Caspase)蛋白首先被激活,活性的Caspase切割GSDM家族成员,从而使GSDM的N端结构域插入细胞膜并形成孔隙,最终诱导细胞膜破裂和细胞死亡[8, 12, 16-17]。
炎性小体是与炎症有关的多元蛋白复合物,是先天的、适应性的和调控宿主反应的关键因子。炎性小体由传感器、衔接蛋白和蛋白酶效应体构成。经典炎性小体由胞内传感器[一般是富含亮氨酸重复序列蛋白、NOD样受体(NLR)或黑色素瘤2缺失(AIM2)样受体],包括含有半胱天冬酶募集结构域(CARD)的凋亡相关斑点样蛋白(apoptosis-associated speck-like protein containing a CARD,ASC或PYCARD)和Caspase-1蛋白前体组成[13, 18-25]。非经典炎性小体是宿主细胞感知细胞质中细菌脂多糖(lipopolysaccharide,LPS)后在胞内形成的大分子蛋白复合体,胞内Caspase-4/5/11前体直接与LPS结合继而发生寡聚化后被激活[20, 26-27]。这种由炎性小体诱导的依赖Caspase-1的细胞焦亡途径被称为经典焦亡途径,而另一种依赖Caspase-4/5/11的细胞焦亡途径被称为非经典焦亡途径。
炎症性肠病(inflammatory bowel disease,IBD)在先天性和适应性免疫中由促炎分子和抗炎分子的异常表达引起,是一种慢性、易复发性和不易治愈的疾病,例如克罗恩病(Crohn’s disease,CD)和溃疡性结肠炎(ulcerative colitis,UC)等[20, 28-31]。炎症分子包括细胞因子、趋化因子、炎性小体和miRNA;肠道微生物分子如抗菌肽、神经肽等的改变均与IBD的发病机制有关[30, 32]。大量研究表明,动物炎性疾病,尤其是IBD的发生与细胞焦亡有关。在IBD中,参与调控细胞焦亡的GSDM家族蛋白在肠道中被激活,其中GSDMD、GSDMB和GSDME蛋白在调节IBD信号通路中发挥着重要作用。
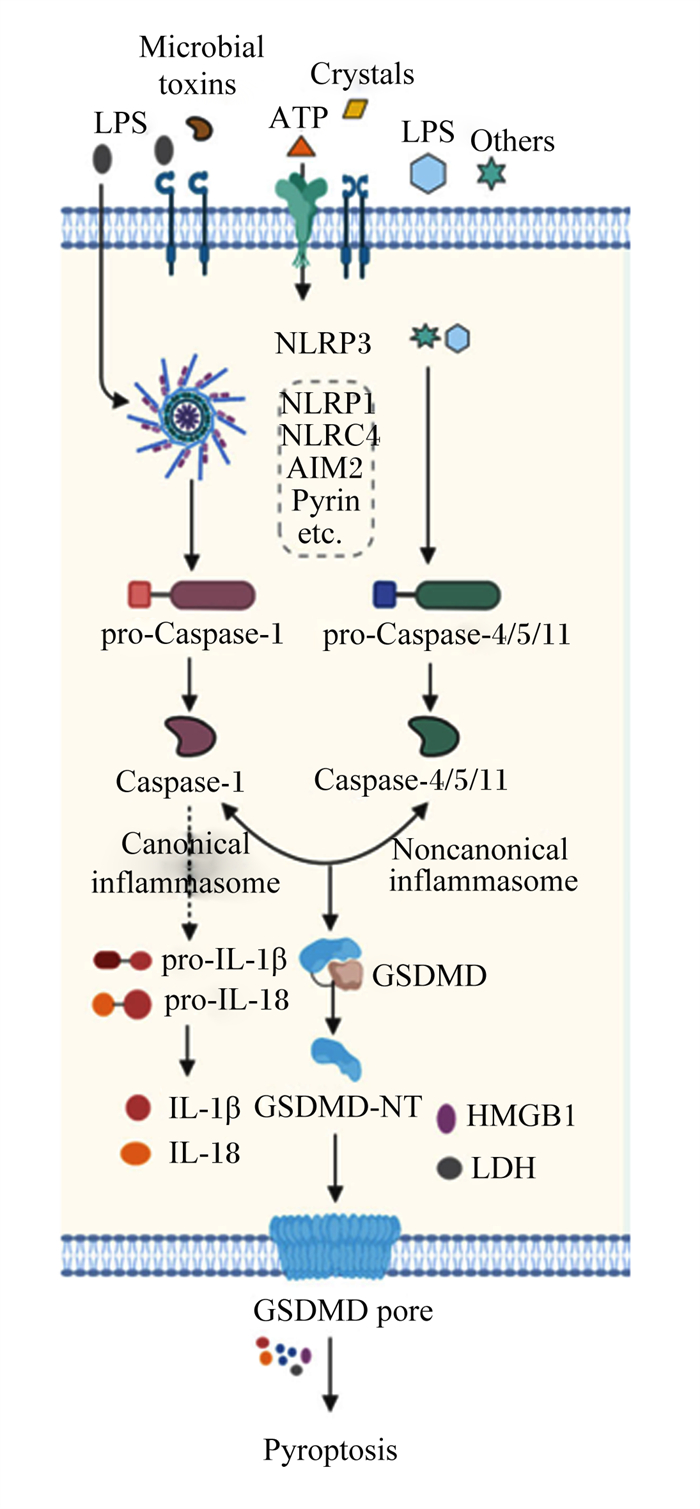
1 经典焦亡途径激活Caspase-1是经典焦亡途径的核心,经典焦亡途径是动物对抗病原微生物感染的一种防御机制,是先天性免疫系统的重要组成部分[19, 33]。目前能引起经典焦亡途径的炎性小体主要包括NLR家族pyrin结构域包含1(NLRP1)、NLR家族pyrin结构域包含3(NLRP3)、NLR家族CARD包含4(NLRC4)、AIM2以及pyrin[13, 22]。炎性小体被激活后,Caspase-1进一步被激活,一方面Caspase-1能将GSDMD切割成GSDMD C端结构域和GSDMD N端结构域,GSDMD的N端结构域能够穿透细胞膜,形成内径为10~14 nm的非选择性孔,并导致细胞肿胀或破裂,从而释放出胞内因子[33-34];另一方面Caspase-1还能切割IL-1β和IL-18的前体,形成成熟的IL-1β和IL-18,通过GSDMD形成的孔被释放,最终诱导细胞焦亡[16, 33, 35]。
2 非经典焦亡途径鼠源Caspase-11和人源Caspase-4/5可诱导非经典细胞焦亡。人源Caspase-4/5与鼠源Caspase-11是同源蛋白,功能相似[26]。革兰氏阴性菌可通过依赖Caspase-11的途径引起细胞焦亡,Caspase-11可直接识别并结合LPS,作用于GSDMD,释放活性GSDMD N端结构域,诱导细胞焦亡[21, 27]。Caspase-11还能激活泛连接蛋白-1(pannexin-1)通道,释放ATP,促进P2×7膜通道的开放,诱导细胞焦亡[36]。此外,由于细胞膜成孔,导致细胞内钾离子外流,钾离子可激活NLRP3炎性小体,进而激活下游Caspase-1,促进炎症因子IL-1β和IL-18释放[37]。在IBD中,Caspase-4/5/11可通过NLRP3-ASC-Caspase-1途径间接调控IL-1β和IL-18的分泌[8, 33]。这揭示经典与非经典炎性小体可能具有协同作用,共同调控IL-1β和IL-18的分泌。迄今为止,大量的研究也证明,在细胞焦亡调控通路中,非经典炎性通路和经典炎性通路具有同等重要作用。
3 GSDMD在细胞焦亡和IBD中的调控作用GSDMD是目前研究最多,较家族其他蛋白机制最完善的焦亡蛋白。GSDMD在经典和非经典炎性通路中均必不可少。GSDMD由8号染色体编码,由1个31 ku的N端和1个22 ku的C端通过肽链连接而成[1, 38]。GSDMD是整个GSDM家族中最重要的蛋白,也是Caspase-1/4/5/11的共同底物蛋白,其中GSDMD的C端结构域和N端结构域在细胞焦亡的调控过程中发挥着重要作用[39]。GSDMD被活性Caspase切割成GSDMD N端结构域和GSDMD C端结构域,GSDMD N端结构域具有亲脂性,可与磷脂酰肌醇磷酸盐、磷脂酰丝氨酸和心磷脂结合,使细胞膜形成大小为10~14 nm的孔,导致细胞内容物流出,诱导焦亡从而杀死细胞[39-42](图 2)。细胞在焦亡发生期间不会伤害邻近细胞,因为GSDMD的C端结构域能够抑制GSDMD N端结构域的活性,正常情况下GSDMD C端结构域与GSDMD N端结构域结合,使GSDMD处于无活性的自抑制状态[43-46]。GSDMD C端结构域以及GSDMD全长蛋白在细胞内过表达均不能引起细胞焦亡[45-46]。
|
LPS:脂多糖lipopolysaccharide;Microbial toxins:微生物毒素;Crystals:晶体;Others:其他;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;NLRP1:NOD样受体家族pyrin结构域包含1 NOD-like receptor family pyrin domain containing 1;NLRC4:NOD样受体家族半胱天冬酶募集结构域包含4 NOD-like receptor family caspase recruitment domain containing 4;AIM2:黑色素瘤2缺失absent in melanoma 2;pro-Caspase-1:半胱天冬酶-1前体precursor of cysteine-aspartic acid protease-1;pro-Caspase-4/5/11:半胱天冬酶-4/5/11前体precursor of cysteine-aspartic acid protease-4/5/11;Caspase-1:半胱天冬酶-1 cysteine-aspartic acid protease-1;Caspase-4/5/11:半胱天冬酶-4/5/11 cysteine-aspartic acid protease-4/5/11;Canonical inflammasome:经典炎性小体;Noncanonical inflammasome:非经典炎性小体;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-IL-18:白细胞介素-18前体precursor of interleukin-18;IL-1β:白细胞介素-1β interleukin-1β;IL-18:白细胞介素-18 interleukin-18;GSDMD:gasdermin D;GSDMD-NT:gasdermin D N端gasdermin D N-terminal;HMGB1:高迁移率族蛋白B1 high-mobility group box 1;LDH:乳酸脱氢酶lactate dehydrogenase;GSDMD pore:gasdermin D孔隙gasdermin D pore;Pyroptosis:细胞焦亡。 图 2 GSDMD诱导的细胞焦亡 Fig. 2 GSDMD-induced pyroptosis[7] |
当肠道受到病毒或细菌侵染时,由GSDMD介导的细胞焦亡在IBD中发挥重要作用,这种免疫模式保证了机体快速去除有害物质并促进机体修复。IBD主要由Caspase-1诱导的经典炎性焦亡途径调控。Caspase-1作用于GSDMD,介导经典细胞焦亡的发生,同时促进促炎细胞因子IL-1β和IL-18的释放[39, 47-49]。Zhu等[50]研究发现,轮状病毒感染机体引起强烈的肠炎,体内缺乏GSDMD的小鼠炎性症状减轻。因为GSDMD在肠炎性疾病中起到重要的免疫调控作用,GSDMD介导的细胞焦亡影响IL-18的分泌。Bulek等[48]、Ma等[51]和Schwarzer等[52]也证明GSDMD是调控IBD的一种重要蛋白,体内缺乏GSDMD的小鼠肠道炎症减轻,这表明对于动物肠道炎症的调控,首先应该考虑调控GSDMD蛋白的表达,降低GSDND蛋白的表达有利于减轻炎性症状,以上研究结果对于动物肠道炎症的防治具有重要的参考价值。
