动物肌肉蛋白质作为维持机体正常生理功能的蛋白质,其在机体内合成和水解的动态平衡是调节肌肉量多少的重要过程,并且这一过程是在严密的信号网络调控下完成的。骨骼肌组织结构精密且具有高度可塑性,占畜禽胴体重量的50%~70%[1],其大小与功能由肌肉蛋白质的合成和降解精细调控。骨骼肌不仅是重要的运动器官,还可作为代谢器官调控机体的能量代谢,这对提高动物的生长性能与改善肉品质具有重要意义[2]。相关研究已经表明,动物肌肉组织蛋白质代谢由多条信号通路参与完成,如胰岛素样生长因子-1(insulin-like growth factors-1,IGF-1)/磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/蛋白激酶B(protein kinase B,Akt)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)/核转录因子-κB(nuclear factor kappa-B,NF-κB)和肌肉生长抑制素(myostatin,MSTN)/Smadt等,而无论是肌肉蛋白质的合成代谢还是分解代谢都与肉品质的形成密切相关。此外,肌肉组织发育也受到如miRNA、腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)、肠道微生物和脂肪酸的调控。基于此,本文对肌肉蛋白质代谢通路、调控机制及其对肉品质的影响进行系统地综述,这对深入探究肌肉组织蛋白质代谢和肉品质都具有重要意义。
1 肌肉蛋白质代谢的信号通路蛋白质是机体的重要组成部分,而肌肉蛋白质的合成和分解代谢由多条信号通路参与完成。
1.1 IGF-1/PI3K/AktIGF-1/PI3K/Akt信号转导首先是通过IGF-1配体与IGF-1受体(IGF-1R)的结合来实现的;这种结合将诱导PI3K发生聚集效应,进而可将膜磷酸肌醇磷酸化,由此产生的磷酸化酪氨酸为胰岛素受体底物1(recombinant insulin receptor substrate 1,IRS1)的募集创造了对接位点,Akt随后活化[3]。Akt家族是一种重要的丝氨酸/苏氨酸蛋白激酶,在肌肉蛋白质代谢方面发挥着至关重要的双向调控作用,它既可以促进蛋白质的合成[4],又可以调控蛋白质的降解[5]。Lai等[6]建立了一种可诱导的Akt模型,该模型证明即使在成年小鼠中,相对短期的Akt激活也会导致骨骼肌的质量增加1倍以上。Akt的3种异构体中,Akt1在平衡肌肉蛋白质方面作用效果显著。相关研究表明,Akt促进肌肉蛋白质的合成作用主要是通过哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)实现的[7],而调节叉头转录因子(forkhead box,FoxO)家族是Akt影响肌肉蛋白质降解的必要靶点[8]。
mTOR是一种重要的蛋白激酶,研究表明,Akt/mTOR可以通过其下游信号分子真核翻译起始因子4E结合蛋白1(eIF4E-binding protein 1,4EBP1)和p70核糖体蛋白S6激酶(p70 ribosomal protein S6 kinase,p70S6K)来调控蛋白质的合成。雷帕霉素受体复合物1(target of rapamycin complex 1,TORC1)通过磷酸化和激活p70S6K以及抑制4EBP1来传播下游信号,其中4EBP1的磷酸化受到TORC1的严格控制[9]。蛋白质合成的翻译过程主要分为起始、延长和终止3个阶段,而mTOR则可恰好通过p70S6K和4EBP1作用于蛋白质翻译的起始和延长阶段[7, 10]。FoxO是降解路径中的重要因子,当Akt磷酸化受到抑制时会导致FoxO去磷酸化,去磷酸化的FoxO激活后进入细胞核,最终导致肌肉蛋白质的降解[11]。
1.2 TNF-α/NF-κB在骨骼肌中,NF-κB以非活性状态停留在细胞质中,当机体中的TNF-α大量释放时,NF-κB被激活并从细胞质进入到细胞核内,进而调控肌肉萎缩F盒基因(muscle atrophy F-box,MAFbx)和肌肉特异性环指蛋白1(muscle ring-finger protein 1,MuRF1)的转录与表达,最终导致蛋白质发生降解[13](图 1)。此外,被激活的NF-κB也可通过泛素-蛋白酶体途径(ubiquitin-proteasome pathway,UPP)诱导MuRF1和MAFbx的表达进而调控肌肉蛋白质的代谢[14]。研究证实,UPP主要通过增加泛素蛋白酶体的活性及促进泛素活化酶E1、结合酶E2和连接酶E3相关调控基因的表达等方面来减少肌肉蛋白质的含量[15]。除TNF-α,还有部分因子可对NF-κB进行激活或抑制。目前还存在另一种理论,即过氧化物酶体增殖物激活受体(peroxisome proliferators-activated receptors,PPAR)γ的激活可以抑制NF-κB活性。Trindade等[16]在对PPAR家族特异性激动剂的筛选试验中发现,PPARγ的激活可有效抑制NF-κB活性。为证明此观点,Coll等[17]在研究报告中指出,GW501516(PPARδ的专一性激动剂)可以有效地抑制由饱和脂肪酸如棕榈酸诱导的NF-κB活性。
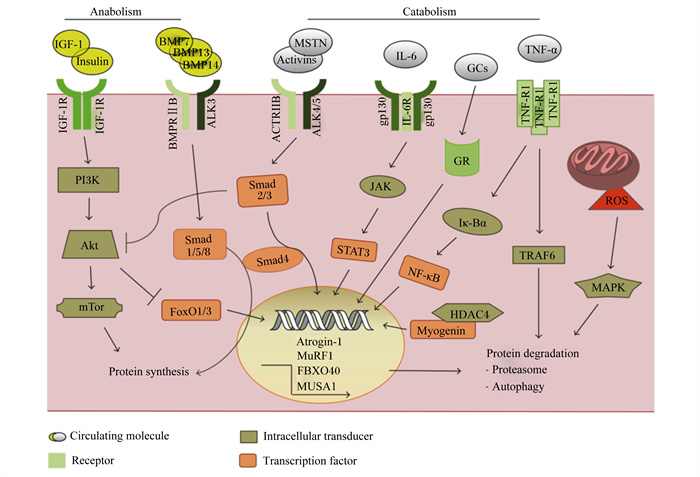
|
Anabolism:合成代谢;Catabolism:分解代谢;IGF-1:胰岛素样生长因子-1 insulin-like growth factors-1;IGF-1R:胰岛素样生长因子-1受体insulin-like growth factor receptor-1;Insulin:胰岛素;PI3K:磷脂酰肌醇3-激酶phosphatidylinositol 3-kinase;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;Akt:蛋白激酶B protein kinase B;protein synthesis:蛋白质合成;BMP7:骨形成蛋白-7 bone morphogenetic protein-7;BMP13:骨形成蛋白-13 bone morphogenetic protein-13;BMP14:骨形成蛋白-14 bone morphogenetic protein-14;ALK3:间变性淋巴瘤激酶3 anaplastic lymphoma kinase-3;BMPRⅡB:骨形态发生蛋白受体ⅡB bone morphogenetic protein receptor ⅡB;FoxO1/3:叉头转录因子1/3 forkhead box O1/3;MSTN:肌肉生长抑制素myostatin;Activins:激活素;ACTRIIB:激活素受体ⅡB activin receptor ⅡB;ALK4/5:间变性淋巴瘤激酶4/5 anaplastic lymphoma kinase-4/5;IL-6:白细胞介素-6 interleukin-6;gp130:信号转导因子糖蛋白130g lycoprotein 130;IL-6R:白介素-6受体interleukin-6 receptor;Atrogin-1:萎缩相关基因-1 atrophy gene-1;MuRF1:肌肉特异性环指蛋白1 muscle ring-finger protein-1;FBXO40:F-框蛋白40重组蛋白F-box 40 recombinant protein;JAK:蛋白酪氨酸激酶protein tyrosine kinase;STAT3:信号传导与转录激活因子3 signal transducer and activator of