益生菌作为绿色饲料添加剂在家禽生产中得到广泛应用。乳酸菌(lactic acid bacteria,LAB)作为益生菌主要菌种,是动物肠道的常驻菌,与其他细菌相比具有独特的生理特性及益生功能[1],产生的酶能将饲粮中的粗蛋白质降解为小肽、游离氨基酸等小分子物质,增强营养物质的消化吸收,提高生长性能,促进免疫器官和免疫系统发育,提高抗病力[2-3]。乳酸菌可产生细菌素、乳酸、抑制蛋白等,抑制有害菌的生长[4];维持消化道微生态平衡,保持畜禽肠道健康,提高养殖的经济效益[5-6]。饲粮中添加乳酸菌可促进雏鸡肠绒毛发育,改变肠黏膜结构,提高营养物质吸收和利用[7];促进短链脂肪酸的形成,降低动物肠道pH,从而提高蛋白质溶解度,促进蛋白酶对蛋白质分解[8]。邓庆庆等[9]研究还发现,肉鸡饲喂乳酸菌制剂可以改善由黄曲霉毒素导致的生长性能下降,提高肉鸡营养物质表观代谢率,从而提高肉鸡生长性能。
乳酸菌菌种不耐高温,其固态制剂的干燥工艺多为真空冷冻干燥,成本过高,限制了其在动物养殖上使用。将乳酸菌菌液直接饲喂动物,则避免了干燥的环节,降低了使用成本。目前,乳酸菌菌液饲喂动物有多种方式,不同饲喂方式的效果缺乏比较。基于此,本试验通过饮水、灌服和拌料3种饲喂方式饲喂肉鸡乳酸菌菌液,研究其对肉鸡生长性能、粗蛋白质表观代谢率及肠道健康的影响,以期为乳酸菌菌液在肉鸡生产中科学应用提供依据。
1 材料与方法 1.1 试验材料试验动物:192只1日龄爱拔益加(AA)肉鸡。
试验菌种:植物乳杆菌(L. plantarum),本实验室前期筛选、鉴定并保存,试验前活化培养,活菌数为4×109 CFU/mL。
基础饲粮:购自洛阳某饲料厂,基础饲粮组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
将192只1日龄肉鸡随机分为4个组,每组6个重复,每个重复8只鸡。对照组(CON组)饲喂基础饲粮;拌料组(MIX组)在基础饲粮中添加乳酸菌菌液;灌服组(OI组)饲喂基础饲粮,并口腔灌服乳酸菌菌液;饮水组(DW组)饲喂基础饲粮,并在饮水中添加乳酸菌菌液。饲养试验在河南科技大学牧场进行,采用2阶段笼养。试验期42 d。肉鸡自由采食、饮水,正常控温光照,全程无免疫。
为保证各组肉鸡每日采食乳酸菌活菌数基本相等(约8×108 CFU)[10]。根据肉鸡在饲养过程中水料比3 ∶ 1原则、国家饲养标准及类似试验参考值,确定AA肉鸡平均日采食量(1~21日龄平均采食量48 g,22~42日龄平均采食量156 g),通过计算确定不同饲喂方式下1~21日龄和22~42日龄的乳酸菌菌液添加量,如表 2所示。MIX组每周及时进行拌料,OI组每日进行1次口腔灌服,DW组根据肉鸡饮水情况及时向水箱中补充混有乳酸菌菌液的饮用水。
|
|
表 2 试验设计 Table 2 Experimental design |
每周记录每个重复投料量、剩料量和饮水量,在21、42日龄禁食12 h后,对每个重复的肉鸡称重,计算平均日饮水量、平均日采食量、平均日增重和料重比。
1.3.2 粗蛋白质表观代谢率测定在21、42日龄时,每个重复取1只鸡,按照Steenfeldt等[11]的试验方法进行代谢试验,收集3 d的粪样及饲粮样品,依据《饲料分析及饲料质量检测技术》[12]利用半自动凯氏定氮仪测定粗蛋白质含量,粗蛋白质表观代谢率计算公式如下:

|
在21、42日龄时,每个重复随机抓取1只肉鸡进行屠宰,分离出空肠、回肠,各取2 cm肠段,生理盐水冲洗后放入4%多聚甲醛中固定保存,进行石蜡切片制作,苏木精-伊红(HE)染色后在光学显微镜下对组织形态进行观察。利用CaseViewer 2.4图像处理系统对肠道绒毛高度、绒毛宽度、隐窝深度进行测量,利用绒毛高度和绒毛宽度计算绒毛表面积,并计算绒毛高度与隐窝深度的比值(绒隐比,CD/VH)。
1.3.4 盲肠内容物菌群的Illumina高通量测序及盲肠活菌培养计数在21、42日龄时,每个重复随机取1只肉鸡屠宰,无菌采取左侧盲肠内容物,液氮保存,送上海元华生物科技有限公司,利用Illumina测序系统对盲肠内容物菌群基因组DNA的16S rRNA V3~V4区进行高通量测序和生物信息学分析[13]。
同时,取0.5 g肠道内容物于1 mL无菌生理盐水中,37 ℃摇床中培养30 min,稀释到10-7~10-2,用平板计数法计算大肠杆菌、志贺氏菌、乳酸菌和双歧杆菌数量。大肠杆菌采用EMB培养基,志贺氏菌采用SS培养基,好氧培养24 h;乳酸菌采用LBS培养基,双歧杆菌采用TPY培养基,厌氧培养48 h。
1.4 统计分析利用SPSS 20.0对试验数据进行统计分析,利用一般线性模型对数据进行单因素方差分析(one-way ANOVA)和Duncan氏法多重比较,数据以“平均值±标准差”表示,P < 0.05表示差异显著。
