2. 中国水产科学研究院淡水渔业研究中心/农业农村部长江下游渔业生态环境监测中心/农业农村部水产品质量安全环境因子风险评估实验室, 无锡 214081
2. Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences/Fishery Eco-Environment Monitoring Center of Lower Reaches of Yangtze River, Ministry of Agriculture/Laboratory of Quality & Safety Risk Assessment for Aquatic Products on Environmental Factors, Ministry of Agriculture, Wuxi 214081, China
集约化养殖过程中因饲料过量投喂易造成饲料营养物质过剩等问题,研究表明其可能引起鱼类肠黏膜屏障功能被破坏以及固有免疫发生改变,进而诱发肠道慢性低度炎性反应[1]。近年来,学者们对鱼类肠道健康有关的研究越来越全面、深入。肠道是动物体内的消化吸收器官,通过其自身屏障功能(机械屏障、化学屏障和微生物屏障)防止机体受到肠腔内物理损伤和有害微生物的伤害[2]。
白藜芦醇(resveratrol,Res)是一种存在于多种植物当中的天然多酚类化合物,广泛存在于葡萄、花生、虎杖、桑葚等植物中[3]。众多研究表明,Res为NAD+依赖的组蛋白去乙酰化酶Sirt1激动剂,具有抗氧化、抗衰老、降脂、炎症免疫调节、抗病毒等众多药理学功能[4-5],被喻为继紫杉醇之后第二大抗癌药物[6]。而EX527作为Sirt1的高效抑制剂[7],可进一步验证Res对Sirt1的调控。有研究证明,用经过Res强化后卤虫喂养斑马鱼,可促进其肠道绒毛发育,提高肠道抗氧化能力,维护肠道健康[8]。胡瑶莲等[9]研究发现,饲粮中添加Res可调节育肥猪结肠菌群,增强机体抗氧化与空肠黏膜抗炎能力,改善肠道健康。Ershad等[10]研究表明,Res可赋予高膳食脂肪引起的肠上皮细胞对炎症的抵抗力,以及改善由此导致的屏障功能障碍,但此类报道较少。因此,本试验以红罗非鱼为研究对象,通过构建高脂模型,饲料中添加Sirt1激活剂Res及抑制剂EX527,探究Res对高脂饲喂下罗非鱼肠道形态和肠道内炎症因子含量的调控情况,为鱼类保护肠道饲料添加剂的开发提供参考依据,同时也为研究Res如何调控Sirt1基因及下游信号通路奠定基础。
1 材料与方法 1.1 试验鱼及饲养试验用鱼为红罗非鱼(O. mossambicus♀×O. niloticus♂),取自中国水产科学研究院淡水渔业研究中心屺亭科研试验基地。用普通饲料于室内循环流水玻璃缸(100 cm×60 cm×50 cm) 内暂养2周,选择健康红罗非鱼[(30.0±0.5)g/尾]为试验对象,分为4组,每组3个玻璃缸,每缸放置30尾鱼,分别投喂不同的饲料。试验9周,每天投喂2次(08:00和16:00),饲料用量占体重比4%,记录每缸鱼的摄食量。缸内注水约200 L,每天换水约1/3,控制水温(28±2) ℃、溶解氧含量为5~7 mg/L、pH 6.8~7.9,试验用水符合《渔业水质标准》(GB 11607—1989)。
1.2 试验饲料参考文献[11]配制试验饲料,其组成及营养水平参见表 1,设置8%脂肪(A组)、10%脂肪(高脂饮食,B组)、10%脂肪+0.025 g/kg Res (C组)、10%脂肪+0.025 g/kg Res+5 mmol/L EX527(D组)4组。Res(98%)购自西安某生物科技有限公司。将各种饲料原料粉碎,经过40目筛,称重、混匀,在高脂饲料中分别添加0.025 g/kg Res和5 mmol/L抑制剂EX527,制成粒径2 mm的普通颗粒沉水饲料,自然晒干后置于-20 ℃冰柜中保存备用。
|
|
表 1 试验饲料组成及营养水平(风干基础) Table 1 Composition and nutrient levels of experimental diets (air-dry basis) |
分别在养殖开始后第3、6、9周进行无菌取样工作。取样前饥饿24 h,MS-222麻醉后称量,参考文献[11]计算增重率(WGR)、特定生长率(SGR)及饲料系数(FCR)。每缸随机取8尾鱼,进行生物学测量,测量体长、体重,将鱼体置于冰上,用剪刀剪开鱼体腹部,随即取出内脏团,用镊子将腹脂剥离,称量腹脂的重量,计算腹脂率,随后将腹脂于-80 ℃冰箱保存。参考文献[12]选择中肠部位,取肠道组织称重,按照1 : 9 (m ∶ V)加入pH 7.4磷酸盐缓冲液(PBS)(已过滤除菌),用电动匀浆器将样本充分研磨匀浆。2 500 r/min离心20 min,收集上清液于灭菌冻存管中,置于-80 ℃冰箱保存。取中肠部位肠道内容物于2 mL离心管,置于-80 ℃冰箱保存。取部分中肠组织用生理盐水冲洗,并用4%甲醛保存固定,按常规方法制成扫描电镜材料,观察并拍照[13-14]。在碎冰上采集尾静脉血1 mL于1.5 mL离心管中静置10 min左右,2 000 r/min离心1~2 min,取上清液保存于-80 ℃冰箱中。