transcription-3;GR:糖皮质激素受体glucocorticoid receptor;GCs:糖皮质激素glucocorticoids;protein degradation:蛋白质降解;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;TNF-R1:肿瘤坏死因子受体1 tumor necrosis factor receptor 1;Iκ-Bα:核转录因子-κB抑制蛋白α nuclear factor kappa-B inhibitor protein α;NF-κB:核转录因子-κB nuclear factor kappa-B;TRAF6:肿瘤坏死因子受体相关蛋白6 receptor associated factor 6;HDAC4:组蛋白去乙酰化酶4 histone deacetylase-4;ROS:活性氧reactive oxygen species;MAPK:丝裂原活化蛋白激酶mitogen-activated protein kinase;Autophagy:自噬;Proteasome:蛋白酶体;Circulating molecule:循环分子;Intracellular transducer:细胞内传感器;Receptor:受体;Transcription factor:转录因子。 图 1 肌肉蛋白质代谢信号通路 Fig. 1 Muscle protein metabolism signaling pathway[12] |
MSTN已被学者认为是调控蛋白质代谢的重要因子[18]。该基因缺乏时易引起动物的双肌现象(图 2)。MSTN除了影响骨骼肌发育外,还对脂肪沉积起调节作用,这对平衡机体脂肪沉积和肌肉蛋白质代谢有重要意义。因此,MSTN逐渐成为目前的研究热点。
负调节通路MSTN/Smads的作用途径是:MSTN通过TGF-β超家族信号蛋白的活性达到抑制肌细胞生成素(myogenin)、生肌决定因子(myogenic differentiation antigen,MyoD)和配对盒转录因子3(paired box,PAX3)的活性及表达的作用,最终抑制肌肉蛋白质的合成(图 1)。在Smads家族中,Smad2、Smad3及Smad4这3种蛋白的高度表达可直接提高MSTN启动子的活性,进而增强MSTN的表达[19-20]。也有研究证实,MSTN与Akt信号通路在一定程度上存在关联。Chelh等[21]研究发现,当小鼠和牛体内缺乏MSTN基因时,Akt信号通路被上调。MSTN的表达可抑制Akt的磷酸化从而改变FoxO活性,抑制蛋白质的同化代谢。FoxO1还可以通过与MSTN启动子区域结合来上调MSTN的表达,进而抑制MyoD的表达,降低肌酸激酶(creatine kinase,CK)、生肌因子5(recombinant myogenic factor 5,Myf5)的活性。MSTN对蛋白质的同化代谢、异化代谢和脂肪沉积都有重要作用,可以用于调节瘦肉和脂肪的比例,因此后续有必要对其进行更深入的研究探讨。
2 肌肉蛋白质代谢的调控机制 2.1 miRNA对肌肉蛋白质代谢的调控miRNA是真核生物中高度保守的非编码RNA,长度约为22个单链RNA。随着对肌肉特异性miRNA(miR-1、miR-133a/b、miR-206、miR-208b、miR-499和miR-486)的深入研究,扩展了蛋白质代谢的分子网络结构。不同miRNA其功能存在差异(图 3)。Chen等[24]的试验首次证实了miRNAs的重要作用,该研究表明,miR-1(作用于IGF-1)和miR-133(作用于IGF-1R)可共同转录,这一项研究从侧面表明miRNA可调控IGF-1/PI3K/Akt信号通路。此外,miR-27a在成肌细胞增殖期间的过度表达可通过抑制MSTN表达来诱导肌肉蛋白质的合成作用[25]。研究表明,miR-199a3p会影响IGF-1和mTOR的表达,这表明抑制IGF-1/Akt/mTOR信号通路是miR-199a-3p调节肌生成的潜在机制之一[26]。此外,miR-128a在骨骼肌中高表达可抑制IGF-1信号通路中的靶基因,包括IRS1和P70S6K及磷酸化Akt水平[27]。Xu等[28]研究显示,miR-486可增加Akt的磷酸化水平,降低小鼠初级肌管中PTEN和FoxO1的蛋白表达水平。
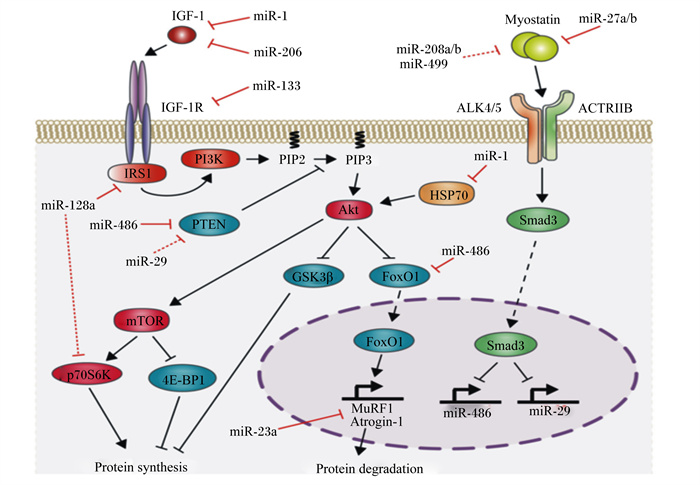
|
IGF-1:胰岛素样生长因子1insulin-like growth factors-1;IGF-1R:胰岛素样生长因子1受体insulin-like growth factor receptor-1;IRS1:胰岛素受体底物1 recombinant insulin receptor substrate 1;PI3K:磷脂酰肌醇3-激酶phosphatidylinositol 3-kinase;PIP2:磷脂酰肌醇二磷酸phosphatidylinositol bisphosphate;PIP3:磷脂酰肌醇三磷酸phosphatidylinositol triphosphate;Akt:蛋白激酶B protein kinase B;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;Myostatin肌肉生长抑制素;Atrogin-1:萎缩相关基因-1 atrophy gene-1;MuRF1:肌肉特异性环指蛋白1 muscle ring-finger protein 1;protein synthesis:蛋白质合成;protein degradation:蛋白质降解;PTEN:蛋白酪氨酸磷酸酶基因phosphatase and tensin homologue deleted on chromosome;GSK3β:糖原合成激酶3β glycogen synthesis kinase 3β;p70S6K:p70核糖体蛋白S6激酶p70 ribosomal protein S6 kinase;4EBP1:真核翻译起始因子4E结合蛋白1 eIF4E-binding protein 1;FoxO1:叉头转录因子1 forkhead box1;HSP70:热应激蛋白70 heat shock protein70;ACTRIIB:激活素受体ⅡB activin receptor ⅡB;ALK4/5:间变性淋巴瘤激酶4/5 anaplastic lymphoma kinase-4/5 图 3 miRNA对肌肉蛋白质的调控作用 Fig. 3 Regulation of miRNA on muscle protein development[29] |
AMPK已被定义为机体内重要的“能量感应器”。近年来,较多的研究表明,AMPK对平衡肌肉蛋白质代谢具有重要作用。Salminen等[30]试验表明,AMPK可调控蛋白质代谢的动态平衡,提示了AMPK可能是影响蛋白质代谢的潜在靶点。部分学者的观点表明,AMPK可促进蛋白质降解,导致蛋白质合成速率降低,因此AMPK对蛋白质的合成有着负调节作用。Kimura等[31]研究结果显示,AMPK促进蛋白质降解很有可能是抑制了mTOR信号通路。也有研究证实,AMPK可通过磷酸化真核细胞延伸因子2(eukaryotic elongation factor 2,eEF2)进而抑制蛋白质合成,其作用机理是AMPK通过改变eEF2激酶的活性进而抑制eEF2与核糖体间的相互作用,从而减少蛋白质的合成[32]。在培养的C2C12细胞中,利用AMPK激活剂进行干预,发现除了MAFbx和MuRF1的基因表达量均增加之外,肌纤维的降解程度也有增加的趋势[33]。王佳明[34]试验证实,热应激条件可激活肉鸡机体内的AMPK,最终导致骨骼肌的生长发育受到抑制。但关于AMPK的作用也有不同观点。Krawiec等[35]通过给小鼠注射5-氨基-4-甲酰胺咪唑核糖核苷酸(AICAR),探究AMPK对肌肉蛋白的作用;结果发现小鼠骨骼肌中MAFbx和MuRF1这些降解基因的mRNA表达量均降低。用亚油酸处理C2C12肌管,发现AMPK活性显著增强,同时伴随着肌肉蛋白质的合成增加[36]。因此,目前关于AMPK对肌肉蛋白质代谢的影响并无明确定论。