GSDM家族其他成员被Caspase切割为GSDM N端结构域后可调控细胞焦亡和IBD,调控机制与GSDMD相似。有证据表明,GSDMB可被Caspase-1、Caspase-3、Caspase-4、Caspase-6和Caspase-7切割,GSDMC可被Caspase-8切割,GSDME可被Caspase-3切割。GSDM家族被Caspase切割成GSDM N端结构域后,GSDM的N端结构域插入细胞膜,诱导细胞膜成孔,造成细胞焦亡(图 3)[17]。
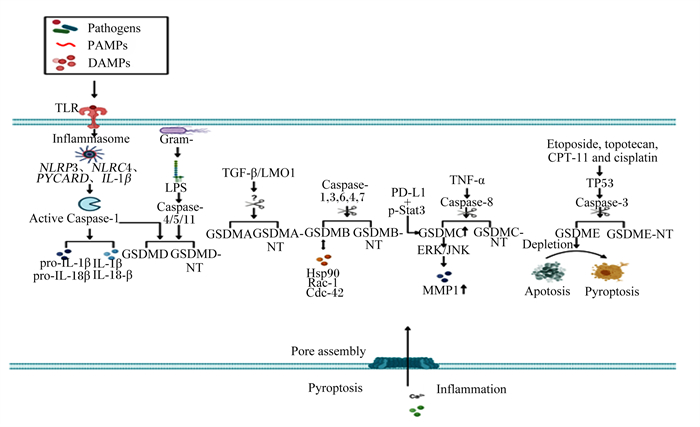
|
Pathogens:病原体;PAMPs:病原体相关的分子模式pathogen-associated molecular patterns;DAMPs:损伤相关的分子模式damage-associated molecular patterns;TLR:Toll样受体Toll-like receptor;Inflammasome:炎性小体;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;NLRC4:NOD样受体家族半胱天冬酶募集结构域包含4 NOD-like receptor family caspase recruitment domain containing 4;PYCARD:含有半胱天冬酶募集结构域的凋亡相关斑点样蛋白apoptosis-associated speck-like protein containing a caspase recruitment domain;IL-1β:白细胞介素-1β interleukin-1β;Active Caspase-1:激活的半胱天冬酶-1 active cysteine-aspartic acid protease-1;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-IL-18:白细胞介素-18前体precursor of interleukin-18;IL-1β:白细胞介素-1β interleukin-1β;IL-18:白细胞介素-18 interleukin-18;Gram-:革兰氏阴性菌Gram-negative bacteria;LPS:脂多糖lipopolysaccharide;Caspase-4/5/11:半胱天冬酶-4/5/11 cysteine-aspartic acid protease-4/5/11;GSDMD:gasdermin D;GSDMD-NT:gasdermin D N端gasdermin D N-terminal;TGF-β:转化生长因子-β transformation growth factor-β;LMO1:LIM域特有蛋白1 LIM domain only 1;GSDMA:gasdermin A;GSDMA-NT:gasdermin A N端gasdermin A N-terminal;Caspase-1, 3, 6, 4, 7:半胱天冬酶-1, 3, 6, 4, 7 cysteine-aspartic acid protease-1, 3, 6, 4, 7;GSDMB:gasdermin B;GSDMB-NT:gasdermin B N端gasdermin B N-terminal;Hsp90:热休克蛋白90 heat shock protein 90;Rac-1:Ras相关的C3肉毒杆菌毒素底物1 Ras-related C3 botulinum toxin substrate 1;Cdc-42:细胞分裂周期蛋白42 cell division cycle 42;PD-L1:程序性细胞死亡配体1 programmed cell death 1 ligand 1;p-Stat3:磷酸化信号转导及转录激活蛋白3 phosphorylated signal transducer and activator of transcription 3;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;Caspase-8:半胱天冬酶-8 cysteine-aspartic acid protease-8;GSDMC:gasdermin C;GSDMC-NT:gasdermin C N端gasdermin C N-terminal;ERK:细胞外信号调节激酶extracellular signal-regulated kinase;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinase;MMP1:基质金属蛋白酶1 matrix metallopeptidase 1;Etoposide, topotecan, CPT-11 and cisplatin:依托泊苷、拓扑替康、伊立替康和顺氯氨铂;Caspase-3:半胱天冬酶-3 cysteine-aspartic acid protease-3;GSDME:gasdermin E;GSDME-NT:gasdermin E N端gasdermin E N-terminal;Depletion:损耗;Apotosis:细胞凋亡;Pyroptosis:细胞焦亡;Pore assembly:孔隙装配;Inflammation:炎症。 图 3 GSDM家族成员的裂解 Fig. 3 Cleavage of GSDM family members[17] |
适度的细胞焦亡在机体抵抗病原体入侵中发挥重要作用,而过度的细胞焦亡将加重炎症程度,破坏机体稳态,损伤机体器官。所以,通过抑制过度焦亡可减轻炎性症状。已有研究报道,有些具有抗炎作用的天然植物成分可以抑制炎性小体的激活进而抑制细胞焦亡的发生。
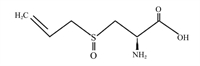
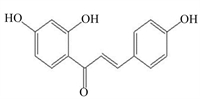
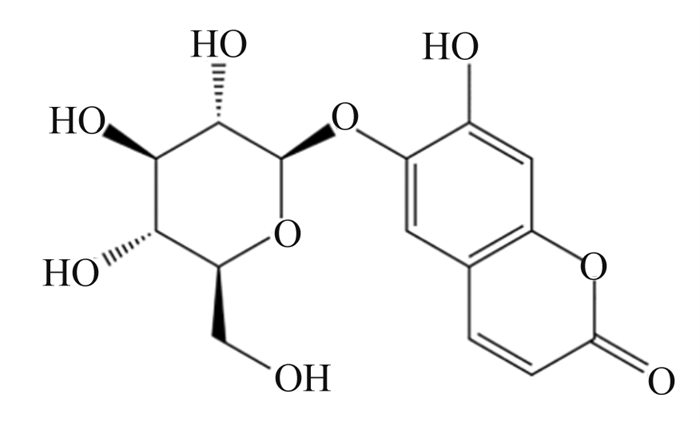
4.1 七叶苷调控GSDMD在细胞焦亡中的作用七叶苷是秦皮的主要成分,是一种具有清除超氧自由基作用和抗氧化作用的羟基香豆素葡萄糖苷(图 4)[53]。Xu等[54]首先构建了大鼠心肌缺血再灌注损伤(MIRI)模型(该模型的主要特征是心肌细胞死亡、氧化损伤、线粒体功能障碍和异常的免疫炎症反应),再向模型中加入3和10 μmol/L的七叶苷,结果发现,七叶苷促进蛋白激酶B(Akt)/糖原合成酶激酶3β(GSK3β)磷酸化,抑制核转录因子-κB(NF-κB)磷酸化,显著降低NLRP3、Caspase-1、GSDMD和IL-1β的表达水平,抑制细胞焦亡,减轻炎性症状(图 5)。
|
图 4 七叶苷化学结构 Fig. 4 Chemical structure of aesculin[53] |
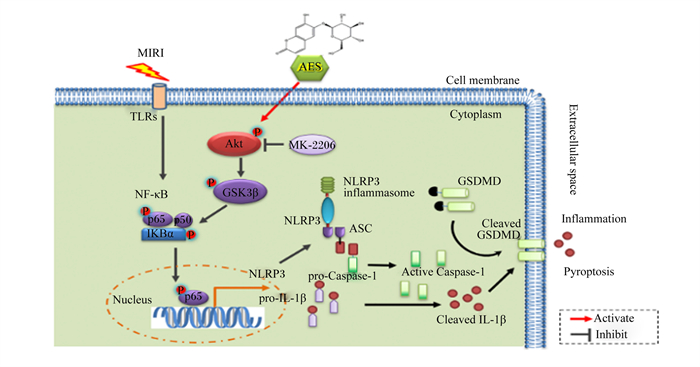
|
MIRI:心肌缺血再灌注损伤myocardial ischemia reperfusion injury;AES:七叶苷aesculin;TLRs:Toll样受体Toll-like receptors;NF-κB:核转录因子-κB nuclear factor-kappa B;IKBα:核转录因子-κB抑制蛋白α nuclear factor-kappa B inhibitor alpha;Akt:蛋白激酶B protein kinase B;MK-2206:一种Akt抑制剂an Akt inhibitor;GSK3β:糖原合成酶激酶3β glycogen synthase kinase-3 beta;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;NLRP3 inflammasome:NLRP3炎性小体;ASC:含有半胱天冬酶募集结构域的凋亡相关斑点样蛋白apoptosis-associated speck-like protein containing a caspase recruitment domain;Nucleus:细胞核;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-Caspase-1:半胱天冬酶-1前体precursor of cysteine-aspartic acid protease-1;Active Caspase-1:激活的半胱天冬酶-1 active cysteine-aspartic acid protease-1;Cell membrane:细胞膜;Cytoplasm:细胞质;Extracellular space:细胞外间隙;GSDMD:gasdermin D;Cleaved GSDMD:裂解GSDMD;Cleaved IL-1β:裂解IL-1β;Inflammation:炎症;Pyroptosis:细胞焦亡;Activate:激活;Inhibit:抑制。 图 5 七叶苷通过调控NLRP3/GSDMD途径抑制细胞焦亡 Fig. 5 Aesculin suppressed pyroptosis by regulating NLRP3/GSDMD pathway[54] |