2 结果 2.1 乳酸菌不同饲喂方式对肉鸡生长性能的影响由表 3可知,在1~21日龄,DW组平均日采食量和平均日饮水量显著低于CON组(P < 0.05),MIX组、OI组和DW组料重比显著低于CON组(P < 0.05)。MIX组、OI组和DW组之间平均日采食量、平均日饮水量、平均日增重无显著差异(P>0.05),MIX组、DW组料重比显著低于OI组(P < 0.05)。
|
|
表 3 乳酸菌不同饲喂方式对肉鸡生长性能的影响 Table 3 Effects of different feeding methods of lactic acid bacteria on growth performance of broilers |
在22~42日龄,MIX组、OI组和DW组平均日增重显著高于CON组(P < 0.05),料重比显著低于CON组(P < 0.05)。MIX组、OI组和DW组之间平均日采食量、平均日饮水量、平均日增重和料重比无显著差异(P>0.05)。
在1~42日龄,DW组和MIX组平均日增重显著高于CON组(P < 0.05),MIX组、OI组和DW组料重比显著低于CON组(P < 0.05)。MIX组、OI组和DW组之间平均日采食量、平均日饮水量、平均日增重和料重比无显著差异(P>0.05)。
2.2 乳酸菌不同饲喂方式对肉鸡粗蛋白质表观代谢率的影响由表 4可知,21和42日龄时,MIX组、OI组和DW组粗蛋白质表观代谢率均显著高于CON组(P < 0.05)。21日龄时,MIX组、OI组和DW组之间粗蛋白质表观代谢率差异不显著(P>0.05);42日龄时,DW组粗蛋白质表观代谢率显著高于OI组(P < 0.05),与MIX组差异不显著(P>0.05)。
|
|
表 4 乳酸菌不同饲喂方式对肉鸡粗蛋白质表观代谢率的影响 Table 4 Effects of different feeding methods of lactic acid bacteria on crude protein apparent metabolic rate of broilers |
由表 5可知,21日龄时,在空肠中,MIX组、OI组和DW组绒毛高度、绒毛表面积和绒隐比均显著高于CON组(P < 0.05),隐窝深度显著低于CON组(P < 0.05)。MIX组、OI组和DW组绒毛高度、绒毛表面积差异不显著(P>0.05);DW组隐窝深度显著低于MIX组(P < 0.05),与OI组差异不显著(P>0.05);DW组绒隐比显著高于OI组(P < 0.05),与MIX组差异不显著(P>0.05)。在回肠中,MIX组绒毛高度、绒毛表面积、绒隐比显著高于CON组(P < 0.05),隐窝深度显著低于CON组(P < 0.05);DW组绒毛表面积、绒隐比显著高于CON组(P < 0.05),隐窝深度显著低于CON组(P < 0.05)。MIX组、OI组和DW组之间绒毛表面积、隐窝深度、绒隐比差异不显著(P>0.05);DW组绒毛高度显著低于MIX组(P < 0.05),与OI组差异不显著(P>0.05)。
|
|
表 5 乳酸菌不同饲喂方式对肉鸡肠道形态的影响 Table 5 Effects of different feeding methods of lactic acid bacteria on intestinal morphology of broilers |
42日龄时,在空肠中,OI组和DW组绒毛高度、绒毛表面积显著高于CON组(P < 0.05);MIX组、OI组和DW组隐窝深度显著低于CON组(P < 0.05),绒隐比显著高于CON组(P < 0.05)。DW组绒毛高度、绒隐比显著高于MIX组和OI组(P < 0.05),绒毛表面积显著高于MIX组(P < 0.05)。在回肠中,MIX组、OI组和DW组绒毛高度和绒隐比显著高于CON组(P < 0.05),MIX组和DW组绒毛表面积显著高于CON组(P < 0.05),DW组隐窝深度显著低于CON组(P < 0.05)。DW组绒毛高度、绒毛表面积、绒隐比显著高于MIX组和OI组(P < 0.05),MIX组、OI组和DW组之间隐窝深度差异不显著(P>0.05)。
2.4 乳酸菌不同饲喂方式对盲肠菌群的影响由表 6可知,21日龄时,各组操作分类单元(OTU)数量在465~692个,MIX组、OI组和DW组Chao指数和Shannon指数与CON组相比有所提高;42日龄时,各组OTU数量在557~650个,MIX组、OI组和DW组Chao指数和Shannon指数与CON组相比均有所提高,表明添加乳酸菌可提高菌落多样性和丰富度。各组样品的覆盖度(coverage)均在0.99以上,说明样品中序列已基本全部检测出。
|
|