2.3 肠道菌群对肌肉蛋白质代谢的调控随着研究的不断深入,发现肠道微生物对蛋白质代谢的作用效果显著。营养物质经过消化道中微生物的分解和代谢后产生的代谢物——短链脂肪酸(short-chain fatty acids,SCFAs),可作为效应分子直接影响蛋白质的代谢和功能[37]。此外,SCFAs可通过增加蛋白激酶B(protein kinase B,PKB)的磷酸化程度以及IRS1的表达,为IGF-1蛋白质代谢信号通路的激活创造条件[1](图 4)。Lahiri等[38]比较了无菌小鼠和常规小鼠(无病原体)的肌肉,发现无菌小鼠肌肉中IGF-1基因的表达量减少,同时与线粒体功能相关基因的表达量降低。Bindels等[39]进行了一项通过改变肠道微生物组成从而影响肌肉组织的研究,他们给小鼠口服含有乳酸杆菌(Lactobacillus)和加氏乳酸杆菌(Lactobacillus gasseri)的益生菌,结果发现,这种益生菌可以降低MuRF1和萎缩相关基因-1(atrophy gene-1,Atrogin-1)的表达。Yan等[40]试验表明,SCFAs的使用可缓解由抗生素引起的蛋白质降解,IGF-1与肌肉质量均可恢复至使用抗生素之前的水平,因此推测微生物是通过SCFAs诱导IGF-1的表达进而影响蛋白质的代谢作用。Jang等[41]给小鼠饲喂清酒乳杆菌后发现,该菌株可诱导AMPK活化,提高沉默信息调节因子1(silent information regulator 1,SIRT1)和过氧化物酶体增殖物激活受体γ共激活因子-1α(peroxisome proliferator-activated receptor gamma co-activator-1α,PGC-1α)的表达,并抑制NF-κB的活化,从而缓解了小鼠肌肉蛋白质的降解作用。还有研究表明,乳酸杆菌可缓解由NF-κB介导的肌肉蛋白质降解[42]。此外,肠道菌群是十分高效的蛋白质代谢系统,大量研究证实,肠道中寄居着大量的有益菌如变形菌门、梭状芽孢杆菌等,都可对蛋白质的吸收和氨基酸的转运产生影响。
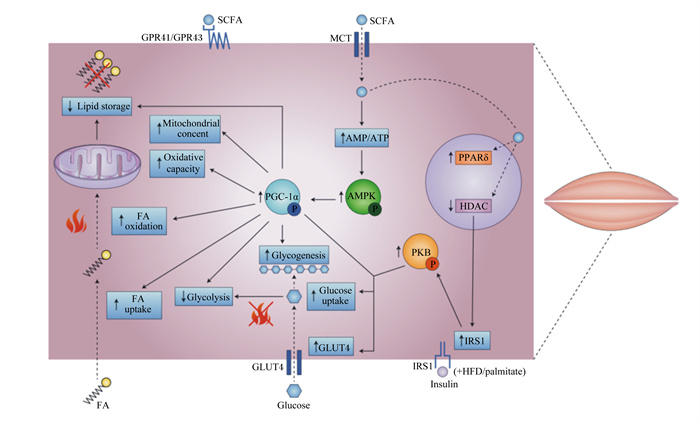
|
SCFA:不饱和脂肪酸short-chain fatty acids;MCT:中链甘油三酯medium chain triglycerides;AMP/ATP磷酸腺苷/三磷酸腺苷adenosine phosphate/adenosine triphosphate;AMPK:腺苷酸激活蛋白激酶AMP-activated protein kinase;PKB:蛋白激酶B protein kinase B;IRS1:胰岛素受体底物1 recombinant insulin receptor substrate 1;Insulin:胰岛素;palmitate:棕榈酸酯;PGC-1α:过氧化物酶体增殖物激活受体γ辅激活因子1α peroxlsome proliferator-activated receptor-γ coactlvator-1α;IRS1:胰岛素受体底物insulin receptor substrate;PPARδ:过氧化物酶体增殖物激活受体δ peroxlsome proliferator-activated receptor δ;HDAC:组蛋白去乙酰化酶histone deacetylase;Lipid storage:脂质储存;Mitochondrial concent:线粒体成分;Oxidative capacity:氧化能力;FA oxidation:脂肪酸氧化;FA uptake:脂肪酸摄取;Glycolysis:糖酵解;Glycogenesis:糖原生成;Glucose uptake:葡萄糖摄取;GPR41/GPR43:G蛋白偶联受体41 G protein-coupled receptor 41/43;GLUT4:葡萄糖转运蛋白;Glucose:葡萄糖;FA:脂肪酸。 图 4 SCFA对蛋白代谢信号通路相关调控因子的作用 Fig. 4 Effects of SCFA on related regulatory factors of protein metabolism signaling pathway[1] |
Seale等[43]研究表明,动物的脂肪细胞和骨骼肌细胞都来源于共同的祖细胞——胚胎间质干细胞(大部分发育为肌肉细胞,小部分发育成脂肪细胞),并且脂肪细胞和肌肉细胞可相互转化。近年来,越来越多的研究揭示了脂肪代谢的产物脂肪酸对蛋白质代谢的重要调控作用。
很多研究表明,脂肪代谢产生的脂肪酸可调控蛋白质的合成与降解,且不同类型的脂肪酸发挥的作用略有差异。如棕榈酸(饱和脂肪酸)会抑制IGF-1信号途径,促进萎缩基因MAFbx的表达,并伴随着转录调节因子FoxO3核定位的增加,从而使得肌肉蛋白质发生降解[44];而二十二碳六烯酸(DHA)可以降低由棕榈酸诱导的肌肉萎缩,其作用机制可能是降低了FoxO3入核的作用以及Atrogin-1/MAFbx基因的表达[45];此外,二十碳五烯酸(EPA)和DHA等不饱和脂肪酸亦可通过诱导Akt/mTOR信号通路使得蛋白质的合成作用加强[46]。Gingras等[47]的研究证明了以上观点,即不饱和脂肪酸EPA和DHA可以通过激活Akt/mTOR信号通路加强蛋白质合成。陈逢[48]研究了添加鱼油对仔猪肌肉蛋白质代谢过程的影响,发现腓肠肌和背最长肌中的蛋白质含量明显提高,同时鱼油增加了Aktl的mRNA表达量,降低了FoxO1和FoxO4的mRNA表达量,因此鱼油可能影响了Akt/FoxO信号通路,最终抑制了肌肉蛋白质的降解。近年来,也有证据表明,骨骼肌中EPA和DHA可以通过抑制白细胞介素-1受体相关激酶(IL-1 receptor associated kinase,IRAK1)磷酸化来阻止NF-κB进入细胞核,抑制MAFbx和MuRF1(蛋白质降解标志基因)的表达。此外,还有相关文献表明,脂肪酸及其衍生物还通过调控氨基酸的转运方式进而平衡蛋白质。Nardi等[49]将亚油酸(C18 ∶ 2)与大鼠L6肌管共同孵育,发现亚油酸显著抑制了中性氨基酸转运蛋白(sodium-coupled neutral amino acid transporter 2,SNAT2)的活化和表达。