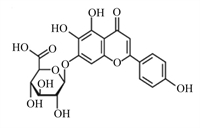
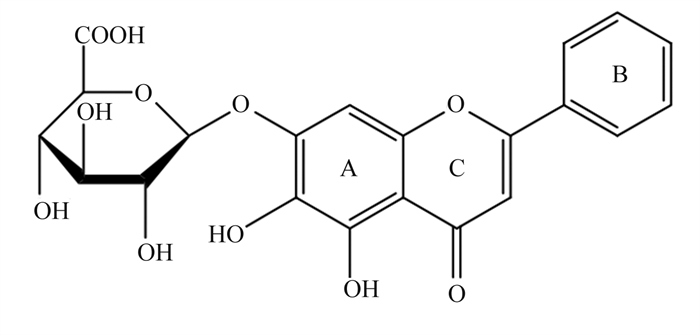
黄芩苷是一种黄酮类化合物(图 6)[55],是中草药黄芩的有效成分,具有显著的抗炎、抗菌和清除自由基的作用。Shi等[56]研究发现,25、50和100 μg/mL黄芩苷可通过抑制NF-κB信号转导和活性氧(ROS)的产生(图 7),从而抑制仔猪单核巨噬细胞NLRP3的活性,下调Caspase-1的表达,阻断GSDMD的激活,阻止IL-1β、IL-18和肿瘤坏死因子-α(TNF-α)等的释放,进而减轻细胞焦亡。Caspase-1的激活依赖NLPR3炎性小体的激活,而GSDM N端结构的表达需要Caspase-1的切割,因此NLPR3和Caspase-1活性调控是调控细胞焦亡的关键一环。此外,Wang等[57]研究证明黄芩苷可显著减轻幽门螺杆菌诱导的大鼠胰腺炎,黄芩苷通过抑制硫氧还蛋白结合蛋白(TXNIP)和NLRP3/Caspase-1的表达,减少GSDMD、IL-1β和IL-18等的表达,从而减轻幽门螺杆菌引起的细胞焦亡和炎症反应。
|
图 6 黄芩苷化学结构 Fig. 6 Chemical structure of baicalin[55] |
|
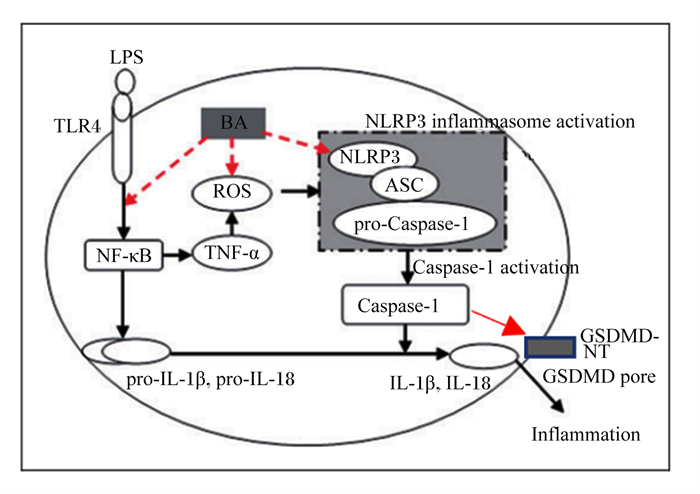
LPS:脂多糖lipopolysaccharide;TLR4:Toll样受体4 Toll-like receptor 4;BA:黄芩苷baicalin;ROS:活性氧reactive oxygen species;NF-κB:核转录因子-κB nuclear factor-kappa B;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;NLRP3 inflammasome activation:NLRP3炎性小体激活;ASC:含有半胱天冬酶募集结构域的凋亡相关斑点样蛋白apoptosis-associated speck-like protein containing a caspase recruitment domain;pro-Caspase-1:半胱天冬酶-1前体precursor of cysteine-aspartic acid protease-1;Caspase-1:半胱天冬酶-1 cysteine-aspartic acid protease-1;Caspase-1 activation:半胱天冬酶-1激活;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-IL-18:白细胞介素-18前体precursor of interleukin-18;IL-1β:白细胞介素-1β interleukin-1β;IL-18:白细胞介素-18 interleukin-18;GSDMD-NT:gasdermin D N端gasdermin D N-terminal;GSDMD pore:gasdermin D孔隙gasdermin D pore;Inflammation:炎症。 图 7 黄芩苷通过调控NLRP3/GSDMD途径抑制仔猪单核吞噬细胞焦亡 Fig. 7 Baicalin suppressed pyrophosis of mononuclear phagocytes in piglets by regulating NLRP3/GSDMD pathway[56] |
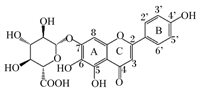
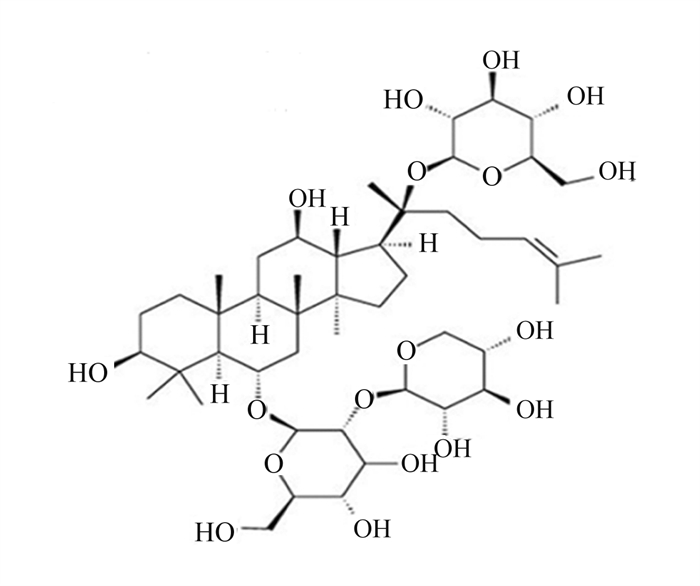
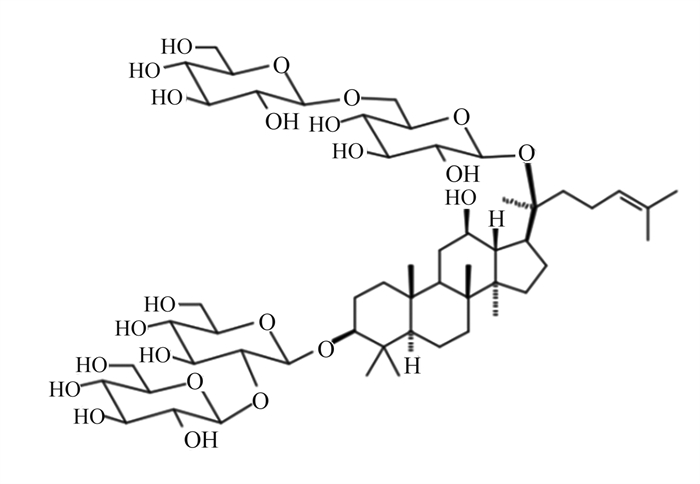
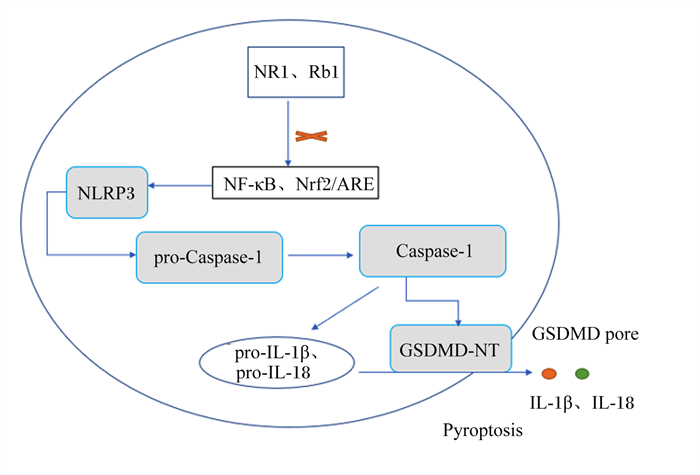
三七皂苷R1是三七中的主要成分,在抗氧化、抗炎和抗凋亡中具有重要作用(图 8)[58]。Tang等[59]研究发现,20 mg/kg的三七皂苷R1可抑制NF-κB信号通路,阻断NLRP3炎性小体的激活,阻止Caspase-1和IL-1β的切割,降低GSDMD N端结构域表达,改善大鼠髓核细胞的细胞功能,抑制细胞焦亡(图 9)。三七中的另一种重要成分人参皂苷Rb1(图 10)[60],可调控核转录因子E2相关因子2(Nrf2)/抗氧化反应元件(ARE)信号通路,减轻人神经母细胞瘤细胞的细胞焦亡,进而保护细胞[61]。细胞内钙离子水平升高可促进NLRP3炎性小体激活,25、50和100 μmol/L人参皂苷Rb1可通过抑制大鼠心肌细胞钙超载,减少心肌细胞焦亡,对乌头碱导致的大鼠心肌病理损伤起到有效的保护作用[62]。
|
图 8 三七皂苷R1化学结构 Fig. 8 Chemical structure of notoginsenoside R1[58] |
|
NR1:三七皂苷R1 notoginsenoside R1;Rb1:人参皂苷Rb1 ginsenoside Rb1;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;NF-κB:核转录因子-κB nuclear factor-kappa B;Nrf2:转录因子E2相关因子2 nuclear factor erythroid 2 related factor 2;ARE:抗氧化反应元件antioxidant response element;pro-Caspase-1:半胱天冬酶-1前体precursor of cysteine-aspartic acid protease-1;Caspase-1:半胱天冬酶-1 cysteine-aspartic acid protease-1;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-IL-18:白细胞介素-18前体precursor of interleukin-18;IL-1β:白细胞介素-1β interleukin-1β;IL-18:白细胞介素-18 interleukin-18;GSDMD-NT:gasdermin D N端gasdermin D N-terminal;GSDMD pore:gasdermin D孔隙gasdermin D pore;Pyroptosis:细胞焦亡。 图 9 三七皂苷R1和人参皂苷Rb1通过调控NLRP3/GSDMD途径抑制细胞焦亡 Fig. 9 Notoginsenoside R1 and ginsenoside Rb1 suppressed pyroptosis by regulating NLRP3/GSDMD pathway |
|
图 10 人参皂苷Rb1化学结构 Fig. 10 Chemical structure of ginsenoside Rb1[60] |
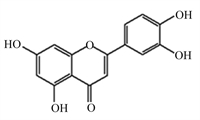
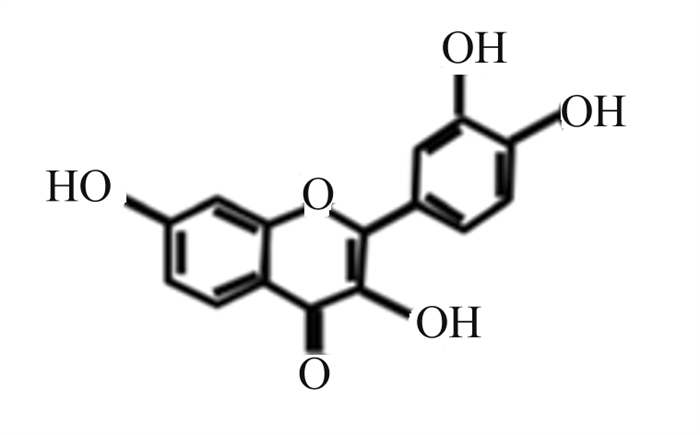
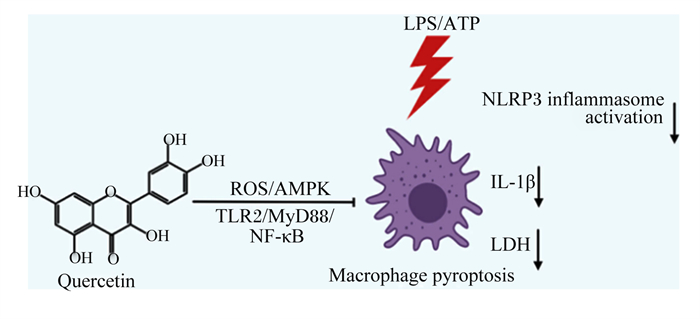
槲皮素是一种天然黄酮类化合物,广泛分布于蔬菜和水果中,具有抗氧化、抗炎等多种生物活性(图 11)[63]。Luo等[64]研究发现,10、20和50 μmol/L槲皮素呈剂量依赖性抑制LPS/ATP诱导的NLRP3炎性小体的激活,抑制GSDMD N端结构域和IL-1β的表达,抑制NLRP3和IL-1β前体的上游蛋白表达,具有调控炎症基因转录功能的p65的核转位和磷酸化。此外,Luo等[64]进一步证实槲皮素通过抑制Toll样受体2(TLR2)/髓样分化因子88(MyD88)/NF-κB和ROS/腺苷酸活化蛋白激酶(AMPK)信号通路,进而抑制NLRP3炎性小体的激活,减少GSDMD蛋白的切割和IL-1β及IL-18的分泌,从而抑制小鼠巨噬细胞焦亡,减轻细胞焦亡带来的损害(图 12)。
|
图 11 槲皮素化学结构 Fig. 11 Chemical structure of quercetin[63] |
|
Quercetin:槲皮素;ROS:活性氧reactive oxygen species;AMPK:腺苷酸活化蛋白激酶AMP-activated protein kinase;TLR2:Toll样受体2 Toll-like receptor 2;MyD88:髓样分化因子88 myeloid differentiation factor 88;NF-κB:核转录因子-κB nuclear factor-kappa B;LPS:脂多糖lipopolysaccharide;NLRP3 inflammasome activation:NLRP3炎性小体激活;IL-1β:白细胞介素-1β interleukin-1β;LDH:乳酸脱氢酶lactate dehydrogenase;Macrophage pyroptosis:巨噬细胞细胞焦亡。 图 12 槲皮素通过调控NLRP3/GSDMD途径抑制细胞焦亡 Fig. 12 Quercetin suppressed pyroptosis by regulating NLRP3/GSDMD pathway[64] |
