中间球海胆(Strongylocentrotus intermedius)又称虾夷马粪海胆,属棘皮动物门(Echinodermata),游走亚门(Eleutherozea),海胆纲(Echinoidea)[1], 原产于日本北海道和俄罗斯远东沿海地区,因其性腺色泽好、味道鲜美、营养丰富而广受消费者喜爱。1989年,大连水产学院(现为大连海洋大学)将其引入中国[2],并相继攻克了中间球海胆的人工育苗、增养殖和病害防控等技术环节,现已成为我国北方沿海特别是辽宁和山东沿海地区重要的海水经济养殖品种[3]。海带、裙带菜、石莼是海胆养殖过程中重要的天然饵料来源,但是,海带等鲜活藻类供应期主要集中于每年的1~7月和12月,夏秋两季由于缺乏优质饵料,海胆体质虚弱,加之高温应激,致使海胆大面积死亡的事件时有发生[4-5]。此外,天然饵料也存在着一些弊端,例如来源不稳定、供应不及时、营养不全面、质量良莠不齐,这极大限制了海胆的生长速度和性腺品质,不利于海胆产业的高质量发展[6]。近年来,本研究团队围绕中间球海胆营养生理和人工配合饲料的开发进行了相关研究,确定了包括蛋白质、脂肪、脂肪酸等主要营养素的需求量,确定了幼胆和成胆饲料中最适脂肪源及其对海胆生长和性腺发育的影响,初步探明了必需脂肪酸合成的路径和营养调控机制[7-11]。
磷脂是一种分子中含有磷酸基团的极性脂,是细胞膜的重要组成部分,能够维持细胞膜的流动性[12-13]。此外,磷脂sn-2位含有丰富的长链多不饱和脂肪酸(LC-PUFA),如花生四烯酸(ARA)、二十碳五烯酸(EPA)和二十二碳六烯酸(DHA),在促进动物生长、免疫、性腺发育和繁殖性能中发挥着重要作用[14-16]。磷脂通常用作配方饲料中的添加剂,以提高饲料的适口性,促进脂质的乳化、消化、吸收以及运输和动员[17]。根据来源不同,磷脂主要分为动物磷脂和植物磷脂[18]。研究表明,不同来源的磷脂其脂肪酸组成和功效也存在差异,从而对水产动物发挥不同的生理功能[19-20]。大豆卵磷脂是饲料中主要的磷脂类型,除具有上述磷脂的功能外,大豆卵磷脂还能增强甲壳动物对渗透胁迫的抵抗力[17],并能提高脂质从肝胰腺转运到卵巢的效率,促进红螯光壳螯虾(Cherax quadricarinatus)的性腺发育,进而提高其繁殖能力[21]。此外,在三疣梭子蟹(Portunus trituberculatus)的研究中发现,适宜水平的大豆卵磷脂可通过调节肝胰腺、血清、卵巢中类固醇激素的合成进而调节未成熟期个体的卵黄发生[22]。Cuesta-Gomez等[23]研究发现,饲料中添加大豆卵磷脂和鱼油对紫海胆(Strongylocentrotus purpuratus)性腺发育有促进作用。磷虾油是一种从南极磷虾(Euphausia superba)中提取出来的动物磷脂,富含大量的n-3LC-PUFA[24]。Lin等[25-26]研究发现,磷虾油较其他磷脂源显著提高了中华绒螯蟹(Eriocheir sinensis)可食用部位(卵巢、肝胰腺和肌肉)的n-3LC-PUFA沉积,这可能是由于磷虾油更有利于极低密度脂蛋白(VLDL)的合成,从而促进肝脏脂质的运输。
随着水产养殖业的迅速发展,鱼油短缺问题日益突出,植物油因其产量稳定、价格低廉成为潜在的替代脂肪源。棕榈油是目前产量最大的植物油,其有益效果已在多种水产动物中得到验证[27-28]。近期研究发现,与鱼油组相比,棕榈油组海胆性腺发育和配子发生明显滞后[10]。这一方面可能是由于棕榈油中缺乏磷脂,另一方面可能是不同来源的磷脂其营养组成和生理功能也存在差异[26]。目前,大豆卵磷脂对海胆生长性能、性腺营养组成和品质影响的研究已在多个海胆种类,如光棘球海胆(Strongylocentrotus nudus)[29]、北方球海胆(Strongylocentrotus droebachiensis)[30]、绿海胆(Lytechinus variegatus)[31]、紫海胆[23]中有所报道。但是,有关不同类型磷脂对海胆生长和性腺发育的影响尚不明确,特别是在棕榈油饲料中添加不同类型磷脂效果的比较研究更是未见报道。因此,本研究旨在探究棕榈油饲料中添加2种磷脂对中间球海胆生长、性腺发育及营养组成的影响,以期为海胆性腺促熟期间饲料中适宜磷脂源的选择提供理论依据。
1 材料与方法 1.1 试验动物试验所用中间球海胆购自大连龙王塘一养殖场。试验前,将海胆在水槽(300 L)中暂养2周,暂养期间每天投喂足量的新鲜海带,每3 d换水、吸底1次。
1.2 试验设计和试验饲料试验以酪蛋白、豆粕为主要蛋白质源,在鱼油饲料中添加大豆卵磷脂作为对照组饲料,在棕榈油饲料中分别添加大豆卵磷脂(PO+SL组)和磷虾油(PO+KO组)作为试验组饲料。试验饲料组成及营养水平见表 1,试验饲料脂肪酸组成及含量见表 2。饲料配制之前,将所有粉状原料粉碎过80目筛,并按照配比将饲料干粉原料充分混合,再将油脂均匀搓至上述干粉原料中。随后,加入30%的水再次混匀,搓好的饲料用双螺旋制粒机(DES-TS1280,济南鼎润机械设备有限公司)压制成直径为1.5 mm的饲料。制作好的饲料于烘箱(55 ℃)中烘干,冷却后用双层塑料袋包装,-20 ℃保存备用。
|
|
表 1 试验饲料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of experimental diets (DM basis) |
|
|
表 2 试验饲料脂肪酸组成及含量(干物质基础) Table 2 Fatty acid composition and contents of experimental diets (DM basis) |
摄食生长试验在农业部北方海水增养殖重点实验室中进行。暂养结束后,随机选取大小相似,健康无病的海胆[初始体重(18.86±0.55) g,壳径(36.21±0.34) mm,壳高(18.66±0.55) mm)]放进已消毒的45个网笼(15 cm×15 cm×35 cm)中,每个网笼放养1只海胆。网笼中放入1个培养皿,防止饲料掉入水底。每种饲料随机投喂15只海胆,每天2次(09:00和17:00)投喂饲料,次日将水槽底部的粪便和残饵吸出,日均换水量为水槽体积的30%。试验期42 d。试验期间水体温度为9~15 ℃,溶氧含量>7 mg/L,盐度30‰。
1.4 样品采集由于无法根据海胆外观判断性别,所以每个组喂养的海胆性别比例是无法确定的。根据以往经验,将所有海胆性腺取一部分分别浸泡在多聚甲醛固定液中,利用组织学分析辨别雌雄。
试验结束前24 h停止投喂,根据组织学分析结果随机选取雌雄各5只海胆,记录生长数据。每组另取雌雄各2只海胆,计算性腺指数,将性腺收集,一部分用于颜色测定,剩余部分液氮速冻后保存于-80 ℃冰箱,用于营养组成分析。