3 肌肉蛋白质对肉品质的影响 3.1 肌肉蛋白质对风味的影响风味是最重要的感官属性,而蛋白质是吸附风味物质的重要基质。在人们的普遍认知中,蛋白质影响风味的物质主要是由于其降解产生的物质,该过程中产生的小分子肽和游离氨基酸赋予肉品独特的风味[50]。然而在更多情况下,肌肉蛋白质在特定的空间构象和结构下,与不同种类风味物质进行物理或者化学吸附,从而改变风味化合物的浓度。这种吸附作用主要是由于氨基酸侧链结构的多样性,使得蛋白质能与不同种类的风味成分进行可逆结合[51]。蛋白质与风味物质的结合位点在蛋白质疏水区[52],图 5显示了蛋白质与挥发性风味物质(醛类和酮类化合物)的相互作用。诱导蛋白质构象发生变化的主要因素还包括蛋白质的种类和浓度、加热温度、pH以及蛋白质氧化,这些因素可直接影响蛋白质与风味物质的相互作用[53],其中蛋白质的种类和浓度是影响二者相互作用的最重要因素[54]。吕彤等[55]通过试验建立了猪肉肌球蛋白-风味化合物作用的复合体系,为后续风味与蛋白质的研究奠定基础。周昌瑜等[54]也对此进行了研究,发现不同浓度肌原纤维蛋白对风味化合物的结合能力不同,如当肌原纤维蛋白浓度在2~6 mg/mL时,二者的结合作用力明显增强;而当肌原纤维蛋白浓度升至8 mg/mL时,吸附能力显著降低。究其原因,前者可能是因为较高的蛋白质浓度改变了风味化合物在液相和气相之间的分配系数;后者可能是由于浓度的增加导致了蛋白质-蛋白质之间的作用增强或表面张力降低,从而削弱了蛋白质与风味物质的结合能力。这与O’neill等[56]的研究结果一致,即在一定范围内,蛋白质浓度与风味的释放呈现负相关关系,推测可能是由于蛋白质浓度上升导致表面张力降低(肌原纤维蛋白是有效的表面张力抑制剂),从而对风味成分释放产生影响。此外,由于氨基酸的组成及侧链结构、蛋白质空间构象不同,不同种类的蛋白质与挥发性成分的结合能力也存在明显差异[57]。Pérez-Juan等[58]在研究肌动球蛋白和肌动蛋白(F-肌动蛋白和G-肌动蛋白)与风味化合物的相互作用时发现,G-肌动蛋白与风味物质基本无结合作用,而肌动球蛋白和F-肌动蛋白作用较为明显,该试验还证实了吸附能力的大小与蛋白质的浓度密切相关。肌肉蛋白质与风味物质的结合也受到风味物质类型(碳链长度、分支程度、官能团)和蛋白质氧化等因素的影响。
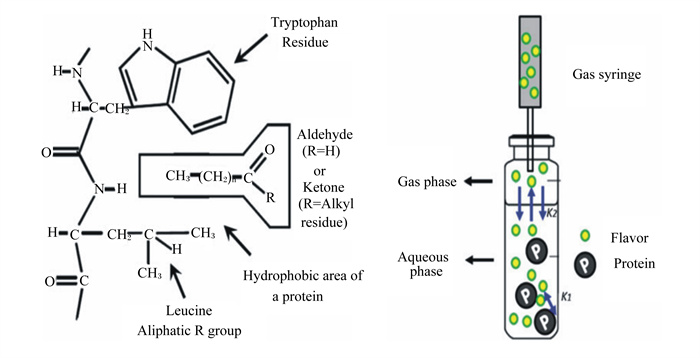
|
Tryptophan residue:色氨酸残基;Aldehyde:醛;ketone:酮;R=Alkyl residue:R=烷基残基;Hydrophobic area of a protein:蛋白质的疏水区域;Leucine:亮氨酸;Aliphatic R group:脂肪族R基团;Gas phase:气相;Aqueous phase:水相;Flavor protein:风味蛋白质;Gas syringe:气体注射器;K1:气相和水相之间的风味分配系数the flavor partition coefficient between the gas phase and the water phase;K2:蛋白质和风味化合物之间的结合系数the binding coefficient between the protein and the flavor compound。 蛋白质与风味物质(醛和酮)的疏水相互作用(左);静态顶空法测定蛋白质和风味化合物之间的结合(右)。 Hydrophobic interaction between protein and flavor substances (aldehydes and ketones) (left); static headspace method to determine the binding between protein and flavor compounds (right). 图 5 肌肉蛋白质与风味化合物 Fig. 5 Muscle protein and flavor compounds[59] |
大量研究表明,肉色稳定性与肌肉中蛋白质密切相关,其中肌红蛋白对肉色的贡献达到80%~90%。研究证实,不同色泽肌肉中高铁肌红蛋白(metmyoglobin,MetMb)的相对含量相差25%~50%[60]。肌肉蛋白质变性是影响肉持水性的重要原因,带有静电荷的肌肉蛋白质具有吸引水分的作用,且蛋白质与蛋白质之间的静电斥力使二者间出现间隙以容纳更多的水分。动物屠宰后肌肉的pH下降到蛋白质的等电点(PI)时(如肌球蛋白PI=5.4),蛋白质-蛋白质分子相互靠近,容纳水分的空间缩小,部分水分被挤出[61]。另外,宰后肌肉持水性与骨架蛋白降解密切相关。魏秀丽等[62]试验显示,宰后肌肉的持水性呈现下降-上升-下降的趋势,进一步研究后发现,钙蛋白酶引起的肌原纤维蛋白降解可影响水的分布及相互迁移,最终导致该现象的发生。
3.3 肌肉蛋白质对嫩度的影响嫩度作为影响消费者对肉品评价的重要指标,在肉品研究中受到广泛关注。目前,普遍认为肌肉蛋白质中肌动蛋白的解离状态是影响肉品嫩度的主要原因。此外,肌原纤维蛋白的主要组成成分如原肌球蛋白、伴肌动蛋白和肌钙蛋白,其结构状态也会直接影响肌球蛋白与肌动蛋白二者的结合,最终对嫩度产生直接影响[63]。近年来,越来越多的证据表明,宰后成熟过程中嫩度的改善与肌肉骨架蛋白的降解作用密切相关。Koohmaraie[64]通过试验推测,畜禽宰后成熟过程中,由于肌球蛋白及肌动蛋白的连接结构较弱,肉的嫩度较好。Takahashi[65]研究发现,兔肉在宰后僵直期只能提取到少量的肌球蛋白和肌动蛋白,因而嫩度较差;而成熟阶段的提取量会明显提高。Okitani等[66]研究发现,当肌肉中含有浓度较多的肌动蛋白及肌球蛋白时,肉的剪切力较低,嫩度较好。也有研究表明参与肌肉收缩的蛋白均能被磷酸化修饰,且这一过程受到成熟时间的影响,因此推测宰后僵直阶段肌原纤维蛋白的磷酸化过程对肉的嫩度具有重要作用[67]。
4 小结综上所述,肌肉蛋白质作为维持动物骨骼肌正常生理功能的重要蛋白质,也直接影响着肉的风味、嫩度和色泽等肉品质指标。大量研究已经证实其代谢过程需要IGF-1/PI3K/Akt、TNF-α/NF-κB和MSTN/Smad等多条信号通路共同参与。此外,基因水平、能量代谢、肠道菌群以及脂肪代谢都会对肌肉蛋白质代谢产生潜在的影响,因此后续研究可以从以上角度出发,深入探究其对蛋白质代谢的影响及作用机制,进而达到提高畜禽生长性能和改善肉品质的目的。
| [1] |
FRAMPTON J, MURPHY K G, FROST G, et al. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function[J]. Nature Metabolism, 2020, 2(9): 840-848. DOI:10.1038/s42255-020-0188-7 |
| [2] |
刘壮. 畜禽骨骼肌生长发育规律及其调控机制[J]. 饲料博览, 2019(9): 8-13. LIU Z. Growth and development of skeletal muscle in livestock and poultry and regulation mechanism[J]. Feed Review, 2019(9): 8-13 (in Chinese). DOI:10.3969/j.issn.1001-0084.2019.09.003 |
| [3] |
SACHECK J M, OHTSUKA A, MCLARY S C, et al. IGF-Ⅰ stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1[J]. American Journal of Physiology.Endocrinology and Metabolism, 2004, 287(4): E591-E601. DOI:10.1152/ajpendo.00073.2004 |
| [4] |
ORELLANA R A, SURYAWAN A, WILSON F A, et al. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs[J]. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 2012, 302(6): R682-R690. DOI:10.1152/ajpregu.00259.2011 |
| [5] |