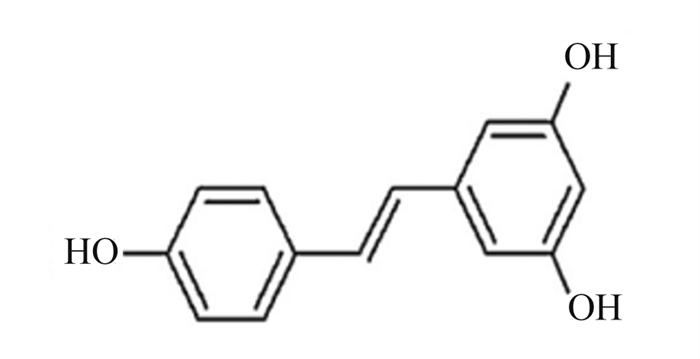
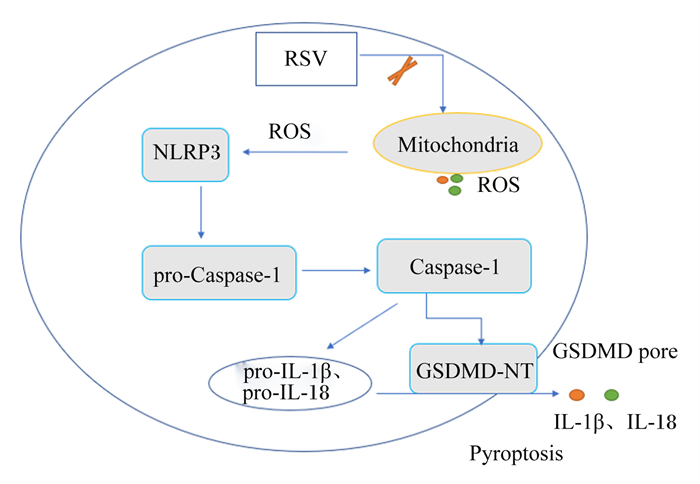
白藜芦醇是一种多酚化合物,在多种植物中天然存在,特别是红葡萄皮(图 13)[65]。白藜芦醇的抗炎、抗氧化、抗癌及心血管保护等生物学功能已被广泛报道。Chang等[66]研究证明,15、30和60 μmol/L的白藜芦醇可显著抑制LPS/ATP诱导的小鼠巨噬细胞焦亡。线粒体产生的ROS导致线粒体通透性转变,并促进线粒体DNA的释放,从而激活NLRP3炎性小体;p62是自噬标志物,自噬的激活依赖NLRP3炎性小体的激活。白藜芦醇通过抑制线粒体ROS的产生,下调p62的表达,保护线粒体完整性和增强自噬,进一步抑制NLRP3炎性小体的激活,从而下调Caspase-1表达,减少GSDMD蛋白的切割,减少IL-1β及IL-18的释放,减轻小鼠巨噬细胞焦亡(图 14)。
|
图 13 白藜芦醇化学结构 Fig. 13 Chemical structure of resveratrol[65] |
|
RSV:白藜芦醇resveratrol;NLRP3:NOD样受体家族pyrin结构域包含3 NOD-like receptor family pyrin domain containing 3;ROS:活性氧reactive oxygen species;Mitochondria:线粒体;pro-Caspase-1:半胱天冬酶-1前体precursor of cysteine-aspartic acid protease-1;Caspase-1:半胱天冬酶-1 cysteine-aspartic acid protease-1;pro-IL-1β:白细胞介素-1β前体precursor of interleukin-1β;pro-IL-18:白细胞介素-18前体precursor of interleukin-18;IL-1β:白细胞介素-1β interleukin-1β;IL-18:白细胞介素-18 interleukin-18;GSDMD-NT:gasdermin D N端gasdermin D N-terminal;GSDMD pore:gasdermin D孔隙gasdermin D pore;Pyroptosis:细胞焦亡。 图 14 白藜芦醇通过调控NLRP3/ GSDMD途径抑制细胞焦亡 Fig. 14 Resveratrol suppressed pyroptosis by regulating NLRP3/GSDMD pathway |
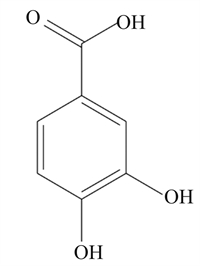
该部分提到的天然植物成分的结构式、调控细胞焦亡的可能途径及剂量等总结见表 1。蒜氨酸是从大蒜中提取的一种有机硫化合物,具有抗氧化和抗炎作用[67];Liu等[68]研究发现,5、10和20 μmol/L蒜氨酸可促进磷酸酶和张力蛋白同系物诱导的激酶1(PINK1)/Parkin介导的线粒体自噬,减少小鼠巨噬细胞内ROS的生成,进而抑制NLRP3炎性小体的激活,减少IL-1β和IL-18分泌,从而减轻LPS诱导的小鼠巨噬细胞焦亡。灯盏乙素具有抗氧化、抗凋亡以及抗炎作用[69];12.5、25.0和50.0 μmol/L灯盏乙素呈剂量依赖性地抑制LPS诱导的小鼠巨噬细胞内Caspase-11的释放和GSDMD N端结构域的产生,从而减轻小鼠巨噬细胞焦亡[70]。姜黄素是从姜黄根茎中提取的一种天然多酚,已被证实具有抗炎、抗癌、抗氧化等多种药理活性;50 mg/kg姜黄素通过调控Toll样受体4(TLR4)/NF-κB信号通路,进一步抑制依赖NLRP3炎性小体介导的小鼠小胶质细胞焦亡,对小鼠小胶质细胞起到保护作用[71]。木犀草素作为一种潜在的生物活性化学物质可用来治疗与细胞焦亡相关的炎症性疾病;100 μmol/L木犀草素具有显著的抗细胞焦亡作用,木犀草素通过抑制Nrf2和NF-κB信号通路,减少ROS的产生,抑制NLRP3炎性小体的激活,减少GSDMD N端结构域和IL-1β的表达,缓解THP-1巨噬细胞焦亡[72]。此外,其他具有抗氧化和抗炎作用的天然植物成分,如芍药苷[73-74]、石蒜碱[75-76]、虫草素[77-78]、小檗碱[79-80]、异甘草素[81-82]以及原儿茶酸[83-84]等天然植物成分通过抑制NLRP3/GSDMD信号通路,抑制细胞焦亡,对相关细胞和器官起到保护作用。
|
|
表 1 部分天然植物成分调控GSDMD在细胞焦亡中的作用 Table 1 Some natural plant components regulated pyroptosis through GSDMD |
综上所述,当动物受到外界病原体侵袭及各种病理损伤后,细胞焦亡发生并加剧机体炎症。具有抗炎作用的天然植物成分,可抑制NLRP3炎性小体的激活,进而抑制GSDMD蛋白的切割,减少炎性细胞因子IL-1β和IL-18等的释放,缓解细胞焦亡,减轻细胞及组织的损伤,缓解炎症。GSDM家族是先天性免疫中的一种重要蛋白,过度表达会加剧机体的炎症反应,造成机体损伤。GSDMD蛋白作为细胞焦亡中的一种标志蛋白,其表达量可以反映机体的炎症水平,天然植物成分主要通过调控NLRP3/GSDMD信号通路调控肠道的细胞焦亡和IBD,该理论为畜禽肠道疾病防治提供了重要思路。
5 小结国内外的研究表明,GSDM作为一个成孔蛋白家族,在驱动细胞死亡方面发挥着重要作用。除PJVK外,GSDM其他5个成员均具有使细胞膜成孔的N端结构域,该结构可以诱导细胞焦亡和调控IBD。已发现部分天然植物成分可通过GSDM调控细胞焦亡和IBD,但试验动物主要集中在鼠上。养畜禽就是养肠道,在畜禽养殖全面禁抗的背景下,如何减少肠道炎症性疾病,保障畜禽肠道健康,是畜牧养殖领域急需面对和解决的问题。因此,开发具有抗炎功能的天然植物成分,探究其抗炎机理,对畜禽健康养殖具有重大意义。
| [1] |
BROZ P, PELEGRÍN P, SHAO F. The gasdermins, a protein family executing cell death and inflammation[J]. Nature Reviews Immunology, 2020, 20(3): 143-157. DOI:10.1038/s41577-019-0228-2 |
| [2] |
LIU X, XIA S Y, ZHANG Z B, et al. Channelling inflammation: gasdermins in physiology and disease[J]. Nature Reviews Drug Discovery, 2021, 20(5): 384-405. DOI:10.1038/s41573-021-00154-z |
| [3] |
FENG S Y, FOX D, MAN S M. Mechanisms of gasdermin family members in inflammasome signaling and cell death[J]. Journal of Molecular Biology, 2018, 430(18 Pt B): 3068-3080. |
| [4] |
KUANG S Y, ZHENG J, YANG H, et al. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(40): 10642-10647. DOI:10.1073/pnas.1708194114 |
| [5] |
AGLIETTI R A, DUEBER E C. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions[J]. Trends in Immunology, 2017, 38(4): 261-271. DOI:10.1016/j.it.2017.01.003 |
| [6] |
DE SCHUTTER E, ROELANDT R, RIQUET F B, et al. Punching holes in cellular membranes: biology and evolution of gasdermins[J]. Trends in Cell Biology, 2021, 31(6): 500-513. DOI:10.1016/j.tcb.2021.03.004 |