1.5 常规营养成分采用AOAC(1995)[32]的方法分析测定饲料和海胆性腺的营养成分,具体为:水分含量的测定采用失重法,105 ℃烘干至恒重;粗蛋白质含量的测定采用凯氏定氮法,将氮含量乘以6.25;粗脂肪含量的测定采用索氏抽提法,抽提剂为乙醚。
1.6 石蜡组织切片采用刘权迪等[33]的方法,将性腺样品在多聚甲醛固定液中浸泡24 h,用手术刀切割成合适大小,利用自动脱水机(RM2016,Leica公司,德国)进行脱水,将组织嵌入石蜡中,利用旋转组织切片机进行切片,切片厚度调成4 μm,然后对切片进行苏木精-伊红(HE)染色,封片。制作好的组织切片可在智能显微镜(Leica公司,德国)下进行性腺成熟度和性别的观察。
1.7 脂肪酸含量测定脂肪酸含量测定采用气相色谱质谱联用仪(Trace 1310 IS,ThermoFisher公司,美国),色谱柱为TG-5MS(30 m×0.25 mm×0.25 μm)。加入2 mL 5%盐酸甲醇溶液,3 mL氯仿-甲醇溶液(体积比1 ∶ 1),100 μL十九烷酸甲酯内标。于85 ℃水浴锅中水浴1 h。水浴完成后,降至室温,在离心管中加入1 mL正己烷,振荡萃取2 min后,静置1 h。取上层清液100 μL,用正己烷定容到1 mL,用0.45 μm滤膜过滤后上机测试。色谱柱升温程序为80 ℃保持1 min,以10 ℃/min的速率升温至200 ℃,继续以5 ℃/min的速率升温至250 ℃,最后以2 ℃/min的速率升到270 ℃,保持3 min。脂肪酸含量表示为g/kg的形式。
1.8 氨基酸含量测定将样品进行冷冻干燥,称取30 mg样品放入20 mL水解管中,加入10 mL 6 mol/L盐酸,将吹氮仪的吹氮口插入装有样品的水解管中,吹氮气15 min,立即在火焰酒精灯下封口。在110 ℃下消解22~24 h,冷却后移出,用0.22 μm有机滤膜过滤0.1 mL于气相瓶中,于60 ℃用氮气吹干溶液中的盐酸和水,加入1 mL 0.02 mol/L盐酸,摇匀,利用氨基酸分析仪(L-8900,Hitachi公司,日本)进行测定,通过计算每个氨基酸的峰值面积,并将其与标准校准曲线进行比对。氨基酸含量表示为g/kg的形式。
1.9 性腺颜色测定取海胆性腺样品,仔细修剪成规格一致的小块,利用色差仪(CM-A145,KonicaMinolta公司,日本)测量亮度(L*)、红度(a*)和黄度(b*)值。根据Mcbride等[34]的方法,利用标准浅橙黄色(L*=68.9、a*=28.7、b*=60.4)和标准浅黄色(L*=74.6、a*=28.7、b*=66.1)进行计算单个性腺颜色和标准颜色之间的总色差(ΔE)。
1.10 计算公式和统计分析
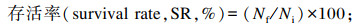
|
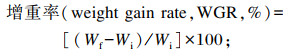
|
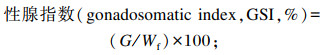
|
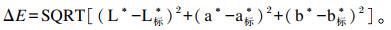
|
式中:Nf和Ni分别为海胆终末数量和初始数量(个);Wf和Wi分别为海胆终末体重和初始体重(g);G为海胆性腺重(g);L标*为标准颜色的亮度(L标*=68.9或74.6);a标*为标准颜色的红色(a标*=28.7);b标*为标准颜色的黄色(b标*=60.4或66.1)。
利用SPSS 22.0软件对数据进行分析,在采用单因素方差分析(one-way ANOVA)法进行显著性检验的基础上,采用Duncan氏多重比较法对组间差异显著性进行检验。试验数据用平均值±标准误(mean±SE)表示,P < 0.05为差异显著。
2 结果 2.1 棕榈油饲料中添加2种磷脂对中间球海胆存活、生长和性腺指数的影响由表 3可见,各组存活率均为100%。各组之间终末体重、增重率和性腺湿重无显著差异(P>0.05)。PO+SL组的性腺指数最低,显著低于对照组(P < 0.05),但与PO+KO组差异不显著(P>0.05)。PO+KO组的性腺指数虽低于对照组,但差异不显著(P>0.05)。
|
|
表 3 棕榈油饲料中添加2种磷脂对中间球海胆存活、生长和性腺指数的影响 Table 3 Effects of two phospholipids addition in palm oil-based diet on survival, growth and gonadosomatic index of Strongylocentrotus intermedius |
由表 4可知,PO+SL组的性腺水分含量显著高于PO+KO组和对照组(P < 0.05)。PO+SL组的性腺粗蛋白质含量略低于PO+KO组(P>0.05),显著低于对照组(P < 0.05)。各组之间性腺粗脂肪含量无显著差异(P>0.05)。此外,PO+KO组和对照组之间性腺水分、粗脂肪和粗蛋白质均差异不显著(P>0.05)。
|
|
表 4 棕榈油饲料中添加2种磷脂对中间球海胆性腺常规营养成分的影响(湿物质基础) Table 4 Effects of two phospholipids addition in palm oil-based diet on gonad proximate nutritional composition of Strongylocentrotus intermedius (WM basis) |
由表 5可知,PO+SL组的性腺L*值最高,对照组的性腺a*值最高,但各组之间性腺L*和a*无显著差异(P>0.05)。PO+SL组的性腺b*值显著低于PO+KO组和对照组(P < 0.05),但PO+KO组和对照组之间无显著差异(P>0.05)。PO+KO组和对照组的性腺ΔE1和ΔE2值无显著差异(P>0.05),且均显著低于PO+SL组(P < 0.05)。
|
|
表 5 棕榈油饲料中添加2种磷脂对中间球海胆性腺颜色的影响 Table 5 Effects of two phospholipids addition in palm oil-based diet on gonad color of Strongylocentrotus intermedius |
如图 1所示,PO+SL组的性腺发育速度较其他各组迟缓,雄性和雌性个体仅发育到Ⅱ期。PO+KO组和对照组的性腺发育速度相当,但雄性和雌性个体之间性腺发育不同步,雄性个体性腺均发育到Ⅲ期,而雌性个体性腺仅发育到Ⅱ期。

|