1.4 血清及肠道中炎症因子及脂多糖(LPS)含量测定采用南京建成生物工程研究所的酶联免疫吸附剂测定(ELISA)试剂盒对4组红罗非鱼中肠组织肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)、白细胞介素-10(IL-10)、转化生长因子-β(TGF-β)含量,及血清与肠道内容物内LPS含量进行检测,操作方法严格按照说明书进行。
1.5 计算公式
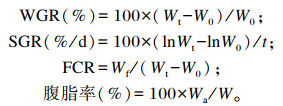
|
式中:W0为初始鱼总重量(g);Wt为终末鱼总重量(g);W为单尾鱼重量(g);Wf为摄入的饲料总量(干物质基础,g);Wa为腹脂重(g); t为饲喂天数(d)。
1.6 统计分析试验数据用SPSS 25进行单因素方差分析(one-way ANOVA)和组间LSD多重比较,数据表示为平均值±标准误,以P < 0.05作为差异显著水平,使用OriginPro 2018进行相关图形的绘制。
2 结果与分析 2.1 Res对红罗非鱼生长性能及腹脂率的影响由表 2可知,第3、6和9周,B组红罗非鱼体重、WGR、SGR均显著高于A组(P < 0.05),C组较B组均显著降低(P < 0.05)。第3周,各组间体长无显著差异(P>0.05),第6和9周,B组体长显著高于A组(P < 0.05)。第3、6和9周,B组FCR显著低于A组(P < 0.05),且C组较B组显著降低(P < 0.05)。
|
|
表 2 Res对红罗非鱼生长性能的影响 Table 2 Effects of Res on growth performance of red tilapia |
|
|
表 3 Res对红罗非鱼腹脂率的影响 Table 3 Effects of Res on abdominal fat rate of red tilapia |
|
|
表 4 100×时视野内GC细胞数量 Table 4 Number of GC number in visual field at 100× |
由表 3可知,第3和6周,B组腹脂率显著高于其他3组(P < 0.05)。第3至9周,C组腹脂率较B组显著降低(P < 0.05),而第3和9周,D组腹脂率较C组有所升高。
2.2 红罗非鱼肠道组织学观察结果分析由图 1可知,A31、B31、D31所示A、B、D组肠道环状皱襞均存在破损现象,D31环状皱襞结构紊乱且向内增生。由C32和A32、B32、D32对比发现,C组中肠杯状细胞(goblet cell, GC)数量多于其他组。
|
A~D分别对应A组、B组、C组、D组;A31、B31、C31、D31为各组第3周4×视野,A32、B32、C32、D32为各组第3周100×视野;箭头处为GC。 A to D corresponds to group A, group B, group C, group D, respectively; A31, B31, C31 and D31 were 4× of visual field in week 3 of each group, and A32, B32, C32 and D32 were 100× of visual field in the week 3 of each group; GC at arrow. 图 1 第3周红罗非鱼中肠横切图(HE染色) Fig. 1 Cross section of red tilapia midgut in week 3 (HE staining) |
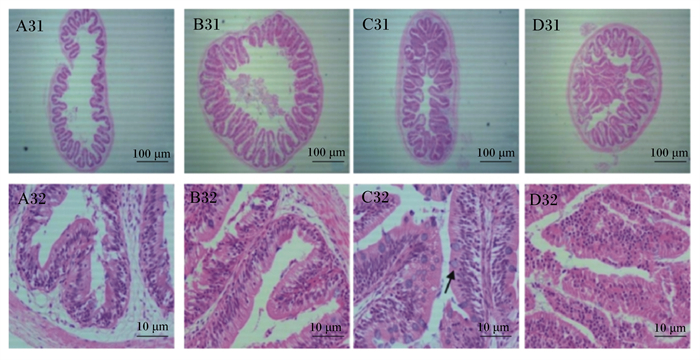
由图 2可知,A61、B61、C61、D61所示A、B、C、D组肠道环状皱襞均存在破损现象,A组环状皱襞结构紊乱且向内增生。根据A62、B62、C62、D62所示,各个组中肠均有GC存在。
|
A~D分别对应A组、B组、C组、D组;A61、B61、C61、D61为各组第6周4×视野,A62、B62、C62、D62为各组第6周100×视野;箭头处为GC。 A to D corresponds to group A, group B, group C, group D, respectively; A61, B61, C61 and D61 were 4× of visual field in the week 6 of each group, and A62, B62, C62 and D62 were 100× of visual field in the week 6 of each group; GC at arrow. 图 2 第6周红罗非鱼中肠横切图(HE染色) Fig. 2 Cross section of red tilapia midgut in week 6 (HE staining) |
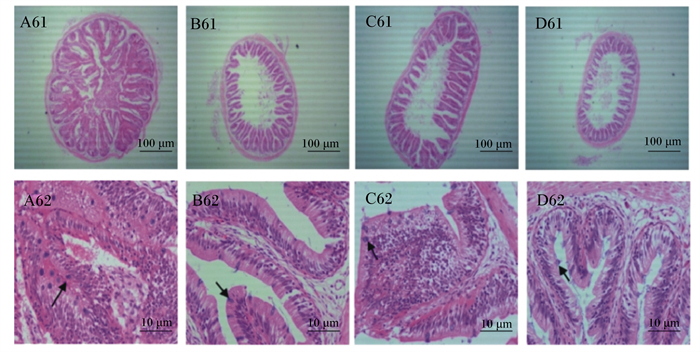
由图 3可知,B91所示B组肠道环状皱襞存在破损现象。由C92和A92、B92、D92对比发现,C组中肠GC数量多于其他组。
|
A~D分别对应A组、B组、C组、D组;A91、B91、C91、D91为各组第9周4×视野,A92、B92、C92、D92为各组第9周100×视野;箭头处为GC。 A to D corresponds to group A, group B, group C, group D, respectively; A91, B91, C91, D91 were 4× of visual field in the week 9 of each group, A92, B92, C92, D92 were 100× of visual field in the week 9 of each group; GC at arrow. 图 3 第9周红罗非鱼中肠横切图(HE染色) Fig. 3 Cross section of red tilapia midgut in week 9 (HE staining) |