WANG X N, HU Z Y, HU J P, et al. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling[J]. Endocrinology, 2006, 147(9): 4160-4168. DOI:10.1210/en.2006-0251 |
| [6] |
LAI K M V, GONZALEZ M, POUEYMIROU W T, et al. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy[J]. Molecular and Cellular Biology, 2004, 24(21): 9295-9304. DOI:10.1128/MCB.24.21.9295-9304.2004 |
| [7] |
BODINE S C, STITT T N, GONZALEZ M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo[J]. Nature Cell Biology, 2001, 3(11): 1014-1019. DOI:10.1038/ncb1101-1014 |
| [8] |
MILAN G, ROMANELLO V, PESCATORE F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy[J]. Nature Communications, 2015, 6: 6670. DOI:10.1038/ncomms7670 |
| [9] |
SARBASSOV D D, GUERTIN D A, ALI S M, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex[J]. Science, 2005, 307(5712): 1098-1101. DOI:10.1126/science.1106148 |
| [10] |
ZANCHI N E, LANCHA A H, J r. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis[J]. European Journal of Applied Physiology, 2008, 102(3): 253-263. DOI:10.1007/s00421-007-0588-3 |
| [11] |
CROSSLAND H, CONSTANTIN-TEODOSIU D, GARDINER S M, et al. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle[J]. The Journal of Physiology, 2008, 586(22): 5589-5600. DOI:10.1113/jphysiol.2008.160150 |
| [12] |
COSTAMAGNA D, COSTELLI P, SAMPAOLESI M, et al. Role of inflammation in muscle homeostasis and myogenesis[J]. Mediators of Inflammation, 2015, 2015: 805172. |
| [13] |
BAKKAR N, GUTTRIDGE D C. NF-kappa B signaling: a tale of two pathways in skeletal myogenesis[J]. Physiological Reviews, 2010, 90(2): 495-511. DOI:10.1152/physrev.00040.2009 |
| [14] |
PIJET B, PIJET M, LITWINIUK A, et al. TNF-α and IFN-s-dependent muscle decay is linked to NF-κB- and STAT-1α-stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes[J]. Mediators of Inflammation, 2013, 2013: 171437. |
| [15] |
LECKER S H, GOLDBERG A L, MITCH W E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states[J]. Journal of the American Society of Nephrology, 2006, 17(7): 1807-1819. DOI:10.1681/ASN.2006010083 |
| [16] |
TRINDADE B C, CHEN G Y. NOD1 and NOD2 in inflammatory and infectious diseases[J]. Immunological Reviews, 2020, 297(1): 139-161. DOI:10.1111/imr.12902 |
| [17] |
COLL C, LAMBERTY G, JENKINS R, et al. An integrative model for the study of developmental competencies in minority children[J]. Child Development, 1996, 67(5): 1891-1914. DOI:10.2307/1131600 |
| [18] |
曹婷, 周汉林, 荀文娟, 等. MSTN基因对猪骨骼肌发育调控的作用及其研究进展[J]. 基因组学与应用生物学, 2017, 36(4): 1511-1517. CAO T, ZHOU H L, XUN W J, et al. The effect of MSTN gene on the regulation of skeletal muscle development of pig and its research progress[J]. Genomics and Applied Biology, 2017, 36(4): 1511-1517 (in Chinese). |
| [19] |
ALLEN D L, UNTERMAN T G. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors[J]. American Journal of Physiology.Cell Physiology, 2007, 292(1): C188-C199. DOI:10.1152/ajpcell.00542.2005 |
| [20] |
阮井玲, 甄鑫, 刘娣, 等. Myostatin通过Smad3下调MyoD的表达来抑制骨骼肌卫星细胞的分化[J]. 中国生物工程杂志, 2008, 28(5): 99-103. RUAN J L, ZHEN X, LIU D, et al. Myostatin inhibits myogenic satellite cell differentiation through down-regulating MyoD expression by Smad3[J]. China Biotechnology, 2008, 28(5): 99-103 (in Chinese). |
| [21] |