A:对照组(♂) control group (♂);B:对照组(♀) control group (♀);C:PO+SL组(♂) PO+SL group (♂);D:PO+SL组(♀) PO+SL group (♀);E:PO+KO组(♂) PO+KO group (♂);F:PO+KO组(♀) PO+KO group (♀)。NP:营养吞噬细胞nutritive phagocyte;SP:精母细胞spermatocyte;S:精子spermatozoa;EV:早期卵黄形成卵母细胞early vitellogenic oocyte。 图 1 各组中间球海胆性腺组织切片 Fig. 1 Gonad tissue section of Strongylocentrotus intermedius in each group |
由表 6可知,PO+SL组的性腺总氨基酸(TAA)含量显著高于PO+KO组和对照组(P < 0.05),但PO+KO组和对照组之间无显著差异(P>0.05)。PO+SL组的性腺必需氨基酸(EAA)含量显著高于PO+KO组和对照组(P < 0.05),且PO+KO组显著低于对照组(P < 0.05)。性腺甜味氨基酸和鲜味氨基酸含量依次为PO+SL组>PO+KO组>对照组,且各组之间均存在显著差异(P < 0.05)。PO+SL组的性腺苦味氨基酸含量显著高于PO+KO组和对照组(P < 0.05),但PO+KO组和对照组之间无显著差异(P>0.05)。
|
|
表 6 棕榈油饲料中添加2种磷脂对中间球海胆性腺氨基酸组成的影响(湿物质基础) Table 6 Effects of two phospholipids addition in palm oil-based diet on composition of amino acid in gonad of Strongylocentrotus intermedius (WM basis) |
由表 7可知,PO+SL组的性腺EPA和DHA含量最低,显著低于PO+KO组和对照组(P < 0.05);PO+KO组的性腺EPA和DHA含量也显著低于对照组(P < 0.05)。PO+SL组的性腺ARA含量与PO+KO组差异不显著(P>0.05),但均显著低于对照组(P < 0.05)。PO+SL组的性腺n-6多不饱和脂肪酸(n-6PUFA)含量显著高于PO+KO组(P < 0.05),且显著低于对照组(P < 0.05)。PO+KO组的性腺n-3多不饱和脂肪酸(n-3PUFA)含量显著高于PO+SL组(P < 0.05),且显著低于对照组(P < 0.05)。PO+KO组的性腺n-3PUFA/n-6PUFA显著高于对照组和PO+SL组(P < 0.05),且对照组显著高于PO+SL组(P < 0.05)。
|
|
表 7 棕榈油饲料中添加2种磷脂对中间球海胆性腺脂肪酸组成的影响(湿物质基础) Table 7 Effects of two phospholipids addition in palm oil-based diet on composition of fatty acid in gonad of Strongylocentrotus intermedius (WM basis) |
有研究表明,不同来源的磷脂其脂肪酸组成存在差异,因而其生理功能也有所不同,从而对水产动物生长性能的影响也不同[19-20]。本研究中,PO+SL组中间球海胆的增重率和性腺指数较低,这与Lin等[26]和Ning等[10]的研究结果相似,可能是由于大豆卵磷脂中含有大量亚油酸(LA,18 ∶ 2n-6),LA具有致炎作用,并且影响类固醇激素的合成,从而导致水生动物生长缓慢,性腺发育迟缓[35-36]。此外,在北方球海胆[37]和中间球海胆[9]的研究中已经证实大量的LA会造成ARA的沉积,过多摄入ARA会对水产动物的生长和性腺发育产生负面影响,这在牙鲆(Paralichthys olivaceus)[38]、夏威夷帽贝(Cellana sandwicensis)[39]、黑海胆(Diadema setosum)[40]和雌性半滑舌鳎(Cynoglossus semilaevis)[41]等水产动物中已得到证实。
3.2 棕榈油饲料中添加2种磷脂对中间球海胆性腺发育的影响性腺不仅是产生配子的生殖器官,还是储存能量和营养物质的器官[42-44]。因此希望海胆性腺发育同步,以确保能够持续生产,满足市场需求[45]。大量研究表明,在促进甲壳动物发育和繁殖过程中,磷脂发挥着重要作用,如磷脂作为底物参与卵黄发生的合成,以促进卵黄蛋白的生成、促进脂质积累及促进卵巢的发育等[21-22, 46]。在本研究中,PO+SL组海胆性腺发育速度较其他各组相对较慢,雌性和雄性个体均停留在Ⅱ期;而PO+KO组和对照组雄性个体发育较快,均进入Ⅲ期,而雌性个体仍停留在Ⅱ期,这说明富含n-3LC-PUFA的磷虾油和鱼油加快了雄性海胆的性腺发育。有关n-3LC-PUFA影响海胆雌性和雄性个体性腺发育差异的机制尚不完全清楚,但在大菱鲆(Scophthalmus maximus)[47]的研究中发现,饲料中高水平的n-3高度不饱和脂肪酸(n-3HUFA)可以提高亲鱼的产卵量,所产卵子卵径大,孵化率高,仔稚鱼的存活率强。因此,在本研究中,棕榈油与磷虾油的联合投喂有利于性腺发育和配子发生,从而有助于提高繁殖期海胆的成熟度。但是,海胆性腺是唯一可食用部分,从食用角度来看,Ⅱ期末Ⅲ期初的性腺最佳,因为此时性腺中营养吞噬细胞达到最大且配子还未分化或刚刚开始分化,大量营养物质如卵黄蛋白和脂类储存在营养吞噬细胞内[48]。随着配子发生,营养吞噬细胞内储存的营养物质逐渐被消耗,导致海胆性腺味道变酸,质地变软[49]。
3.3 棕榈油饲料中添加2种磷脂对中间球海胆性腺颜色的影响海胆性腺颜色是市场售卖时最重要的指标,其不受欢迎和不稳定的颜色都会导致海胆经济价值大大下降[50]。海胆性腺是唯一可食用的部分,亮黄色、亮橙色、芒果黄的性腺是消费者喜爱的颜色[51]。先前的研究表明,性腺的颜色会受到饲料种类和营养成分的影响[52]。在本研究中,a*值在22~24,b*值在40~51,PO+SL组和PO+KO组的性腺L*值处于60左右,高于其他种类的海胆性腺L*值[53-56]。
关于颜色的差异,△E越低,就越接近标准颜色[55]。海胆性腺的颜色取决于体内黄色和橙色类胡萝卜素的沉积[57],磷脂可能通过改变细胞膜的通透性来提高细胞吸收效率,从而提高类胡萝卜素的生物利用度[58-59]。本研究中,PO+KO组的性腺△E1和△E2低于PO+SL组,可能因为磷虾油中含有大量虾青素,虾青素是一种类胡萝卜素,具有强抗氧化活性和显著的着色能力[60],这使得PO+KO组海胆性腺更接近标准颜色。Cuesta-Gomez等[23]研究发现,饲料中同时含有鱼油和大豆卵磷脂,△E较低,与标准颜色相近,这与本研究结果相似。