由表 4可知,第3和6周,B组中肠GC数量较正常组有所降低但差异不显著(P>0.05);第3和9周,C组中肠GC数量显著多于其他各组(P < 0.05)。第6周,C组中肠GC细胞数量显著低于A、B组且少于第3和9周。
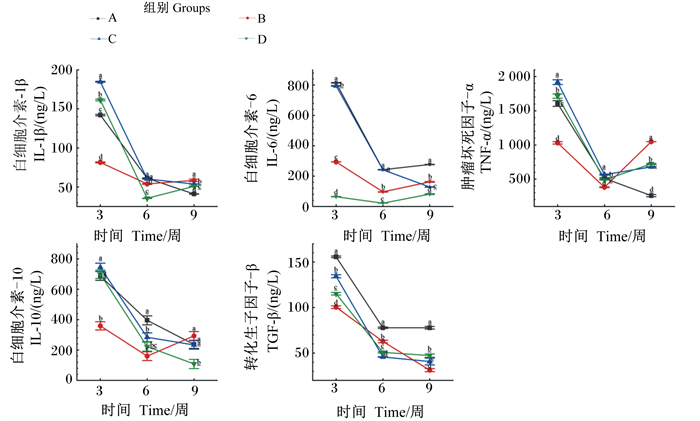
2.3 红罗非鱼肠道内炎症因子含量分析由图 2可知,促炎因子中,投喂3周后C组红罗非鱼中肠组织IL-1β含量显著高于其他组(P < 0.05);6周后D组含量显著低于B组(P < 0.05);9周后B组含量最高。投喂3周后A组中肠组织IL-6含量最高,B组含量显著高于D组(P < 0.05);6周后D组含量显著低于B组(P < 0.05);9周后A组含量最高,C、D组显著低于B组(P < 0.05),且C组含量均随时间呈下降趋势。投喂3和6周后C组中肠组织TNF-α含量均最高,9周后B组含量显著高于其他组(P < 0.05)。
抗炎因子中,投喂3和6周后,B组中肠组织IL-10含量显著低于A组,C组含量显著高于B组(P < 0.05)。投喂3、6、9周后各组间中肠组织TGF-β含量均存在显著差异(P < 0.05),且B组含量均显著低于A组,第3和9周C、D组含量均显著高于B组(P < 0.05)。
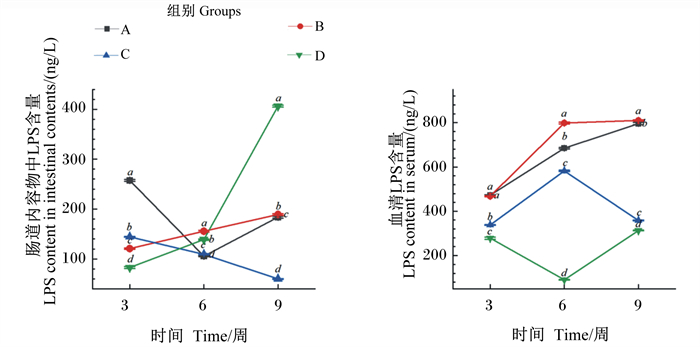
2.4 红罗非肠道血清及肠道内容物LPS含量分析由图 5可知,投喂3周后,各组间肠道内容物中LPS含量均存在显著差异(P < 0.05),其中A组含量显著高于其他组(P < 0.05),D组含量显著低于B组(P < 0.05)。血清中LPS含量A组和B组无显著差异(P < 0.05),但显著高于C组和D组(P < 0.05)。
|
图中标注不同小写字母表示差异显著(P < 0.05)。图 5同。 Different small letters in the figure mean significant difference (P < 0.05). The same as Fig. 5. 图 4 红罗非鱼肠道组织匀浆中不同细胞因子含量 Fig. 4 Contents of different cytokines in intestinal tissue homogeneity of red tilapia |
|
图 5 红罗非鱼肠道血清和肠道内容物中LPS含量 Fig. 5 LPS content in serum and intestinal contents of red tilapia |
投喂6周后,血清及肠道内容物中LPS含量均B组最高,且各组间存在显著差异(P < 0.05)。
投喂9周后,血清及肠道内容物LPS含量各组间均存在显著差异(P < 0.05)。其中血清LPS含量B组显著高于其他组(P < 0.05);肠道内容物中LPS含量D组显著高于其他组(P < 0.05),C组显著低于A组和B组(P < 0.05),且随时间呈下降趋势。
3 讨论 3.1 Res对高脂饲料红罗非鱼生长性能及腹脂率的影响Res作为一种天然的植物源抗毒素,越来越多的研究表明其具有调控动物生长性能的作用。本试验中,Res添加组的体重、SGR和WGR显著低于高脂组,Res添加组腹脂率显著低于高脂组,初步说明Res能够调控高脂胁迫下红罗非鱼的体重。而高脂红罗非鱼腹脂沉积过多可能与高脂导致鱼体内脂肪代谢紊乱,脂质的利用和储存不平衡密切相关[15]。研究表明Res对鱼体内脂肪代谢具有调控作用,并能通过减少鱼体内的脂肪沉积来降低增重[16]。之前我们的试验表明Res在一定程度上促进肠道健康并提高饲料利用率[17-18],本试验中添加Res后红罗非鱼FCR也显著降低。这提示Res对腹脂沉积有较好的调控作用,但Res如何缓解高脂胁迫下脂肪代谢紊乱还有待进一步研究。
3.2 Res对高脂饲料红罗非鱼肠道结构的影响鱼类主要靠肠道吸收营养物质,影响肠道消化吸收能力主要因素包括黏膜褶皱的长短和疏密、纹状缘的发达程度以及GC数量的多少[19]。GC是产生和分泌黏蛋白的单细胞腺。黏蛋白形成黏液层,将腔内物质与肠上皮隔开,以各种方式防止病原微生物入侵,对发挥正常的肠黏膜屏障功能有重要意义。有研究表明,长期食用富含饱和脂肪酸成分的高脂饮食,通过诱导肥胖和高胰岛素血症使野生型小鼠的GC数量减少[20]。这与本试验中投喂高脂饲料后GC数量低于对照组的结果相似,这些结果都提示摄入高脂饲料(红罗非鱼饲喂10%脂肪)可能会导致鱼体肠道通透性增加,肠黏膜被破坏。而添加Res后第3和9周,GC细胞数量和尺寸均明显多于高脂组和添加抑制剂组,与Zhang等[21]研究发现小鼠口服Res后的结果(表现为GC特征黏液相关蛋白质mRNA表达量显著提高)相似,从侧面说明Res可能通过GC缓解因高脂导致的肠黏膜损伤。有报道称畜禽饲粮添加植物提取物可提高动物肠道GC数量,改善肠道屏障作用[22],但大多数结果都表明这种改善作用可能与作用方式和处理时间相关。由于目前探讨Res作用的多数研究采用的干预时间为8~16周[23],本研究第6周高脂添加Res后GC数量少于第3和9周,可能与Res投喂6周后,由于时间较短,Res未来得及发挥修复高脂导致的红罗非鱼肠道损伤作用,而随着时间的推移,在第9周肠道损伤有所缓解有关。由此推测,Res能和天然多酚类植物提取物具有多种肠道生物活性一样,增加GC数量,缓解肠道损伤,但用量和时间不同可能效果不一致[17]。