表 6 盲肠内容物16S rRNA测序结果 Table 6 Sequencing results of 16S rRNA in cecal contents |
在门水平上,各组优势菌群如图 1所示。21日龄时,各组核心菌门为厚壁菌门、拟杆菌门;CON组厚壁菌门相对丰度最高,为88.37%,DW组拟杆菌门相对丰度最高,为25.50%。42日龄时,各组核心菌门为厚壁菌门、拟杆菌门、变形菌门、无壁菌门等;CON组厚壁菌门、变形菌门、无壁菌门相对丰度较高,分别为88.37%、2.75%、2.68%,DW组拟杆菌门相对丰度最高,为25.50%。
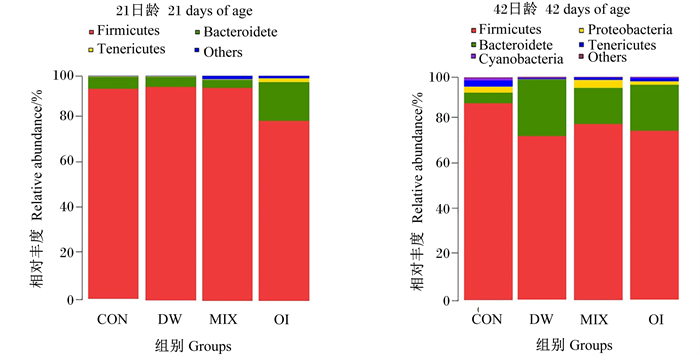
|
Firmicutes:厚壁菌门;Bacteroidete:拟杆菌门;Tenericutes:无壁菌门;Proteobacteria:变形菌门;Cyanobacteria:蓝细菌;Others:其他。 图 1 21、42日龄肉鸡盲肠内容物菌群结构(门水平) Fig. 1 Flora structure of cecal contents of broilers at 21 and 42 days of age (at phylum level) |
在属水平上,各组优势菌群如图 2所示。21日龄时,各组盲肠菌群主要菌属为粪球菌属;CON组粪球菌属相对丰度为36.77%,MIX组为18.75%,OI组为18.91%,DW组为33.67%。毛螺菌科未分类属也为优势菌属,MIX组毛螺菌科未分类属相对丰度为12.34%,OI组为9.86%,DW组为12.76%。CON组粪球菌属、梭菌属相对丰度较高,分别为36.77%、6.08%。MIX组克里斯滕森菌科R-7群相对丰度最高,为16.12%。OI组令枝菌属、乳杆菌属相对丰度较高,分别为8.58%、4.58%。DW组毛螺菌科未分类属、瘤胃球菌科UCG-014相对丰度较高,分别为12.76%、9.07%。
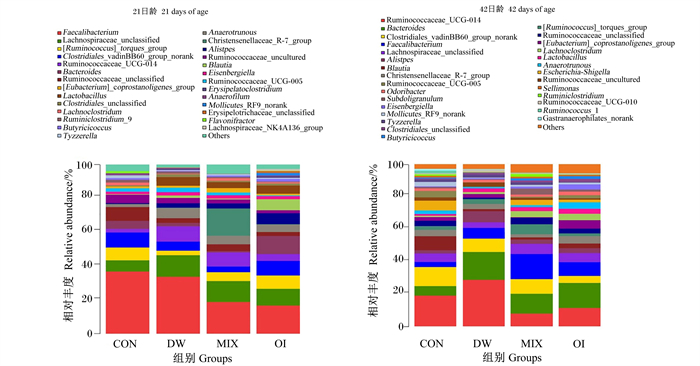
|
Faecalibacterium:粪球菌属;Lachnospiraceae_unclassified:毛螺菌科未分类属;[Ruminococcus]_torques_group:链瘤胃球菌群;Clostridiales_vadinBB60_group_norank:梭菌属;Ruminococcaceae_UCG-014:瘤胃球菌科UCG-014;Bacteroides:拟杆菌属;Ruminococcaceae_unclassified:瘤胃球科未分类属;Anaerotrunous:厌氧棍状菌属;Christensenellaceae_R-7_group:克里斯滕森菌科R-7群;Alistpes:令枝菌属;Ruminococcaceae_uncultured:瘤胃球科未分类属;Blautia:布劳特氏菌;Eisenbergiella:艾森伯格氏菌属;Ruminococcaceae_UCG-005:瘤胃球菌科UCG-005;[Eubacterium]_coprostanoligenes_group:真杆菌属;Lactobacillus:乳杆菌属;Clostridiales_unclassified:梭状芽胞杆菌未分类属;Lachnoclostridum:蓝绿藻菌属;Ruminiclostridium_9:瘤胃梭菌-9属;Butyricicoccus:丁酸球菌属;Tyzzerella:泰泽拉菌属;Erysipelatoclostridium:丹毒荚膜菌属;Anaerofilum:细杆菌属;Mollicutes_RF9_norank:柔膜细菌RF9属;Erysipelotrichaceae_unclassified:丹毒菌科未分类属;Flavonifractor:黄腐菌属;Lachnospiraceae_NK4A136_group:毛螺菌科NK4A136群;Escherichia-Shigella:埃希氏菌属;Odoribacter:内脏臭气杆菌;Subdoligranulum:罕见小球菌属;Sellimonas:肠道粪便单胞菌属;Ruminiclostridium:瘤胃梭菌属;Ruminococcaceae_UCG-010:瘤胃球菌科属UCG-010;Ruminococcus_1:链瘤胃球菌1属;Gastranaerophilates_norank:胃嗜气菌属。 图 2 21、42日龄肉鸡盲肠内容物菌群结构(属水平) Fig. 2 Flora structure of cecal contents of broilers at 21 and 42 days of age (at genus level) |