3.4 棕榈油饲料中添加2种磷脂对中间球海胆性腺氨基酸组成的影响氨基酸的组成和含量是评价食物营养价值重要的指标之一[61]。人们根据氨基酸在口腔中的感受将其分为甜味氨基酸、鲜味氨基酸、苦味氨基酸三大类。其中,苏氨酸、丝氨酸、甘氨酸、丙氨酸、脯氨酸属于甜味氨基酸;天冬氨酸、谷氨酸属于鲜味氨基酸;蛋氨酸、异亮氨酸、亮氨酸、酪氨酸、苯丙氨酸、赖氨酸、组氨酸、精氨酸属于苦味氨基酸[23]。有研究表明,饲料中添加鱼油或者鱼粉会对海胆性腺味道产生负面影响[62-63]。在本研究中,对照组的性腺甜味氨基酸和鲜味氨基酸含量最少,但在促进生长、性腺发育方面优于其他各组;对照组的性腺缬氨酸和蛋氨酸的含量显著高于PO+KO组。在苦味氨基酸中,缬氨酸和蛋氨酸等氨基酸显著降低性腺风味,已被报道为其他海胆性腺苦味的促进剂,使其更苦更酸[64-65]。
本研究中,PO+SL组的性腺TAA含量高于PO+KO组和对照组,这可能与不同饲料组n-3PUFA含量差异有关,PO+KO组和对照组的性腺n-3PUFA含量高于PO+SL组。已有研究表明,饲料中n-3LC-PUFA能显著促进海胆性腺发育和配子发生,在配子发生过程中,累积在营养吞噬细胞中的氨基酸会被大量消耗[11, 49]。此外,n-3LC-PUFA主要用于构建体内生物膜,而较少用于氧化供能,为了满足能量需要,机体将氨基酸用于供能的比例可能会增加,从而导致TAA含量降低[66-67]。PO+KO组EAA含量低于PO+SL组,这可能是由于磷虾油的添加提高了海胆性腺发育速度,致使较多必需氨基酸用于配子发生。EAA/TAA值也是评价水产产品中蛋白质营养价值的重要参考指标,一般认为,EAA/TAA值理想状态为0.4,可代表高质量蛋白质[68]。本研究中,PO+SL组的EAA/TAA值为0.35,对照组EAA/TAA值为0.39,这表明PO+SL组和对照组的蛋白质营养价值要优于PO+KO组。
3.5 棕榈油饲料中添加2种磷脂对中间球海胆性腺脂肪酸组成的影响众所周知,脂肪酸在天然饵料或配方饲料中的含量会影响特定组织的生存、生长、发育以及生殖性能[69]。有研究表明,n-3LC-PUFA有益于人类,如促进大脑和神经系统的发育,一定程度上降低冠心病和某种癌症的发病率,增加免疫力,促进人类持续健康[70-76]。由于磷虾油和鱼油中富含n-3LC-PUFA,因此PO+KO组和对照组的性腺n-3PUFA含量,尤其是EPA和DHA含量较高,而PO+SL组由于缺乏n-3LC-PUFA致使性腺中这些脂肪酸沉积量较少,从而大大降低了海胆性腺的脂肪酸营养价值,这与三疣梭子蟹的研究结果[68]相似。在人类饮食结构中,相比较n-3PUFA,大量摄入n-6PUFA被证实会增加一些疾病的可能性[77]。某些n-6PUFA,如LA,可能代谢转化为ARA,导致低密度脂蛋白(LDL)氧化和促炎症介质生成增加,这可能对动物健康产生不良影响[78]。在本研究中,PO+SL组的性腺n-6PUFA含量高于PO+KO组。n-3PUFA/n-6PUFA值被认为是评价海洋生物相对营养价值的重要指标,较高的n-3PUFA/n-6PUFA值对预防心血管疾病、肥胖症和类风湿性关节炎具有重要意义[77, 79]。本研究中,PO+KO组的性腺n-3PUFA/n-6PUFA值最高,这与Lin等[26]的研究结果一致。
对野生海胆性腺脂肪酸组成分析发现,LC-PUFA以ARA和EPA为主,而DHA含量较低[80-82]。在本研究中,PO+KO组的性腺EPA和DHA含量显著高于PO+SL组,主要是因为磷虾油的添加,磷虾油中富含n-3LC-PUFA[24]。值得一提的是,在饲料脂肪酸组成中,PO+SL组饲料中没有检测到ARA、EPA和DHA,但在PO+SL组海胆性腺中发现是有一定量的ARA、EPA和DHA,这表明中间球海胆性腺可能在试验前就具有一定量的ARA、EPA和DHA。此外,海胆可能具有合成LC-PUFA的能力[9, 83]。同时,也具有沉积在消化道中的LC-PUFA,通过磷脂重塑,即在磷脂酶A2(PLA2)和溶血磷脂酰胆碱酰基转移酶(LPCAT)的作用下,在磷脂sn-2位脱下不合适的脂肪酸,结合消化道中的LC-PUFA,并且转运到性腺进行性腺发育的可能性。在美国龙虾(Homarus americanus)中,已表明磷脂可能促进血淋巴中胆固醇的吸收和转运过程[84]。此外,日本对虾(Penaeus japonicus)的研究中进一步发现,磷脂在一定程度上能够促进脂质从肠道向其他组织转运,推测磷脂是参与转运过程的脂蛋白结构中的重要物质,为脂蛋白的形成提供主要的极性脂来源[85]。综合考虑原料成本和性腺脂肪酸营养价值,本研究认为可以考虑先投喂PO+SL组饲料,上市之前投喂PO+KO组饲料进行强化,这样不仅能降低原料成本,而且能够提高中间球海胆性腺脂肪酸营养。
4 结论棕榈油饲料中添加大豆卵磷脂或磷虾油并未显著影响中间球海胆的增重率,磷虾油较大豆卵磷脂能显著提高中间球海胆性腺指数、发育速度及性腺必需脂肪酸含量。
| [1] |
常亚青, 丁君, 宋坚, 等. 海参、海胆生物学研究与养殖[M]. 北京: 海洋出版社, 2004. CHANG Y Q, DING J, SONG J, et al. Biological research and culture of sea cucumber and sea urchin[M]. Beijing: China Ocean Press, 2004 (in Chinese). |
| [2] |
经晨晨, 张伟杰, 宋坚, 等. 中间球海胆与紫海胆种间杂交的受精、孵化和幼体发育研究[J]. 大连海洋大学学报, 2015, 30(6): 620-626. JING C C, ZHANG W J, SONG J, et al. Fertilization, hatching and larval development of sea urchin hybrid between Strongylocentrotus intermedius and Anthocidaris crassispina[J]. Journal of Dalian Fisheries University, 2015, 30(6): 620-626 (in Chinese). |
| [3] |
常亚青, 王子臣, 王国江. 温度和藻类饵料对虾夷马粪海胆摄食及生长的影响[J]. 水产学报, 1999, 23(1): 69-76. CHANG Y Q, WANG Z C, WANG G J. Effect of temperature and algae on feeding and growth in sea urchin, Strongylocentrotus intermedius[J]. Journal of Fisheries of China, 1999, 23(1): 69-76 (in Chinese). |
| [4] |