| [7] |
DU T T, GAO J, LI P L, et al. Pyroptosis, metabolism, and tumor immune microenvironment[J]. Clinical and Translational Medicine, 2021, 11(8): e492. |
| [8] |
SHI J J, GAO W Q, SHAO F. Pyroptosis: gasdermin-mediated programmed necrotic cell death[J]. Trends in Biochemical Sciences, 2017, 42(4): 245-254. DOI:10.1016/j.tibs.2016.10.004 |
| [9] |
SARRIÓ D, MARTÍNEZ-VAL J, MOLINA-CRESPO Á, et al. The multifaceted roles of gasdermins in cancer biology and oncologic therapies[J]. Biochimica et Biophysica Acta: Reviews on Cancer, 2021, 1876(2): 188635. DOI:10.1016/j.bbcan.2021.188635 |
| [10] |
RUAN J W, WANG S J, WANG J B. Mechanism and regulation of pyroptosis-mediated in cancer cell death[J]. Chemico-Biological Interactions, 2020, 323: 109052. DOI:10.1016/j.cbi.2020.109052 |
| [11] |
ZHOU C B, FANG J Y. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection[J]. Biochimica et Biophysica Acta: Reviews on Cancer, 2019, 1872(1): 1-10. DOI:10.1016/j.bbcan.2019.05.001 |
| [12] |
KOVACS S B, MIAO E A. Gasdermins: effectors of pyroptosis[J]. Trends in Cell Biology, 2017, 27(9): 673-684. DOI:10.1016/j.tcb.2017.05.005 |
| [13] |
YU P, ZHANG X, LIU N, et al. Pyroptosis: mechanisms and diseases[J]. Signal Transduction and Targeted Therapy, 2021, 6(1): 128. DOI:10.1038/s41392-021-00507-5 |
| [14] |
WANG Y Q, KANNEGANTI T D. From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways[J]. Computational and Structural Biotechnology Journal, 2021, 19: 4641-4657. DOI:10.1016/j.csbj.2021.07.038 |
| [15] |
KESAVARDHANA S, MALIREDDI R K S, KANNEGANTI T D. Caspases in cell death, inflammation, and pyroptosis[J]. Annual Review of Immunology, 2020, 38: 567-595. DOI:10.1146/annurev-immunol-073119-095439 |
| [16] |
BERGSBAKEN T, FINK S L, COOKSON B T. Pyroptosis: host cell death and inflammation[J]. Nature Reviews Microbiology, 2009, 7(2): 99-109. DOI:10.1038/nrmicro2070 |
| [17] |
ZOU J, ZHENG Y X, HUANG Y, et al. The versatile gasdermin family: their function and roles in diseases[J]. Frontiers in Immunology, 2021, 12: 751533. DOI:10.3389/fimmu.2021.751533 |
| [18] |
HU B, ELINAV E, HUBER S, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(50): 21635-21640. DOI:10.1073/pnas.1016814108 |
| [19] |
LAMKANFI M, DIXIT V M. Inflammasomes and their roles in health and disease[J]. Annual Review of Cell and Developmental Biology, 2012, 28: 137-161. DOI:10.1146/annurev-cellbio-101011-155745 |
| [20] |
LEI-LESTON A C, MURPHY A G, MALOY K J. Epithelial cell inflammasomes in intestinal immunity and inflammation[J]. Frontiers in Immunology, 2017, 8: 1168. DOI:10.3389/fimmu.2017.01168 |
| [21] |
WU J S, SUN J Y, MENG X L. Pyroptosis by caspase-11 inflammasome-gasdermin D pathway in autoimmune diseases[J]. Pharmacological Research, 2021, 165: 105408. DOI:10.1016/j.phrs.2020.105408 |
| [22] |
BROZ P, DIXIT V M. Inflammasomes: mechanism of assembly, regulation and signalling[J]. Nature Reviews Immunology, 2016, 16(7): 407-420. DOI:10.1038/nri.2016.58 |
| [23] |
RATHINAM V A K, FITZGERALD K A. Inflammasome complexes: emerging mechanisms and effector functions[J]. Cell, 2016, 165(4): 792-800. DOI:10.1016/j.cell.2016.03.046 |
| [24] |
RATHINAM V A K, CHAN F K M. Inflammasome, inflammation, and tissue homeostasis[J]. Trends in Molecular Medicine, 2018, 24(3): 304-318. DOI:10.1016/j.molmed.2018.01.004 |
| [25] |
CHEN G Y, NÚÑEZ G. Inflammasomes in intestinal inflammation and cancer[J]. Gastroenterology, 2011, 141(6): 1986-1999. DOI:10.1053/j.gastro.2011.10.002 |
| [26] |
SHI J J, ZHAO Y, WANG Y P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS[J]. Nature, 2014, 514(7521): 187-192. DOI:10.1038/nature13683 |
| [27] |
YANG J L, ZHAO Y, SHAO F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity[J]. Current Opinion in Immunology, 2015, 32: 78-83. DOI:10.1016/j.coi.2015.01.007 |