42日龄时,CON组布劳特氏菌属、梭菌属、埃希氏菌属相对丰度较高,分别为11.20%、8.63%、8.10%。MIX组毛螺菌科未分类属、粪球菌属相对丰度较高,分别为15.49%、9.24%。OI组乳杆菌属、克里斯滕森菌科R-7群相对丰度较高,分别为8.34%、4.60%。DW组梭菌属、拟杆菌属、令枝菌属相对丰度较高,分别为28.65%、17.17%、6.90%。
由表 7可知,21、42日龄时,MIX组、OI组和DW组盲肠双歧杆菌数量均显著高于CON组(P < 0.05),大肠杆菌数量显著低于CON组(P < 0.05)。21日龄时,DW组盲肠乳酸菌、双歧杆菌数量显著高于MIX组和OI组(P < 0.05),志贺氏菌数量显著低于CON组、MIX组和OI组(P < 0.05),大肠杆菌数量显著低于MIX组(P < 0.05)。42日龄时,DW组、OI组盲肠双歧杆菌数量显著高于MIX组(P < 0.05),大肠杆菌数量显著低于MIX组(P < 0.05)。
|
|
表 7 盲肠活菌计数结果 Table 7 Counting results of viable bacteria in cecum |
很多研究认为,乳酸菌可附着于动物肠黏膜表面,改善肠道结构,提高消化酶活性,促进蛋白质等有机物的消化吸收,从而提高对饲粮的代谢及利用率,在肠道中可以起到良好的益生作用[14-16]。研究发现,乳酸菌可通过提高采食量而提高肉鸡生长性能[17-18]。本试验在1~21日龄时,DW组平均日采食量显著低于CON组,与OI组和MIX组差异不显著。产生差异的原因可能是乳酸菌可提高营养物质代谢率,进而降低采食量。因为有研究发现,饲喂肉鸡嗜酸乳杆菌可显著提高粗蛋白质表观代谢率[19]。本试验42日龄时,MIX组、OI组和DW组粗蛋白质表观代谢率和平均日增重均高于CON组,也证实了这种可能性。此外,菌株的繁殖能力、能否代谢活性物质及产生有机酸的不同,也可能对结果产生影响[20]。本研究发现,DW组和MIX组生长性能和粗蛋白质表观代谢率无显著差异,且均优于OI组,可能是在饲喂过程中口腔灌服会导致肉鸡应激,使其精神萎靡,采食量减少,饲料利用率降低[21]。
3.2 乳酸菌不同饲喂方式对肉鸡肠道健康的影响肠道绒毛高度、隐窝深度和绒隐比是评价肠道功能的重要指标[22-23]。谷巍等[24]研究发现,乳酸菌通过增加肠道绒隐比以增强小鼠免疫力,降低小鼠腹泻率。肉鸡饲粮中添加乳酸菌,也可提高绒隐比,降低呕吐毒素污染饲粮对肉鸡的不良影响[25]。本研究中,以饮水方式饲喂乳酸菌菌液,肉鸡小肠绒毛表面积、绒隐比显著高于其他饲喂方式。分析其原因可能为:虽然本试验设计初为各组提供了等量乳酸菌,但受肉鸡采食习性影响(漏料、滴水等),加之拌料后菌种活菌数与菌液直接保存也存在细微差异,导致各组肉鸡每日实际摄入活菌数量存在一定差异,影响饲喂效果。其中,以饮水方式饲喂乳酸菌菌液可保证菌液混合均匀,减少误差,避免肉鸡应激,提高乳酸菌饲喂效果。
乳酸菌在肠道内增殖,代谢出乳酸,抑制致病菌的增殖,调节肠道菌群平衡[26]。本研究发现,肉鸡盲肠菌群在门水平上主要有厚壁菌门、拟杆菌门、无壁菌门、变形菌门,在属水平上主要有粪球菌属、拟杆菌属,与前人研究结果[27-28]一致。研究发现,厚壁菌门和拟杆菌门存在着共生关系,可共同促进宿主吸收或储存能量,若比例过高会导致宿主产生代谢综合征[29]。本试验发现,饲喂乳酸菌能降低厚壁菌门和拟杆菌门的相对丰度,促进肉鸡健康。
本试验发现,在属水平上,MIX组、OI组和DW组大肠杆菌、志贺氏菌等致病菌属相对丰度均低于CON组,与刘凤美等[30]研究结果一致。其中,以饮水方式饲喂乳酸菌菌液能显著降低有害菌数量。DW组瘤胃球菌科UCG-014相对丰度最高,其主要发酵产物为乙酸、甲酸,可以降低肠道pH,减少有害菌的增殖[31]。有报道表明,小鼠肠道菌群在乳酸菌干预后,乳杆菌属丰富度显著增加[32]。本试验中,不同方式饲喂乳酸菌均可增加盲肠乳杆菌属相对丰度,其中OI组、DW组盲肠乳杆菌属相对丰度较高,与活菌计数结果相一致。
4 结论本试验条件下,3种乳酸菌饲喂方式均可不同程度提高肉鸡生长性能和粗蛋白质表观代谢率,改善肠道形态和肠道菌群结构,增加有益菌数量,降低有害菌数量,其中以饮水方式饲喂效果更佳。
| [1] |
鲁宇橦, 陈群. 乳酸菌在畜禽生产中应用的研究进展[J]. 黑龙江畜牧兽医, 2019(3): 45-46, 51. LU Y T, CHEN Q. Research progress on application of lactic acid bacteria in livestock and poultry production[J]. Heilongjiang Animal Science and Veterinary Medicine, 2019(3): 45-46, 51 (in Chinese). |
| [2] |