COOK E J, HUGHES A D, ORR H, et al. Influence of dietary protein on essential fatty acids in the gonadal tissue of the sea urchins Psammechinus miliaris and Paracentrotus lividus (Echinodermata)[J]. Aquaculture, 2007, 273(4): 586-594. DOI:10.1016/j.aquaculture.2007.10.032 |
| [5] |
苏延明, 蔡学新, 孙俭, 等. 用几种饲料原料饲喂中间球海胆稚胆的效果[J]. 大连水产学院学报, 2008, 23(3): 242-246. SU Y M, CAI X X, SUN J, et al. Effects of several kinds of feedstuff on growth and digestibility of juvenile sea urchin Strongylocentrotus intermedius[J]. Journal of Dalian Fisheries University, 2008, 23(3): 242-246 (in Chinese). DOI:10.3969/j.issn.1000-9957.2008.03.017 |
| [6] |
孔泳滔, 王琦, 程振明, 等. 盐渍裙带菜替代鲜海带投喂虾夷马粪海胆的试验[J]. 水产养殖, 2001(1): 7-8. KONG Y T, WANG Q, CHENG Z M, et al. Experiment on the feed of salted-Undaria pinnatifida Suringar instead of fresh kelp to Strongylocentrotus intermedius[J]. Journal of Aquaculture, 2001(1): 7-8 (in Chinese). DOI:10.3969/j.issn.1004-2091.2001.01.003 |
| [7] |
李广, 李世顺, 李敏, 等. 饲料中花生四烯酸对中间球海胆存活、生长和肠道菌群组成的影响[J]. 中国渔业质量与标准, 2018, 8(3): 42-51. LI G, LI S S, LI M, et al. Effects of dietary arachidonic acid on survival and growth performance and the intestinal bacterial profile of adult sea urchin (Strongylocentrotus intermedius)[J]. China Fishery Quality And Standards, 2018, 8(3): 42-51 (in Chinese). DOI:10.3969/j.issn.2095-1833.2018.03.006 |
| [8] |
左然涛, 李广, 吴反修, 等. 饲料中不同蛋白源对中间球海胆生长、消化酶活力、性腺指数及氨基酸组成的影响[J]. 饲料工业, 2017, 38(22): 17-21. ZUO R T, LI G, WU F X, et al. Effects of different protein sources on the growth performance, digestive enzyme activities, gonadosomatic index and amino acid profile in juvenile sea urchin (Strongylocentrotus intermedius)[J]. Feed Industry, 2017, 38(22): 17-21 (in Chinese). |
| [9] |
LI M, ZHANG F, DING J, et al. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius)[J]. Aquaculture Nutrition, 2021, 27(1): 28-38. DOI:10.1111/anu.13162 |
| [10] |
NING Y C, ZHANG F, TANG L, et al. Effects of dietary lipid sources on the growth, gonad development, nutritional and organoleptic quality, transcription of fatty acid synthesis related genes and antioxidant capacity during cold storage in adult sea urchin (Strongylocentrotus intermedius)[J]. Aquaculture, 2022, 548(Part 2): 737688. |
| [11] |
LV D L, ZHANG F, DING J, et al. Effects of dietary n-3 LC-PUFA on the growth performance, gonad development, fatty acid profile, transcription of related genes and intestinal microflora in adult sea urchin (Strongylocentrotus intermedius)[J]. Aquaculture Research, 2021, 52(4): 1431-1441. DOI:10.1111/are.14997 |
| [12] |
EBERT T A. Growth and survival of post-settlement sea urchins[M]//LAWRENCE J M. Developments in aquaculture and fisheries science: edible sea urchins: biology and ecology. Amsterdam: Elsevier, 2001: 79-102.
|
| [13] |
MARSH A G, WATTS S A. Energy metabolism and gonad development[M]//LAWRENCE J M. Developments in aquaculture and fisheries science: edible sea urchins: biology and ecology. Amsterdam: Elsevier, 2001: 27-42.