CHELH I, PICARD B, HOCQUETTE J F, et al. Myostatin inactivation induces a similar muscle molecular signature in double-muscled cattle as in mice[J]. Animal, 2011, 5(2): 278-286. DOI:10.1017/S1751731110001862 |
| [22] |
KAMBADUR R, SHARMA M, SMITH T P, et al. Mutations in myostatin (GDF8) in double-muscled Belgian blue and Piedmontese cattle[J]. Genome Research, 1997, 7(9): 910-916. DOI:10.1101/gr.7.9.910 |
| [23] |
MOSHER D S, QUIGNON P, BUSTAMANTE C D, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs[J]. PLoS Genetics, 2007, 3(5): e79. DOI:10.1371/journal.pgen.0030079 |
| [24] |
CHEN J F, MANDEL E M, THOMSON J M, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation[J]. Nature Genetics, 2006, 38: 228-233. DOI:10.1038/ng1725 |
| [25] |
HUANG Z Q, CHEN X L, YU B, et al. MicroRNA-27a promotes myoblast proliferation by targeting myostatin[J]. Biochemical and Biophysical Research Communications, 2012, 423(2): 265-269. DOI:10.1016/j.bbrc.2012.05.106 |
| [26] |
JIA L, LI Y F, WU G F, et al. MiRNA-199a-3p regulates C2C12 myoblast differentiation through IGF-1/AKT/mTOR signal pathway[J]. International Journal of Molecular Sciences, 2013, 15(1): 296-308. DOI:10.3390/ijms15010296 |
| [27] |
MOHAMED J S, HAJIRA A, PARDO P S, et al. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1α network in skeletal muscle[J]. Diabetes, 2014, 63(5): 1546-1559. DOI:10.2337/db13-1364 |
| [28] |
XU J, LI R S, WORKENEH B, et al. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486[J]. Kidney International, 2012, 82(4): 401-411. DOI:10.1038/ki.2012.84 |
| [29] |
HITACHI K, TSUCHIDA K. Role of microRNAs in skeletal muscle hypertrophy[J]. Frontiers in Physiology, 2013, 4: 408. |
| [30] |
SALMINEN A, KAARNIRANTA K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network[J]. Ageing Research Reviews, 2012, 11(2): 230-241. DOI:10.1016/j.arr.2011.12.005 |
| [31] |
KIMURA N, TOKUNAGA C, DALAL S, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway[J]. Genes to Cells, 2003, 8(1): 65-79. DOI:10.1046/j.1365-2443.2003.00615.x |
| [32] |
刘启梁. eEF2K与肿瘤[J]. 生命的化学, 2016, 36(5): 633-638. LIU Q L. Eukaryotic elongation factor 2 kinase and cancer[J]. Chemistry of Life, 2016, 36(5): 633-638 (in Chinese). |
| [33] |
JENSEN T E, WOJTASZEWSKI J F P, RICHTER E A. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient?[J]. Acta Physiologica, 2009, 196(1): 155-174. DOI:10.1111/j.1748-1716.2009.01979.x |
| [34] |
王佳明. 热应激下ABCG2介导AMPK通路调节肉鸡肌肉生长发育及机理[D]. 硕士学位论文. 合肥: 安徽农业大学, 2019. WANG J M. ABCG2-mediated AMPK pathway regulates muscle growth and development in broilers under heat stress[D]. Master's Thesis. Hefei: Anhui Agricultural University, 2019. (in Chinese) |
| [35] |
KRAWIEC B J, NYSTROM G J, FROST R A, et al. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells[J]. American Journal of Physiology.Endocrinology and Metabolism, 2007, 292(6): E1555-E1567. DOI:10.1152/ajpendo.00622.2006 |
| [36] |
任阳. 饱和与不饱和脂肪酸对猪肌纤维组成的影响及其AMPK途径研究[D]. 博士学位论文. 杭州: 浙江大学, 2014. REN Y. Effect of saturated and unsaturated fatty acids on porcine muscle fiber composition and AMPK expression[D]. Ph. D. Thesis. Hangzhou: Zhejiang University, 2014. (in Chinese) |
| [37] |
MARIÑO E, RICHARDS J L, MCLEOD K H, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes[J]. Nature Immunology, 2017, 18(5): 552-562. DOI:10.1038/ni.3713 |
| [38] |