PERCEVAL C, SZAJEWSKA H, INDRIO F, et al. Prophylactic use of probiotics for gastrointestinal disorders in children[J]. The Lancet Child & Adolescent Health, 2019, 3(9): 655-662. |
| [3] |
FABERSANI E, ABEIJON-MUKDSI M C, ROSS R, et al. Specific strains of lactic acid bacteria differentially modulate the profile of adipokines in vitro[J]. Frontiers in Immunology, 2017, 8: 266. |
| [4] |
YEH R H, HSIEH C W, CHEN K L. Screening lactic acid bacteria to manufacture two-stage fermented feed and pelleting to investigate the feeding effect on broilers[J]. Poultry Science, 2018, 97(1): 236-246. DOI:10.3382/ps/pex300 |
| [5] |
夏亿, 张元可, 徐晶云, 等. 发酵乳杆菌和凝结芽孢杆菌对产气荚膜梭菌感染肉鸡生长性能和肠道健康的影响[J]. 中国畜牧兽医, 2019, 46(10): 2927-2936. XIA Y, ZHANG Y K, XU J Y, et al. Effects of Lactobacillus fermentum and Bacillus coagulans on the growth performance and gut health of broilers challenged with Clostridium perfringens[J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(10): 2927-2936 (in Chinese). |
| [6] |
聂荷敏, 饶雷, 王媚, 等. 不同乳酸菌制剂对禽类感染空肠弯曲杆菌防治效果的研究[J]. 黑龙江畜牧兽医, 2019(3): 125-129, 182. NIE H M, RAO L, WANG M, et al. Effect of different lactic acid bacteria preparation on Campylobacter jejuni infection in poultry[J]. Heilongjiang Animal Science and Veterinary Medicine, 2019(3): 125-129, 182 (in Chinese). |
| [7] |
谢文惠, 姜宁, 张爱忠. 复合益生菌制剂对肉鸡生长性能、屠宰性能和免疫指标的影响[J]. 动物营养学报, 2018, 30(1): 360-367. XIE W H, JIANG N, ZHANG A Z. Effects of compound probiotics on growth performance, slaughter performance and immune indices of broilers[J]. Chinese Journal of Animal Nutrition, 2018, 30(1): 360-367 (in Chinese). |
| [8] |
袁磊, 唐瑜, 刘晓庚. 蛋白质消化率的影响因素研究[J]. 粮食科技与经济, 2015, 40(3): 43-46, 61. YUAN L, TANG Y, LIU X G. Research on the factors affecting digestibility of protein[J]. Grain Science and Technology and Economy, 2015, 40(3): 43-46, 61 (in Chinese). |
| [9] |
邓庆庆, 刘宁, 江青东, 等. 乳酸菌对饲喂黄曲霉毒素B1日粮的肉鸡生长性能、养分消化率和屠宰性能的影响[J]. 中国畜牧兽医, 2016, 43(5): 1194-1200. DENG Q Q, LIU N, JIANG Q D, et al. Effect of lactic acid bacteria on the growth performance, nutrient apparent digestibility and slaughter performance of broilers fed diets contaminated with AFB1[J]. China Animal Husbandry & Veterinary Medicine, 2016, 43(5): 1194-1200 (in Chinese). |