|
| [14] |
ZHAO Y, CHEN Y Q, BONACCI T M, et al. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase[J]. Journal of Biological Chemistry, 2008, 283(13): 8258-8265. DOI:10.1074/jbc.M710422200 |
| [15] |
邓玉英, 刘桂森, 方治山, 等. 在断奶仔猪日粮中添加不同剂量大豆磷脂的饲喂效果[J]. 当代畜牧, 2009(6): 28-29. DENG Y Y, LIU G S, FANG Z S, et al. Feeding effect of adding different doses of soybean lecithin in the diet of weaned piglets[J]. Contemporary Animal Husbandry, 2009(6): 28-29 (in Chinese). |
| [16] |
罗士津, 瞿明仁. 日粮中多不饱和脂肪酸对动物免疫功能影响的研究[J]. 饲料与畜牧, 2007(8): 10-13. LUO S J, QU M R. Study on the effects of dietary polyunsaturated fatty acids on animals immune functions[J]. Feed and Husbandry, 2007(8): 10-13 (in Chinese). DOI:10.3969/j.issn.1006-6314-B.2007.08.007 |
| [17] |
GARCÍA-GALANO T, VILLARREAL-COLMENARES H, FENUCCI J L. Manual de ingredientes proteicos y aditivos empleados en la formulación de alimentos balanceados para camarones peneidos[J]. EUDEM, Mar del Plata, 2007, 143: 22-29. |
| [18] |
徐含颖. 饲料磷脂源及水平对拟穴青蟹(Scylla paramamosain)早期幼蟹生长、体组成及抗氧化能力的影响[D]. 硕士学位论文. 舟山: 浙江海洋大学, 2019. XU H Y. Effects of dietary phospholipids sources and levels on growth, body composition and antioxidant capacity of the early juvenile green mud crab, Scylla paramamosain (Estampador)[D]. Master's Thesis. Zhoushan: Zhejiang Ocean University, 2019. (in Chinese) |
| [19] |
MIYASHITA K, NARA E, OTA T. Comparative study on the oxidative stability of phosphatidylcholines from salmon egg and soybean in an aqueous solution[J]. Bioscience, Biotechnology, and Biochemistry, 1994, 58(10): 1772-1775. DOI:10.1271/bbb.58.1772 |
| [20] |
NARA E, MIYASHITA K, OTA T. Oxidative stability of liposomes prepared from soybean PC, chicken egg PC, and salmon egg PC[J]. Bioscience, Biotechnology, and Biochemistry, 1997, 61(10): 1736-1738. DOI:10.1271/bbb.61.1736 |
| [21] |
WANG L M, ZUO D, LV W W, et al. Effects of dietary soybean lecithin on gonadal development and vitellogenin mRNA expression in the female redclaw crayfish Cherax quadricarinatus (von Martens) at first maturation[J]. Aquaculture Research, 2013, 44(8): 1167-1176. DOI:10.1111/j.1365-2109.2012.03128.x |
| [22] |
SONG D Y, SHI B, DING L Y, et al. Regulation of dietary phospholipids on growth performance, antioxidant activities, phospholipid metabolism and vitellogenesis in prereproductive phase of female swimming crabs, Portunus trituberculatus[J]. Aquaculture, 2019, 511: 734230. DOI:10.1016/j.aquaculture.2019.734230 |
| [23] |
CUESTA-GOMEZ D M, LAZO J P, SÁNCHEZ-SAAVEDRA M D P. Effects of dietary fish oil and soya bean lecithin on gonad index, colour and biochemical composition of the purple sea urchin, Strongylocentrotus purpuratus (Stimpson 1857)[J]. Aquaculture Research, 2020, 51(8): 3384-3402. |
| [24] |
TOU J C, JACZYNSKI J, CHEN Y C. Krill for human consumption: nutritional value and potential health benefits[J]. Nutrition Reviews, 2007, 65(2): 63-77. |
| [25] |
LIN Z D, QI C L, HAN F L, et al. Selecting suitable phospholipid source for female Eriocheir sinensis in pre-reproductive phase[J]. Aquaculture, 2020, 528: 735610. |
| [26] |
LIN Z D, HAN F L, LU J T, et al. Influence of dietary phospholipid on growth performance, body composition, antioxidant capacity and lipid metabolism of Chinese mitten crab, Eriocheir sinensis[J]. Aquaculture, 2020, 516: 734653. |
| [27] |
何凌云. 大豆油或棕榈油替代鱼油对斜带石斑鱼生长性能、脂肪酸组成及脂肪代谢的影响[D]. 硕士学位论文. 厦门: 集美大学, 2019. HE L Y. Effects of replacing fish oil with soybean oil or palm oil in diets on growth performance, fatty acid composition and fat metabolism of grouper (Epinephelus coioides)[D]. Master's Thesis. Xiamen: Jimei University, 2019. (in Chinese) |
| [28] |
程民杰. 棕榈油替代鱼油对半滑舌鳎生长、生理生化和肌肉营养品质影响的研究[D]. 硕士学位论文. 天津: 天津农学院, 2014. CHENG M J. Effect of substitution of fish oil by palm oil on growth performance, physiological and biochemical parameters and muscle nutritional quality in Cynoglossus semilaevis[D]. Master's Thesis. Tianjin: Tianjin Agricultural University, 2014. (in Chinese) |
| [29] |
LAWRENCE J M, LAWRENCE A L, WATTS S A. Chapter 7 feeding, digestion, and digestibility[M]//LAWRENCE J M. Developments in aquaculture and fisheries science: edible sea urchins: biology and ecology. Amsterdam: Elsevier, 2007: 135-158.
|
| [30] |
GONZÁLEZ-DURÁN E, CASTELL J D, ROBINSON S M C, et al. Effects of dietary lipids on the fatty acid composition and lipid metabolism of the green sea urchin Strongylocentrotus droebachiensis[J]. Aquaculture, 2008, 276(1/4): 120-129. |
| [31] |
GIBBS V K, WATTS S A, LAWRENCE A L, et al. Dietary phospholipids affect growth and production of juvenile sea urchin Lytechinus variegatus[J]. Aquaculture, 2009, 292(1/2): 95-103. |
| [32] |
AO AC. Official methods of analysis of association of official analytical chemists[M]. 6th ed. Arlington: Association of Official Analytical Chemists AOAC International, 1995.
|
| [33] |
刘权迪, 宁延昶, 温斌, 等. 饲料中脂肪水平对海湾扇贝性腺发育、脂肪酸组成和组织结构的影响[J]. 上海海洋大学学报, 2021, 30(6): 981-991. LIU Q D, NING Y C, WEN B, et al. Effects of dietary lipid on the gonad development, fatty acid composition, and histological structure of Argopecten irradias[J]. Journal of Shanghai University, 2021, 30(6): 981-991 (in Chinese). |
| [34] |