LAHIRI S, KIM H, GARCIA-PEREZ I, et al. The gut microbiota influences skeletal muscle mass and function in mice[J]. Science Translational Medicine, 2019, 11(502): eaan5662. DOI:10.1126/scitranslmed.aan5662 |
| [39] |
BINDELS L B, BECK R, SCHAKMAN O, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model[J]. PLoS One, 2012, 7(6): e37971. DOI:10.1371/journal.pone.0037971 |
| [40] |
YAN J, HERZOG J W, TSANG K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(47): E7554-E7563. DOI:10.1073/pnas.1607235113 |
| [41] |
JANG H M, HAN S K, KIM J K, et al. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation[J]. Molecular Nutrition & Food Research, 2019, 63(6): e1800978. |
| [42] |
RUSSELL S T, TISDALE M J. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide[J]. Molecular and Cellular Biochemistry, 2009, 330(1/2): 171-179. |
| [43] |
SEALE P, BJORK B, YANG W L, et al. PRDM16 controls a brown fat/skeletal muscle Switch[J]. Nature, 2008, 454(7207): 961-967. DOI:10.1038/nature07182 |
| [44] |
BRYNER R W, WOODWORTH-HOBBS M E, WILLIAMSON D L, et al. Docosahexaenoic acid protects muscle cells from palmitate-induced atrophy[J]. ISRN Obesity, 2012, 2012: 647348. |
| [45] |
WOODWORTH-HOBBS M E, HUDSON M B, RAHNERT J A, et al. Docosahexaenoic acid prevents palmitate-induced activation of proteolytic systems in C2C12 myotubes[J]. The Journal of Nutritional Biochemistry, 2014, 25(8): 868-874. DOI:10.1016/j.jnutbio.2014.03.017 |
| [46] |
ANDRÉE-ANNE G, WHITE P J, CHOUINARD P Y, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity[J]. The Journal of Physiology, 2007, 579(1): 269-284. DOI:10.1113/jphysiol.2006.121079 |
| [47] |
GINGRAS A A, WHITE P J, CHOUINARD P Y, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-Mtor-S6K1 pathway and insulin sensitivity[J]. The Journal of Physiology, 2007, 579(1): 269-284. DOI:10.1113/jphysiol.2006.121079 |
| [48] |
陈逢. 鱼油通过TLR4和NOD信号通路对脂多糖诱导的仔猪肠道、肝脏损伤和肌肉蛋白质降解的调控作用[D]. 硕士学位论文. 武汉: 武汉轻工大学, 2013. CHEN F. Regulative role of fish oil on intestinal and liver injury, and muscle protein degradation of piglets after lipopolysaccharide challenge through TLR4 and NOD signaling pathway[D]. Master's Thesis. Wuhan: Wuhan Polytechnic University, 2013. (in Chinese) |
| [49] |
NARDI F, HOFFMANN T M, STRETTON C, et al. Proteasomal modulation of cellular SNAT2 (SLC38A2) abundance and function by unsaturated fatty acid availability[J]. The Journal of Biological Chemistry, 2015, 290(13): 8173-8184. DOI:10.1074/jbc.M114.625137 |
| [50] |
CHEN Q, LIU Q, SUN Q X, et al. Flavour formation from hydrolysis of pork sarcoplasmic protein extract by a unique LAB culture isolated from Harbin dry sausage[J]. Meat Science, 2015, 100: 110-117. DOI:10.1016/j.meatsci.2014.10.001 |
| [51] |
GUICHARD E. Interactions between flavor compounds and food ingredients and their influence on flavor perception[J]. Food Reviews International, 2002, 18(1): 49-70. DOI:10.1081/FRI-120003417 |
| [52] |
WU S Y, PÉREZ M D, PUYOL P, et al. Beta-lactoglobulin binds palmitate within its central cavity[J]. The Journal of Biological Chemistry, 1999, 274(1): 170-174. DOI:10.1074/jbc.274.1.170 |
| [53] |
BOYER C, JOANDEL S, ROUSSILHES V, et al. Heat-induced gelation of myofibrillar proteins and myosin from fast- and slow-twitch rabbit muscles[J]. Journal of Food Science, 1996, 61(6): 1138-1143. DOI:10.1111/j.1365-2621.1996.tb10948.x |