| [28] |
NEURATH M F. Cytokines in inflammatory bowel disease[J]. Nature Reviews Immunology, 2014, 14(5): 329-342. DOI:10.1038/nri3661 |
| [29] |
PARK J H, PEYRIN-BIROULET L, EISENHUT M, et al. IBD immunopathogenesis: a comprehensive review of inflammatory molecules[J]. Autoimmunity Reviews, 2017, 16(4): 416-426. DOI:10.1016/j.autrev.2017.02.013 |
| [30] |
CASSINOTTI A, SARZI-PUTTINI P, FICHERA M, et al. Immunity, autoimmunity and inflammatory bowel disease[J]. Autoimmunity Reviews, 2014, 13(1): 1-2. DOI:10.1016/j.autrev.2013.06.007 |
| [31] |
SONG Y J, ZHAO Y G, MA Y M, et al. Biological functions of NLRP3 inflammasome: a therapeutic target in inflammatory bowel disease[J]. Cytokine & Growth Factor Reviews, 2021, 60: 61-75. |
| [32] |
DI SABATINO A, LENTI M V, GIUFFRIDA P, et al. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract[J]. Autoimmunity Reviews, 2015, 14(12): 1161-1169. DOI:10.1016/j.autrev.2015.08.004 |
| [33] |
HE W T, WAN H Q, HU L C, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion[J]. Cell Research, 2015, 25(12): 1285-1298. DOI:10.1038/cr.2015.139 |
| [34] |
BURDETTE B E, ESPARZA A N, ZHU H, et al. Gasdermin D in pyroptosis[J]. Acta Pharmaceutica Sinica B, 2021, 11(9): 2768-2782. DOI:10.1016/j.apsb.2021.02.006 |
| [35] |
TSUCHIYA K, HOSOJIMA S, HARA H, et al. Gasdermin D mediates the maturation and release of IL-1α downstream of inflammasomes[J]. Cell Reports, 2021, 34(12): 108887. DOI:10.1016/j.celrep.2021.108887 |
| [36] |
GONG W H, SHI Y, REN J J. Research progresses of molecular mechanism of pyroptosis and its related diseases[J]. Immunobiology, 2020, 225(2): 151884. DOI:10.1016/j.imbio.2019.11.019 |
| [37] |
KAYAGAKI N, WARMING S, LAMKANFI M, et al. Non-canonical inflammasome activation targets Caspase-11[J]. Nature, 2011, 479(7371): 117-121. DOI:10.1038/nature10558 |
| [38] |
WANG K, SUN Q, ZHONG X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis[J]. Cell, 2020, 180(5): 941-955.20. DOI:10.1016/j.cell.2020.02.002 |
| [39] |
SHI J J, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526(7575): 660-665. DOI:10.1038/nature15514 |
| [40] |
KAYAGAKI N, STOWE I B, LEE B L, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling[J]. Nature, 2015, 526(7575): 666-671. DOI:10.1038/nature15541 |
| [41] |
LIU X, ZHANG Z B, RUAN J B, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores[J]. Nature, 2016, 535(7610): 153-158. DOI:10.1038/nature18629 |
| [42] |
SBORGI L, RVHL S, MULVIHILL E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death[J]. EMBO Journal, 2016, 35(16): 1766-1778. DOI:10.15252/embj.201694696 |
| [43] |
DING J J, WANG K, LIU W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family[J]. Nature, 2016, 535(7610): 111-116. DOI:10.1038/nature18590 |
| [44] |
LIU Z H, WANG C P, YANG J, et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization[J]. Immunity, 2019, 51(1): 43-49.e4. DOI:10.1016/j.immuni.2019.04.017 |
| [45] |
XIA S Y. Biological mechanisms and therapeutic relevance of the gasdermin family[J]. Molecular Aspects of Medicine, 2020, 76: 100890. DOI:10.1016/j.mam.2020.100890 |
| [46] |
LIU Z H, WANG C P, RATHKEY J K, et al. Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition[J]. Structure, 2018, 26(5): 778-784.e3. DOI:10.1016/j.str.2018.03.002 |
| [47] |
SHARMA D, KANNEGANTI T D. Inflammatory cell death in intestinal pathologies[J]. Immunological Reviews, 2017, 280(1): 57-73. DOI:10.1111/imr.12602 |
| [48] |
BULEK K, ZHAO J J, LIAO Y, et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis[J]. The Journal of Clinical Investigation, 2020, 130(8): 4218-4234. |
| [49] |
BATISTA S J, STILL K M, JOHANSON D, et al. Gasdermin-D-dependent IL-1α release from microglia promotes protective immunity during chronic Toxoplasma gondii infection[J]. Nature Communications, 2020, 11(1): 3687. DOI:10.1038/s41467-020-17491-z |