MCBRIDE S C, PRICE R J, TOM P D, et al. Comparison of gonad quality factors: color, hardness and resilience, of Strongylocentrotus franciscanus between sea urchins fed prepared feed or algal diets and sea urchins harvested from the Northern California fishery[J]. Aquaculture, 2004, 233(1/2/3/4): 405-422. |
| [35] |
ZUO R T, MAI K S, XU W, et al. Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the marine finfish Larimichthys crocea[J]. Lipids, 2015, 50(2): 149-163. DOI:10.1007/s11745-014-3970-z |
| [36] |
FEI S Z, LIU C, XIA Y, et al. The effects of dietary linolenic acid to linoleic acid ratio on growth performance, tissues fatty acid profile and sex steroid hormone synthesis of yellow catfish Pelteobagrus fulvidraco[J]. Aquaculture Reports, 2020, 17(1): 1-9. |
| [37] |
CASTELL J D, KENNEDY E J, ROBINSON S M C, et al. Effect of dietary lipids on fatty acid composition and metabolism in juvenile green sea urchins (Strongylocentrotus droebachiensis)[J]. Aquaculture, 2004, 242(1/4): 417-435. |
| [38] |
FURUITA H, YAMAMOTO T, SHIMA T, et al. Effect of arachidonic acid levels in broodstock diet on larval and egg quality of Japanese flounder Paralichthys olivaceus[J]. Aquaculture, 2003, 220(1/4): 725-735. |
| [39] |
HUA N T, AKO H. Reproductive biology and effect of arachidonic acid level in broodstock diet on final maturation of the Hawaiian Limpet Cellana sandwicensis[J]. Aquaculture Research Development, 2014, 5(5): 256-264. |
| [40] |
NHAN H T, NHU T Q, DUC P M, et al. Effects of dietary arachidonic acid on final maturation, spawning and composition of gonad of black sea urchin (Diadema setosum) (Leske, 1778)[J]. Aquaculture Nutrition, 2020, 26(2): 1771-1779. |
| [41] |
XU H G, CAO L, ZHANG Y Q, et al. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage[J]. Aquaculture, 2017, 468: 378-385. DOI:10.1016/j.aquaculture.2016.11.002 |
| [42] |
HUGHES A D, KELLY M S, BARNES D K A, et al. The dual functions of sea urchin gonads are reflected in the temporal variations of their biochemistry[J]. Marine Biology, 2006, 148(4): 789-798. DOI:10.1007/s00227-005-0124-0 |
| [43] |
RUSSELL M P. Resource allocation plasticity in sea urchins: rapid, diet induced, phenotypic changes in the green sea urchin, Strongylocentrotus droebachiensis (Müller)[J]. Journal of Experimental Marine Biology and Ecology, 1998, 220(1): 1-14. DOI:10.1016/S0022-0981(97)00079-8 |
| [44] |
SILIANI S, MELIS R, LOI B, et al. Influence of seasonal and environmental patterns on the lipid content and fatty acid profiles in gonads of the edible sea urchin Paracentrotus lividus from Sardinia[J]. Marine Environmental Research, 2016, 113: 124-133. DOI:10.1016/j.marenvres.2015.12.001 |
| [45] |
SPIRLET C, GROSJEAN P, JANGOUX M. Optimization of gonad growth by manipulation of temperature and photoperiod in cultivated sea urchins, Paracentrotus lividus (Lamarck) (Echinodermata)[J]. Aquaculture, 2000, 185(1/2): 85-99. |
| [46] |
SUI L Y, WU X G, WILLE M, et al. Effect of dietary soybean lecithin on reproductive performance of Chinese mitten crab Eriocheir sinensis (H.Milne-Edwards) Broodstock[J]. Aquaculture International, 2009, 17(1): 45-56. DOI:10.1007/s10499-008-9178-6 |
| [47] |
马爱军, 陈超, 雷霁霖, 等. 饲料蛋白质含量和n-3HUFA水平对大菱鲆亲鱼产卵的影响[J]. 海洋水产究, 2005(1): 7-12. MA A J, CHEN C, LEI J L, et al. The effect of protein and n-3HUFA on the reproduction of turbot (Scophthalmus maximus)[J]. Marine Fisheries Research, 2005(1): 7-12 (in Chinese). |
| [48] |
MARSH A G, WATTS S A. Biochemical and energy requirements of gonad development[J]. Developments in Aquaculture and Fisheries Science, 2013, 37(7): 35-53. |
| [49] |
UNUMA T, WALKER C W. Relationship between gametogenesis and food quality in sea urchin gonads[C]//Proceedings of the Thirty-Sixth U.S. -Japan Aquaculture Panel Symposium. Durham: U.S. Dept. Commerce, 2009: 45-54.
|
| [50] |
LAWRENCE J M, LAWRENCE A L, MCBRIDE S C, et al. Developments in the use of prepared feeds in sea-urchin aquaculture[J]. World Aquaculture, 2001, 32(3): 34-39. |
| [51] |
CHEN Y C, CHEN T Y, CHIOU T K, et al. Seasonal variation on general composition, free amino acids and fatty acids in the gonad of Taiwan's sea urchin Tripneustes gratilla[J]. Journal of Marine Science and Technology, 2013, 21(6): 723-732. |
| [52] |
ONOMU A J, VINE N J, CYRUS M D, et al. The effect of fresh seaweed and a formulated diet supplemented with seaweed on the growth and gonad quality of the collector sea urchin, Tripneustes gratilla, under farm conditions[J]. Aquaculture Research, 2020, 51(10): 4087-4102. DOI:10.1111/are.14752 |
| [53] |
AZAD A K, PEARCE C M, MCKINLEY R C. Effects of diet and temperature on ingestion, absorption, assimilation, gonad yield, and gonad quality of the purple sea urchin (Strongylocentrotus purpuratus)[J]. Aquaculture, 2011, 317(1/2/3/4): 187-196. |
| [54] |
ROBINSON S M C, CASTELL J D, KENNEDY E J. Developing suitable colour in the gonads of cultured green sea urchins (Strongylocentrotus droebachiensis)[J]. Aquaculture, 2002, 206(3/4): 289-303. |
| [55] |
WOODS C M C, JAMES P J, MOSS G A, et al. A comparison of the effect of urchin size and diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes[J]. Aquaculture International, 2008, 16(1): 49-68. DOI:10.1007/s10499-007-9124-z |
| [56] |
COOK E J, KELLY M S. Co-culture of the sea urchin Paracentrotus lividus and the edible mussel Mytilus edulis L. on the west coast of scotland, United Kingdom[J]. Journal of Shellfish Research, 2009, 28(3): 553-559.