| [54] |
周昌瑜, 蒋娅婷, 曹锦轩, 等. 肌原纤维蛋白浓度对风味物质吸附能力的影响[J]. 核农学报, 2016, 30(5): 904-911. ZHOU C Y, JIANG Y T, CAO J X, et al. Effect of the myofibril proteins concentrations on the adsorbing capacity for the flavor compounds[J]. Journal of Nuclear Agricultural Sciences, 2016, 30(5): 904-911 (in Chinese). |
| [55] |
吕彤, 林俊杰, 周昌瑜, 等. 热处理强度对猪肉肌球蛋白结构及风味成分吸附特性的影响[J]. 农业工程学报, 2016, 32(8): 285-291. LV T, LIN J J, ZHOU C Y, et al. Effect of heat treatment intensity on structure and binding capacity of volatile compounds of myosin[J]. Transactions of the Chinese Society of Agricultural Engineering, 2016, 32(8): 285-291 (in Chinese). |
| [56] |
O'NEILL E, MORRISSEY P A, MULVIHILL D M. The surface-active properties of muscle proteins[J]. Food Chemistry, 1990, 35(1): 1-12. DOI:10.1016/0308-8146(90)90125-N |
| [57] |
TAN Y, SIEBERT K J. Modeling bovine serum albumin binding of flavor compounds (alcohols, aldehydes, esters, and ketones) as a function of molecular properties[J]. Journal of Food Science, 2007, 73(1): 56-63. DOI:10.1111/j.1750-3841.2007.00591.x |
| [58] |
PÉREZ-JUAN M, FLORES M, TOLDRÁ F. Binding of aroma compounds by isolated myofibrillar proteins: effect of protein concentration and conformation[J]. Food Chemistry, 2007, 105(3): 932-939. DOI:10.1016/j.foodchem.2007.04.051 |
| [59] |
WANG K, ARNTFIELD S D. Effect of protein-flavour binding on flavour delivery and protein functional properties: a special emphasis on plant-based proteins[J]. Flavour and Fragrance Journal, 2017, 32(2): 92-101. DOI:10.1002/ffj.3365 |
| [60] |
LEDWARD D A. Post-slaughter influences on the formation of metyyoglobin in beef muscles[J]. Meat Science, 1985, 15(3): 149-171. DOI:10.1016/0309-1740(85)90034-8 |
| [61] |
OFFER G, KNIGHT P, JEACOCKE R, et al. The structural basis of the water-holding, appearance and toughness of meat and meat products[J]. Food Struture, 1989, 8(1): 17. |
| [62] |
魏秀丽, 谢小雷, 张春晖, 等. 猪宰后肌肉体系中μ-calpain及肌原纤维蛋白理化特性的变化规律[J]. 中国农业科学, 2015, 48(12): 2428-2438. WEI X L, XIE X L, ZHANG C H, et al. The variations inμ-calpain and physico-chemical characteristics of myofibrillar proteins in postmortem porcine muscle[J]. Scientia Agricultura Sinica, 2015, 48(12): 2428-2438 (in Chinese). DOI:10.3864/j.issn.0578-1752.2015.12.016 |
| [63] |
HO C Y, STROMER M H, ROBSON R M. Identification of the 30 kDa polypeptide in post mortem skeletal muscle as a degradation product of troponin-T[J]. Biochimie, 1994, 76(5): 369-375. DOI:10.1016/0300-9084(94)90110-4 |
| [64] |
KOOHMARAIE M. Biochemical factors regulating the toughening and tenderization processes of meat[J]. Meat Science, 1996, 43(S1): 193-201. |
| [65] |
TAKAHASHI K. Structural weakening of skeletal muscle tissue during post-mortem ageing of meat: the non-enzymatic mechanism of meat tenderization[J]. Meat Science, 1996, 43(S1): 67-80. |
| [66] |
OKITANI A, ICHINOSE N, ITOH J, et al. Liberation of actin from actomyosin in meats heated to 65 ℃[J]. Meat Science, 2009, 81(3): 446-450. DOI:10.1016/j.meatsci.2008.09.008 |
| [67] |
HUANG H G, LARSEN M R, PALMISANO G, et al. Quantitative phosphoproteomic analysis of porcine muscle within 24h postmortem[J]. Journal of Proteomics, 2014, 106: 125-139. DOI:10.1016/j.jprot.2014.04.020 |