| [10] |
徐基利, 许丽. 不同乳酸菌及其添加水平对肉仔鸡生长性能、免疫机能和肠道结构的影响[J]. 动物营养学报, 2011, 23(11): 1976-1983. XU J L, XU L. Lactobacillus: strain and supplemental level on growth performance, immune function and intestinal structure of broilers[J]. Chinese Journal of Animal Nutrition, 2011, 23(11): 1976-1983 (in Chinese). |
| [11] |
STEENFELDT S, GONZÁLEZ Z, BACH KNUDSEN K E, et al. Effects of inclusion with blue lupins (Lupinus angustifolius) in broiler diets and enzyme supplementation on production performance, digestibility and dietary AME content[J]. Animal Feed Science and Technology, 2003, 110(1/2/3/4): 185-200. |
| [12] |
张丽英. 饲料分析及饲料质量检测技术[M]. 3版. 北京: 中国农业大学出版社, 2007: 52-60. ZHANG L Y. Feed analysis and feed detection technology[M]. 3rd ed. Beijing: China Agricultural University Press, 2007: 52-60 (in Chinese). |
| [13] |
SHI Y, MA D Y, ZHAI S W. Revealing the difference of intestinal microbiota composition of cultured European eels (Anguilla anguilla) with different growth rates[J]. Israeli Journal of Aquaculture-Bamidgeh, 2020, 72: 1-12. |
| [14] |
HORACKOVA S, VESELA K, KLOJDOVA I, et al. Bile salt hydrolase activity, growth characteristics and surface properties in Lactobacillus acidophilus[J]. European Food Research and Technology, 2020, 246(8): 1627-1636. DOI:10.1007/s00217-020-03518-8 |
| [15] |
张炎达, 潘慧青, 李珠金. 家禽无抗养殖中饲用乳酸菌应用研究进展[J]. 中国家禽, 2018, 40(11): 41-46. ZHANG Y D, PAN H Q, LI Z J. Research advances on application of feed Lactobacillus in antibiotic-free breeding in poultry[J]. China Poultry, 2018, 40(11): 41-46 (in Chinese). |
| [16] |
POORBAGHI S L, GHEISARI H, DADRAS H, et al. Effects of simple and microencapsulated Lactobacillus acidophilus with or without inulin on the broiler meat quality infected by avian influenza virus (H9N2)[J]. Probiotics and Antimicrobial Proteins, 2016, 8(4): 221-228. DOI:10.1007/s12602-016-9224-z |
| [17] |