|
| [57] |
KELLY M S, SYMONDS R C. Carotenoids in sea urchins[J]. Developments in Aquaculture and Fisheries Science, 2013, 38: 171-177. |
| [58] |
HAUSER H. Short-chain phospholipids as detergents[J]. Biochimica et Biophysica Acta-Biomembranes, 2000, 1508(1/2): 164-181. |
| [59] |
STAFFORD R E, DENNIS E A. Lysophospholipids as biosurfactants[J]. Colloids and Surfaces, 1987, 30(1): 47-64. DOI:10.1016/0166-6622(87)80203-2 |
| [60] |
谈俊晓, 赵永强, 李来好, 等. 南极磷虾综合利用研究进展[J]. 广东农业科学, 2017, 44(3): 143-150. TAN J X, ZHAO Y Q, LI L H, et al. Research progress on comprehensive utilization of Antarctic krill[J]. Guangdong Agricultural Sciences, 2017, 44(3): 143-150 (in Chinese). |
| [61] |
DONG Z G, ZHANG M, WEI S F, et al. Effect of farming patterns on the nutrient composition and farming environment of loach, Paramisgurnus dabryanus[J]. Aquaculture, 2018, 497: 214-219. DOI:10.1016/j.aquaculture.2018.07.061 |
| [62] |
HOSHIKAWA H, TAKAHASHI K, SUGIMOTO T, et al. The effects of fish meal feeding on the gonad quality of cultivated sea urchins, Strongylocentrotus nudus (A. Agassiz)[J]. Scientific Reports of Hokkaido Fisheries Experimental Station (Japan), 1998, 52: 17-24. |
| [63] |
PEARCE C M, DAGGETT T L, ROBINSON S M C. Effect of binder type and concentration on prepared feed stability and gonad yield and quality of the green sea urchin, Strongylocentrotus droebachiensis[J]. Aquaculture, 2002a, 205: 301-323. DOI:10.1016/S0044-8486(01)00685-8 |
| [64] |
PEARCE C M, DAGGETT T L, ROBINSON S M C. Effect of urchin size and diet on gonad yield and quality in the green sea urchin (Strongylocentrotus droebachiensis)[J]. Aquaculture, 2004, 233(1/2/3/4): 337-367. |
| [65] |
PHILLIPS K, BREMER P, SILCOCK P, et al. Effect of gender, diet and storage time on the physical properties and sensory quality of sea urchin (Evechinus chloroticus) gonads[J]. Aquaculture, 2009, 288(3/4): 205-215. |
| [66] |
XIAO Y F, KE Q, WANG S Y, et al. Single point mutations affect fatty acid block of human myocardial sodium channel α subunit Na+ channels[J]. Proceedings of the National Academy of Sciences, 2001, 98(6): 3606-3611. DOI:10.1073/pnas.061003798 |
| [67] |
NEWGARD C B. Interplay between lipids and branched-chain amino acids in development of insulin resistance[J]. Cell metabolism, 2012, 15(5): 606-614. DOI:10.1016/j.cmet.2012.01.024 |
| [68] |
YUAN Y, WANG X X, JIN M, et al. Modification of nutritional values and flavor qualities of muscle of swimming crab (Portunus trituberculatus): application of a dietary lipid nutrition strategy[J]. Food Chemistry, 2020, 308: 125607. DOI:10.1016/j.foodchem.2019.125607 |
| [69] |
HYNE R V, SÁNCHEZ-BAYO F, BRYAN A D, et al. Fatty acid composition of the estuarine amphipod, Melita plumulosa (Zeidler): ink between diet and fecundity[J]. Environmental Toxicology and Chemistry, 2010, 28(1): 123-132. |
| [70] |
CARBONI S, HUGHES A D, ATACK T, et al. Fatty acid profiles during gametogenesis in sea urchin (Paracentrotus lividus): effects of dietary inputs on gonad, egg and embryo profiles[J]. Comparative Biochemistry and Physiology A: Molecular and Integrative Physiology, 2013, 164(2): 376-382. DOI:10.1016/j.cbpa.2012.11.010 |
| [71] |
KANG J X, LEAF A. The cardiac antiarrhythmic effects of polyunsaturated fatty acid[J]. Lipids, 1996, 31(1): S41-S44. DOI:10.1007/BF02637049 |
| [72] |
TAVAZZI L, MAGGSIONI A P, MARCHIOLI R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GSISSI-HF trial): a randomised, double-blind, placebo-controlled trial[J]. Lancet, 2008, 372(9645): 1223-1230. DOI:10.1016/S0140-6736(08)61239-8 |
| [73] |
CARLSON S E, WERKMAN S H, RHODES P G, et al. Visual-acuity development in healthy preterm infants: effect of marine-oil supplementation[J]. American Journal of Clinical Nutrition, 1993, 58(1): 35-42. DOI:10.1093/ajcn/58.1.35 |
| [74] |
KELLEY D S. Modulation of human immune and inflammatory responses by dietary fatty acids[J]. Nutrition, 2001, 17(7/8): 669-673. |
| [75] |
KIMURA Y, KONO S, TOYOMURA K, et al. Meat, fish and fat intake in relation to subsite-specific risk colorectal cancer: the fukuoka colorectal cancer study[J]. Cancer Science, 2007, 98(4): 590-597. DOI:10.1111/j.1349-7006.2007.00425.x |
| [76] |
NILSEN D, HARRIS W S L. N-3 fatty acids and cardiovascular disease[J]. American Journal of Clinical Nutrition, 2008, 93(6): 807-812. |
| [77] |
PATTERSON E, WALL R, FITZGERALD G F, et al. Health implications of high dietary omega-6 polyunsaturated fatty acids[J]. Journal of Nutrition and Metabolism, 2012, 2012(2): 1-16. |
| [78] |
LARSEN R, EILERTSEN K E, ELVEVOLL E O. Health benefits of marine foods and ingredients[J]. Biotechnology Advances, 2011, 29(5): 508-518. DOI:10.1016/j.biotechadv.2011.05.017 |
| [79] |
从娇娇, 庾庭驰, 于立志, 等. 饲料中植物油替代鱼油对中华绒螯蟹脂肪酸组成的影响[J]. 上海海洋大学学报, 2020, 29(4): 559-567. CONG J J, YU T C, YU L Z, et al. Effects of dietary replacement of fish oil by vegetable oil on fatty acid composition of Chinese mitten crab (Eriocheir sinensis)[J]. Journal of Shanghai Ocean University, 2020, 29(4): 559-567 (in Chinese). |
| [80] |
FERNANDEZ C. Effect of diet on the biochemical composition of Paracentrotus lividus (Echinodermata: Echinoidea) under natural and rearing conditions (effect of diet on biochemical composition of urchins)[J]. Comparative Biochemistry and Physiology Part A Physiology, 2018, 118(4): 1377-1384. |
| [81] |
KENNEDY E J, ROBINSON S M C, PARSONS G J, et al. Studies on feed formulations to maximize somatic growth rates of juvenile green sea urchins (Strongylocentrotus droebachiensis)[J]. Special Publication Aquaculture Association of Canada, 2000(4): 68-71. |
| [82] |
GEORGE S B, FOX C, WAKEHAM S. Fatty acid composition of larvae of the sand dollar Dendraster excentricus (Echinodermata) might reflect FA composition of the diets[J]. Aquaculture, 2008, 285(1/2/3/4): 167-173. |
| [83] |
ZUO R T, LI M, DING J, et al. Higher dietary arachidonic acid levels improved the growth performance, gonad development, nutritional value, and antioxidant enzyme activities of adult sea urchin (Strongylocentrotus intermedius)[J]. Journal of Ocean University of China, 2018, 17(4): 932-940. DOI:10.1007/s11802-018-3606-7 |
| [84] |
BAUM N A, CONKLIN D E, CHANG E S. Effect of dietary lecithin in combination with casein or crab protein on cholesterol uptake and transport in the lobster Homarus americanus[J]. Journal of the World Aquaculture Society, 2010, 21(4): 277-287. |
| [85] |
TESHIMA S, KANAZAWA A, KAKUTA Y. Role of dietary phospholipids in the transport of [14C] tripalmitin in the prawn[J]. Bulletin of the Japanese Society of Scientific Fisheries, 1986, 52(3): 519-524. DOI:10.2331/suisan.52.519 |