3.3 Res对高脂饲料红罗非鱼炎症因子含量的影响炎症是机体对刺激的一种防御反应。有许多细胞因子参与炎症反应应答,促炎和抗炎因子在多种炎症反应中起重要作用[24]。促炎因子中,IL-1β主要是由单核细胞、巨噬细胞分泌的一种能激活多种免疫和炎症细胞的高效炎性细胞因子[25]。IL-6是一种与免疫有关的多效炎症细胞因子[26]。TNF-α是一种能引起生物和细胞反应,如淋巴细胞和白细胞的活化等的有效细胞因子[27]。抗炎因子中,IL-10具有抑制巨噬细胞和Th1细胞分泌因子以及促进B细胞增殖和抗体生成等功能[28]。而TGF-β具有调控细胞的生长、代谢、凋亡以及分化等功能[29-30]。促-抗炎因子的动态变化决定机体炎症反应程度,一般正常情况下,两者维持在相对平衡的状态。
研究表明高脂饮食不仅会引起血脂代谢紊乱,还会使机体长期处于慢性炎症状态[31]。本试验中,第9周高脂组的肠组织IL-1β、TNF-α含量高于正常脂肪组而TGF-β含量较正常组显著降低,表明高脂饲料刺激红罗非鱼机体中的炎症细胞因子比例失衡而导致炎症产生[32]。而添加Res后,第9周肠组织IL-1β、IL-6和TNF-α含量低于高脂组而TGF-β含量较高脂组显著升高,仅从炎症信号通路看,说明Res可能通过上调细胞因子中的抗炎细胞因子和下调促炎细胞因子含量,缓解肠道炎症损伤[32]。因为在小鼠上得到了类似结果[33],从作用机制方面来说,Res能够在一定程度上上调小鼠组织或细胞中Sirt1的表达,进而调控炎症因子及相关信号传导通路来抑制炎症反应[34-35]。除此以外,还有很多研究表明Res能够通过氧化应激信号通路缓解高脂所带来的肠道损伤[8, 11],若想以本试验结果为基础,探究Res是否仅调控Sirt1及其下游通路,还需设计更为严谨的试验来进一步探究。
3.4 Res对高脂饲料红罗非鱼肠道内LPS含量的影响LPS又称内毒素,是革兰氏阴性菌细胞壁的主要成分。高脂饮食可引起肠道菌群失调,致使结肠内LPS含量增加[36]。本试验中,第6~9周,高脂组血清和肠道内容物中LPS含量显著高于正常组,且肠道内容物中LPS含量随时间呈上升趋势,提示高脂饲粮可能会引起肠道微生物群变化,扰乱肠道免疫系统[37]。而LPS含量增加在短时间内会引起细菌感染症状和刺激巨噬细胞释放许多炎症因子,如TNF-α、白细胞介素-1(IL-1)、IL-6等大量分泌[38-39],引起炎症反应和组织损伤。本试验中,第6~9周,高脂饮食组炎症因子IL-1β、IL-6含量随着肠道内容物LPS含量的升高而升高。翟秋红[40]的试验也表明,LPS会增加细胞中炎症细胞因子mRNA的表达量,以上结果进一步验证了LPS会诱导细胞引发炎症。而添加Res后6~9周,肠道内容物及血清中LPS含量、促炎因子含量随着时间逐渐降低,添加抑制剂EX527后,LPS含量逐渐升高,因抑制剂具有阻碍作用,表明Res能够降低LPS含量及其诱导的炎症反应。还有研究表明,Res作为一种天然化合物,可通过下调核转录因子-κB(NF-κB)的活化来调节肠道细胞对细菌衍生分子(如LPS)的炎症反应[41]。Res会不会调节NF-κB信号通路等机制研究仍需进一步探讨。
4 结论① 高脂饲料会引起红罗非鱼腹腔脂肪沉积,还会导致LPS含量增加从而造成肠道炎症反应并损伤肠道结构组织。
② 高脂饲料添加Res后可促进红罗非鱼的生长,降低腹脂率,同时调节促炎和抗炎因子的平衡,增加中肠段GC数量,减少LPS含量,从而保护肠道功能完整性,有益于肠道健康。
| [1] |
KORTMAN G A M, MULDER M L M, RICHTERS T J W, et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens[J]. European Journal of Immunology, 2015, 45(9): 2553-2567. DOI:10.1002/eji.201545642 |
| [2] |
呙于明, 刘丹, 张炳坤. 家禽肠道屏障功能及其营养调节[C]//中国畜牧兽医学会动物营养学分会第七届中国饲料营养学术研讨会论文集. 郑州: [s. n], 2014: 363. GOU Y M, LIU D, ZHANG B K, et al. Intestinal barrier function and nutritional regulation in poultry[C]//The Seventh Chinese Symposium on Feed Nutrition, Animal Nutrition Branch of Chinese Animal Husbandry and Veterinary Association. Zhengzhou: [s. n], 2014: 363. (in Chinese) |
| [3] |