| [50] |
ZHU S, DING S Y, WANG P H, et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells[J]. Nature, 2017, 546(7660): 667-670. DOI:10.1038/nature22967 |
| [51] |
MA C M, YANG D X, WANG B W, et al. Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation[J]. Science Advances, 2020, 6(21): eaaz6717. DOI:10.1126/sciadv.aaz6717 |
| [52] |
SCHWARZER R, JIAO H P, WACHSMUTH L, et al. FADD and Caspase-8 regulate gut homeostasis and inflammation by controlling MLKL-and GSDMD-mediated death of intestinal epithelial cells[J]. Immunity, 2020, 52(6): 978-993.e6. DOI:10.1016/j.immuni.2020.04.002 |
| [53] |
BILJALI S, HADJIMITOVA V A, TOPASHKA-ANCHEVA M N, et al. Antioxidant and antiradical properties of esculin, and its effect in a model of epirubicin-induced bone marrow toxicity[J]. Folia Medica, 2012, 54(3): 42-49. DOI:10.2478/v10153-011-0096-4 |
| [54] |
XU X N, JIANG Y, YAN L Y, et al. Aesculin suppresses the NLRP3 inflammasome-mediated pyroptosis via the Akt/GSK3β/NF-κB pathway to mitigate myocardial ischemia/reperfusion injury[J]. Phytomedicine, 2021, 92: 153687. DOI:10.1016/j.phymed.2021.153687 |
| [55] |
CHAO J B, WANG X L, XU M, et al. Characterization and enhanced antioxidant activity of the inclusion complexes of baicalin with p-sulfonatocalix[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2019, 93(3): 361-370. |
| [56] |
SHI H L, ZHANG Y L, XING J, et al. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis[J]. International Immunopharmacology, 2020, 81: 106195. DOI:10.1016/j.intimp.2020.106195 |
| [57] |
WANG X Y, CAI H, CHEN Z Y, et al. Baicalein alleviates pyroptosis and inflammation in hyperlipidemic pancreatitis by inhibiting NLRP3/Caspase-1 pathway through the miR-192-5p/TXNIP axis[J]. International Immunopharmacology, 2021, 101(Part B): 108315. |
| [58] |
QIAN D L, SHAO X K, LI Y C, et al. Notoginsenoside R1 protects WI-38 cells against lipopolysaccharide-triggered injury via adjusting the miR-181a/TLR4 axis[J]. Journal of Cellular Biochemistry, 2019, 120(12): 19764-19774. DOI:10.1002/jcb.29282 |
| [59] |
TANG K, SU W H, HUANG C H, et al. Notoginsenoside R1 suppresses inflammatory response and the pyroptosis of nucleus pulposus cells via inactivating NF-κB/NLRP3 pathways[J]. International Immunopharmacology, 2021, 101(Part B): 107866. |
| [60] |
AHMED T, RAZA S H, MARYAM A, et al. Ginsenoside Rb1 as a neuroprotective agent: a review[J]. Brain Research Bulletin, 2016, 125: 30-43. DOI:10.1016/j.brainresbull.2016.04.002 |
| [61] |
王睿智, 陈志文, 邵乐, 等. 人参皂苷Rb1通过调控Nrf2/ARE信号通路抗氧糖剥夺/复氧后神经细胞焦亡的研究[J]. 中国药理学通报, 2021, 37(10): 1383-1390. WANG R Z, CHEN Z W, SHAO L, et al. Ginsenoside Rb1 inhibits neuronal pyroptosis after oxygen-glucose deprivation/reoxygenation via Nrf2/ARE signaling pathway[J]. Chinese Pharmacological Bulletin, 2021, 37(10): 1383-1390 (in Chinese). |
| [62] |
WANG M, WANG R Y, SUN H, et al. Ginsenoside Rb1 ameliorates cardiotoxicity triggered by aconitine via inhibiting calcium overload and pyroptosis[J]. Phytomedicine, 2021, 83: 153468. DOI:10.1016/j.phymed.2021.153468 |
| [63] |
CHEN X L, LIANG D H, HUANG Z Q, et al. Quercetin regulates skeletal muscle fiber type switching via adiponectin signaling[J]. Food & Function, 2021, 12(6): 2693-2702. |
| [64] |
LUO X, BAO X Y, WENG X Z, et al. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway[J]. Life Sciences, 2022, 291: 120064. DOI:10.1016/j.lfs.2021.120064 |
| [65] |
SALEHI B, MISHRA A P, NIGAM M, et al. Resveratrol: a double-edged sword in health benefits[J]. Biomedicines, 2018, 6(3): 91. DOI:10.3390/biomedicines6030091 |
| [66] |
CHANG Y P, KA S M, HSU W H, et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy[J]. Journal of Cellular Physiology, 2015, 230(7): 1567-1579. DOI:10.1002/jcp.24903 |
| [67] |