谢童, 董涛, 王伟唯, 等. 复合乳酸菌制剂对黄羽肉鸡生长性能、胃肠道pH、肠道形态和盲肠微生物的影响[J]. 动物营养学报, 2022, 34(1): 254-263. XIE T, DONG T, WANG W W, et al. Effects of compound Lactobacillus preparation on growth performance, gastrointestinal pH, intestinal morphology and cecal microorganism of yellow-feathered broilers[J]. Chinese Journal of Animal Nutrition, 2022, 34(1): 254-263 (in Chinese). |
| [18] |
刘伟敏. 乳酸菌对仔猪生长性能、免疫功能和肠道菌群的影响[J]. 中国饲料, 2022(2): 33-37. LIU W M. Effect of lactic acid bacteria on immune function, anti-inflammatory factors and intestinal flora of piglets[J]. China Feed, 2022(2): 33-37 (in Chinese). |
| [19] |
祁凤华, 周立强, 马红, 等. 嗜酸乳杆菌对黄羽肉鸡生长性能及营养物质代谢率的影响[J]. 畜牧与兽医, 2014, 46(4): 17-19. QI F H, ZHOU L Q, MA H, et al. Effects of Lactobacillus acidophilus on growth performance and nutrient metabolic rate in yellow broilers[J]. Animal Husbandry & Veterinary Medicine, 2014, 46(4): 17-19 (in Chinese). |
| [20] |
刘婷, 李宗军, 廖勇, 等. 饲喂乳酸菌和茶多酚对肉鸡生长及生理生化的影响[J]. 中国农学通报, 2014, 30(11): 6-10. LIU T, LI Z J, LIAO Y, et al. Effects of Lactobacillus and tea polyphenols by gavage on growth and physiological and biochemical in broilers[J]. Chinese Agricultural Science Bulletin, 2014, 30(11): 6-10 (in Chinese). |
| [21] |
许丛霞. 引起肉鸡应激的几种非疾病性原因及预防措施[J]. 现代畜牧科技, 2016(7): 91. XU C X. Several non disease causes of stress in broilers and preventive measures[J]. Modern Animal Husbandry Science & Technology, 2016(7): 91 (in Chinese). |
| [22] |
EL AIDY S, VAN DEN BOGERT B, KLEEREBEZEM M. The small intestine microbiota, nutritional modulation and relevance for health[J]. Current Opinion in Biotechnology, 2015, 32: 14-20. DOI:10.1016/j.copbio.2014.09.005 |
| [23] |
徐静, 张子儒, 王德贺, 等. 饮水中添加大蒜精油对蛋鸡生长性能、肠道组织形态及盲肠菌群的影响[J]. 动物营养学报, 2021, 33(1): 308-316. XU J, ZHANG Z R, WANG D H, et al. Effects of adding garlic essential oil into drinking water on growth performance, intestinal tissue morphology and cecum microbial flora of layer hens[J]. Chinese Journal of Animal Nutrition, 2021, 33(1): 308-316 (in Chinese). |
| [24] |