PARK E J, PEZZUTO J M. The pharmacology of resveratrol in animals and humans[J]. Biochimica et Biophysica Acta, 2015, 1852(6): 1071-1113. DOI:10.1016/j.bbadis.2015.01.014 |
| [4] |
MAGYAR K, HALMOSI R, PALFI A, et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease[J]. Clinical Hemorheology and Microcirculation, 2012, 50(3): 179-187. DOI:10.3233/CH-2011-1424 |
| [5] |
BO S, CICCONE G, CASTIGLIONE A, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial[J]. Current Medicinal Chemistry, 2013, 20(10): 1323-1331. DOI:10.2174/0929867311320100009 |
| [6] |
李先宽, 李赫宇, 李帅, 等. 白藜芦醇研究进展[J]. 中草药, 2016, 47(14): 2568-2578. LI X K, LI H Y, LI S, et al. Advances in study on resveratrol[J]. Chinese Traditional and Herbal Drugs, 2016, 47(14): 2568-2578 (in Chinese). DOI:10.7501/j.issn.0253-2670.2016.14.030 |
| [7] |
VELÁSQUEZ D A, MARTÍNEZ G, ROMERO A, et al. The central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin[J]. Diabetes, 2011, 60(4): 1177-1185. DOI:10.2337/db10-0802 |
| [8] |
董婧, 王洋, 郭锦晓, 等. 白藜芦醇强化卤虫对斑马鱼肠道的形态结构和抗氧化能力的影响[J]. 沈阳农业大学学报, 2016, 47(4): 488-492. DONG J, WANG Y, GUO J X, et al. Effects of resveratrol enriched artemia on intestinal morphology and anti-oxidative capacities of zebrafish (Barchydanio rerio)[J]. Journal of Shenyang Agricultural University, 2016, 47(4): 488-492 (in Chinese). |
| [9] |
胡瑶莲, 张恒志, 陈代文, 等. 白藜芦醇对生长育肥猪抗氧化能力、空肠黏膜免疫及结肠菌群的影响[J]. 动物营养学报, 2019, 31(1): 459-468. HU Y L, ZHANG H Z, CHEN D W, et al. Effects of resveratrol on antioxidant capacity, jejunal mucosal immunity and colonic microflora of growing-finishing pigs[J]. Chinese Journal of Animal Nutrition, 2019, 31(1): 459-468 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.01.054 |
| [10] |
ERSHAD M, SHIGENAGA M K, BANDY B. Differential protection by anthocyanin-rich bilberry extract and resveratrol against lipid micelle-induced oxidative stress and monolayer permeability in Caco-2 intestinal epithelial cells[J]. Food & Function, 2021, 12(7): 2950-2961. |
| [11] |
龚福来, 林雪, 王红权. 不同维生素C源对吉富罗非鱼生长性能、抗氧化能力和免疫力的影响[J]. 动物营养学报, 2021, 33(4): 2378-2389. GONG F L, LIN X, WANG H Q. Effects of different vitamin C sources on growth performance, antioxidant capacity and immunity of genetically improved farmed tilapia[J]. Chinese Journal of Animal Nutrition, 2021, 33(4): 2378-2389 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.04.055 |