SHI L M, LIN Q L, LI X H, et al. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation[J]. Molecular Nutrition & Food Research, 2017, 61(9): 10. |
| [68] |
LIU M T, LU J, YANG S T, et al. Alliin alleviates LPS-induced pyroptosis via promoting mitophagy in THP-1 macrophages and mice[J]. Food and Chemical Toxicology, 2022, 160: 112811. DOI:10.1016/j.fct.2022.112811 |
| [69] |
MO J, YANG R H, LI F, et al. Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation[J]. Phytomedicine, 2018, 42: 66-74. DOI:10.1016/j.phymed.2018.03.021 |
| [70] |
YE J Z, ZENG B, ZHONG M Y, et al. Scutellarin inhibits Caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling[J]. Acta Pharmaceutica Sinica B, 2021, 11(1): 112-126. DOI:10.1016/j.apsb.2020.07.014 |
| [71] |
ZHENG Y L, ZHANG J W, ZHAO Y, et al. Curcumin protects against cognitive impairments in a rat model of chronic cerebral hypoperfusion combined with diabetes mellitus by suppressing neuroinflammation, apoptosis, and pyroptosis[J]. International Immunopharmacology, 2021, 93: 107422. DOI:10.1016/j.intimp.2021.107422 |
| [72] |
ZOU Y P, LUO X, FENG Y, et al. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation[J]. Chemico-Biological Interactions, 2021, 345: 109573. DOI:10.1016/j.cbi.2021.109573 |
| [73] |
DAI W Y, LUO Z P. Paeoniflorin inhibits pyroptosis of nucleus pulposus cells in an acidic environment and alleviates the degeneration of the intervertebral disc in rats[J]. Cellular Signalling, 2022, 91: 110243. DOI:10.1016/j.cellsig.2022.110243 |
| [74] |
XU L, WANG H, YU Q Q, et al. The monomer derivative of paeoniflorin inhibits macrophage pyroptosis via regulating TLR4/NLRP3/GSDMD signaling pathway in adjuvant arthritis rats[J]. International Immunopharmacology, 2021, 101(Part A): 108169. |
| [75] |
LIANG Q, CAI W Y, ZHAO Y X, et al. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis[J]. Pharmacological Research, 2020, 158: 104884. DOI:10.1016/j.phrs.2020.104884 |
| [76] |
ROY M, LIANG L, XIAO X J, et al. Lycorine: a prospective natural lead for anticancer drug discovery[J]. Biomedicine & Pharmacotherapy, 2018, 107: 615-624. |
| [77] |
SUN Y, HUANG W M, TANG P C, et al. Neuroprotective effects of natural cordycepin on LPS-induced Parkinson's disease through suppressing TLR4/NF-κB/NLRP3-mediated pyroptosis[J]. Journal of Functional Foods, 2020, 75: 104274. DOI:10.1016/j.jff.2020.104274 |
| [78] |
RABIE A M. Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication[J]. ACS Omega, 2022, 7(3): 2960-2969. DOI:10.1021/acsomega.1c05998 |
| [79] |
ZHAO Y N, LI Z K, LU E R, et al. Berberine exerts neuroprotective activities against cerebral ischemia/reperfusion injury through up-regulating PPAR-γ to suppress NF-κB-mediated pyroptosis[J]. Brain Research Bulletin, 2021, 177: 22-30. DOI:10.1016/j.brainresbull.2021.09.005 |
| [80] |
NEAG M A, MOCAN A, ECHEVERRÍA J, et al. Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders[J]. Frontiers in Pharmacology, 2018, 9: 557. DOI:10.3389/fphar.2018.00557 |
| [81] |
LV X F, FAN C, JIANG Z X, et al. Isoliquiritigenin alleviates P. gingivalis-LPS/ATP-induced pyroptosis by inhibiting NF-κB/NLRP3/GSDMD signals in human gingival fibroblasts[J]. International Immunopharmacology, 2021, 101(Part B): 108338. |
| [82] |
CAO Z X, WEN Y, HE J L, et al. Isoliquiritigenin, an orally available natural FLT3 inhibitor from licorice, exhibits selective anti-acute myeloid leukemia efficacy in vitro and in vivo[J]. Molecular Pharmacology, 2019, 96(5): 589-599. DOI:10.1124/mol.119.116129 |
| [83] |
GUO Y, YANG J H, HE Y, et al. Protocatechuic aldehyde prevents ischemic injury by attenuating brain microvascular endothelial cell pyroptosis via lncRNA Xist[J]. Phytomedicine, 2022, 94: 153849. DOI:10.1016/j.phymed.2021.153849 |
| [84] |
KAKKAR S, BAIS S. A review on protocatechuic acid and its pharmacological potential[J]. ISRN Pharmacology, 2014, 2014: 952943. |