谷巍, 王丽荣, 孙明杰, 等. 嗜酸乳杆菌发酵的复方中药对腹泻小鼠肠道菌群和结构的影响[J]. 中兽医医药杂志, 2020, 39(1): 8-13. GU W, WANG L R, SUN M J, et al. Effect of traditional Chinese medicine fermented by Lactobacillus on intestinal flora and structure of diarrhea mice[J]. Journal of Traditional Chinese Veterinary Medicine, 2020, 39(1): 8-13 (in Chinese). |
| [25] |
段永乐. 乳酸菌对采食呕吐毒素污染饲粮肉鸡肠道健康的影响[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2018. DUAN Y L. The effect of lactic acid bacteria on intestine healthy of broiler chickens challenged with deoxynivalenol[D]. Master's Thesis. Yangling: Northwest A&F University, 2018. (in Chinese) |
| [26] |
陈双双, 司华哲, 李光玉, 等. 动物肠道菌群与营养物质代谢的研究进展[J]. 饲料工业, 2018, 39(2): 33-36. CHEN S S, SI H Z, LI G Y, et al. Research progress of animal gut microbiota and nutrient metabolism[J]. Feed Industry, 2018, 39(2): 33-36 (in Chinese). |
| [27] |
XIAO Y P, XIANG Y, ZHOU W D, et al. Microbial community mapping in intestinal tract of broiler chicken[J]. Poultry Science, 2017, 96(5): 1387-1393. DOI:10.3382/ps/pew372 |
| [28] |
WEI S, MORRISON M, YU Z. Bacterial census of poultry intestinal microbiome[J]. Poultry Science, 2013, 92(3): 671-683. DOI:10.3382/ps.2012-02822 |
| [29] |
HAN G G, LEE J Y, JIN G D, et al. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing[J]. Applied Microbiology and Biotechnology, 2017, 101(14): 5903-5911. DOI:10.1007/s00253-017-8304-7 |
| [30] |
刘凤美, 张磊, 黄彬. 日粮添加益生菌对肉鸡生产性能、免疫功能和肠道菌群的影响[J]. 中国饲料, 2018(24): 39-43. LIU F M, ZHANG L, HUANG B. Effects of probiotics on production performance, immunologic function and gut bacteria of broiler chickens[J]. China Feed, 2018(24): 39-43 (in Chinese). |
| [31] |
黄东旭. 不同饮食方式下肠道菌群结构及粪便脂肪酸的关系[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2018. HUANG D X. The relationship between the structure of intestinal microbiota and fecal fatty acids with different diet patterns[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2018. (in Chinese) |
| [32] |
MOHD SHAUFI M A, SIEO C C, CHONG C W, et al. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses[J]. Gut Pathogens, 2015, 7: 4. DOI:10.1186/s13099-015-0051-7 |