| [12] |
YANG G, JIAN S Q, CAO H Z, et al. Changes in microbiota along the intestine of grass carp (Ctenopharyngodon idella): community, interspecific interactions, and functions[J]. Aquaculture, 2019, 498: 151-161. DOI:10.1016/j.aquaculture.2018.08.062 |
| [13] |
闫亚楠, 夏斯蕾, 田红艳, 等. 白藜芦醇对高脂胁迫团头鲂抗氧化能力、非特异免疫机能和抗病力的影响[J]. 水生生物学报, 2017, 41(1): 155-164. YAN Y N, XIA S L, TIAN H Y, et al. Effects of resveratrol supplementation on growth performance, immunity, antioxidant capability and disease resistance of blunt snout bream fed high-fat diet[J]. Acta Hydrobiologica Sinica, 2017, 41(1): 155-164 (in Chinese). |
| [14] |
贺艳辉, 张红燕, 龚赟翀, 等. 我国罗非鱼养殖品种及养殖发展分析[J]. 水产养殖, 2009, 30(2): 12-14. HE Y H, ZHANG H Y, GONG Y C, et al. Analysis on species and development of tilapia culture in China[J]. Journal of Aquaculture, 2009, 30(2): 12-14 (in Chinese). DOI:10.3969/j.issn.1004-2091.2009.02.005 |
| [15] |
鲁康乐. 高脂日粮诱导团头鲂脂肪肝发生机制及黄连素调控的研究[D]. 博士学位论文. 南京: 南京农业大学, 2014. LU K L. Formation mechanism and regulation berberine for fatty liver induced by high-fat diet in blunt snout bream Megalobrama amblycephala[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2014. (in Chinese) |
| [16] |
闫亚楠. 白藜芦醇和槲皮素对高脂诱导团头鲂脂代谢、生长性能以及抗氧化能力的影响[D]. 硕士学位论文. 南京: 南京农业大学, 2017. YAN Y N. Effects of resveratrol and quercetin on lipid metabolism, growth performance, antioxidant capacity of blunt snout bream (Megalobrama amblycephala) fed high fat diet[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2017. (in Chinese) |
| [17] |
赵志祥, 史磊磊, 张聪, 等. 饲料添加白藜芦醇对吉富罗非鱼生长免疫和肝脏结构的影响[J]. 中国农学通报, 2018, 34(8): 111-117. ZHAO Z X, SHI L L, ZHANG C, et al. Effects of resveratrol on growth performance, immunity and liver microstructure of GIFT (Oreochromis niloticus)[J]. Chinese Agricultural Science Bulletin, 2018, 34(8): 111-117 (in Chinese). |
| [18] |
李巍伟. 白藜芦醇抑菌作用及其机制研究[D]. 硕士学位论文. 石家庄: 河北医科大学, 2008. LI W W. Antibacterial effect of resveratrol and mechanism research[D]. Master's Thesis. Shijiazhuang: Hebei Medical University, 2008. (in Chinese) |
| [19] |
楼允东. 组织胚胎学[M]. 北京: 中国农业出版社, 1996: 101-104. LOU Y D. Histology and embryology[M]. Beijing: China Agriculture Press, 1996: 101-104 (in Chinese). |
| [20] |
ANDRES S F, SANTORO M A, MAH A T, et al. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2015, 308(2): G100-G111. DOI:10.1152/ajpgi.00287.2014 |
| [21] |
ZHANG B W, XU Y C, LV H, et al. Intestinal pharmacokinetics of resveratrol and regulatory effects of resveratrol metabolites on gut barrier and gut microbiota[J]. Food Chemistry, 2021, 357: 129532. DOI:10.1016/j.foodchem.2021.129532 |
| [22] |
唐茂妍, 吴海坤. 畜禽肠道杯状细胞的作用及其影响因素[J]. 畜牧产业, 2021(2): 75-78. TANG M Y, WU H K. The role of intestinal goblet cells in livestock and poultry and its influencing factors[J]. Animal Agriculture, 2021(2): 75-78 (in Chinese). |
| [23] |
于飞, 李岩溪, 任亚浩, 等. 白藜芦醇对高脂模型小鼠脂代谢影响[J]. 中国公共卫生, 2010, 26(11): 1401-1402. YU F, LI Y X, REN Y H, et al. Short-term effects of resveratrol on lipid metabolism in mice with high-fat diet[J]. Chinese Journal of Public Health, 2010, 26(11): 1401-1402 (in Chinese). DOI:10.11847/zgggws2010-26-11-28 |
| [24] |
ZHAO L, CEN F, TIAN F, et al. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway[J]. Experimental and Therapeutic Medicine, 2017, 14(6): 5942-5948. |
| [25] |
ELLUL P, BOYER L, GROC L, et al. Interleukin-1β-targeted treatment strategies in inflammatory depression: toward personalized care[J]. Acta Psychiatrica Scandinavica, 2016, 134(6): 469-484. DOI:10.1111/acps.12656 |
| [26] |
PAPANICOLAOU D A, WILDER R L, MANOLAGAS S C, et al. The pathophysiologic roles of interleukin-6 in human disease[J]. Annals of Internal Medicine, 1998, 128(2): 127-137. DOI:10.7326/0003-4819-128-2-199801150-00009 |
| [27] |
BAUD V, KARIN M. Signal transduction by tumor necrosis factor and its relatives[J]. Trends in Cell Biology, 2001, 11(9): 372-377. DOI:10.1016/S0962-8924(01)02064-5 |
| [28] |
杨汉春. 动物免疫学[M]. 2版. 北京: 中国农业大学出版社, 2003: 95-108. YANG H C. Animal immunology[M]. 2nd ed. Beijing: China Agricultural University Press, 2003: 95-108 (in Chinese). |
| [29] |
ZHANG S. The role of transforming growth factor β in T helper 17 differentiation[J]. Immunology, 2018, 155(1): 24-35. DOI:10.1111/imm.12938 |
| [30] |
GAITANTZI H, MEYER C, RAKOCZY P, et al. Ethanol sensitizes hepatocytes for TGF-β-triggered apoptosis[J]. Cell Death & Disease, 2018, 9(2): 51. |
| [31] |
PORET J M, SOUZA-SMITH F, MARCELL S J, et al. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats[J]. International Journal of Obesity, 2018, 42(3): 535-541. DOI:10.1038/ijo.2017.280 |
| [32] |
刘继法, 胡渊龙, 邱占军, 等. 中药有效成分治疗脓毒症急性肺损伤作用机制研究进展[J]. 辽宁中医药大学学报, 2021, 23(1): 171-176. LIU J F, HU Y L, QIU Z J, et al. Advances in the pathogenesis of active ingredients of traditional Chinese medicine in the treatment of acute lung injury in sepsis[J]. Journal of Liaoning University of Traditional Chinese Medicine, 2021, 23(1): 171-176 (in Chinese). |
| [33] |
ANDRADE J M O, PARAÍSO A F, DE OLIVEIRA M V M, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation[J]. Nutrition, 2014, 30(7/8): 915-919. |
| [34] |
YOSHIZAKI T, MILNE J C, IMAMURA T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes[J]. Molecular and Cellular Biology, 2009, 29(5): 1363-1374. DOI:10.1128/MCB.00705-08 |
| [35] |
PFLUGER P T, HERRANZ D, VELASCO-MIGUEL S, et al. Sirt1 protects against high-fat diet-induced metabolic damage[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(28): 9793-9798. DOI:10.1073/pnas.0802917105 |
| [36] |
董娇娥, 刘洁, 胡璇, 等. 高脂饮食对肠道功能的影响[J]. 生命的化学, 2019, 39(6): 1098-1106. DONG J E, LIU J, HU X, et al. The effect of high-fat diet on intestinal function[J]. Chemistry of Life, 2019, 39(6): 1098-1106 (in Chinese). |
| [37] |
STENGEL S, MESSNER B, FALK-PAULSEN M, et al. Regulated proteolysis as an element of ER stress and autophagy: implications for intestinal inflammation[J]. Biochimica et Biophysica Acta: Molecular Cell Research, 2017, 1864(11 Pt B): 2183-2190. |
| [38] |
YANG J W, TIAN G, CHEN D W, et al. Involvement of PKA signalling in anti-inflammatory effects of chitosan oligosaccharides in IPEC-J2 porcine epithelial cells[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(1): 252-259. DOI:10.1111/jpn.12686 |
| [39] |
SHI L, FANG B, YONG Y H, et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway[J]. Carbohydrate Polymers, 2019, 219: 269-279. DOI:10.1016/j.carbpol.2019.05.036 |
| [40] |
瞿秋红. 白藜芦醇对猪肠道微生物区系、炎症相关基因和紧密连接蛋白表达的影响研究[D]. 硕士学位论文. 南宁: 广西大学, 2020. QU Q H. Study on the effect of resveratrol on gut microflora, the expression of the genes related to inflammation and tight junction protein in pigs[D]. Master's Thesis. Nanning: Guangxi University, 2020. (in Chinese) |
| [41] |
CIANCIULLI A, CALVELLO R, CAVALLO P, et al. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression[J]. Toxicology in Vitro, 2012, 26(7): 1122-1128. DOI:10.1016/j.tiv.2012.06.015 |




