随着全球气候变暖趋势的加剧,热应激已成为影响畜牧业发展的重要因素之一。反刍动物、猪、家禽是恒温动物,在热中性区通过自身体温调节机制维持体温,当环境温度达到动物等热区上限,超过机体自身调节能力时,引发一系列非特异性免疫应答反应,导致畜禽热应激,从而增加畜禽养殖成本、损害畜禽健康、降低生产力和产品品质,最终造成经济损失。
作为畜禽机体功能性必需氨基酸之一,色氨酸(Trp)及其代谢产物可以提高采食量、增加机体蛋白质沉积和消化吸收功能、提高生长性能、增强免疫功能、缓解肠道黏膜屏障损伤、提高机体抗应激能力[1-4]。本文从热应激的产生机理和对畜禽的影响、色氨酸的代谢及其代谢产物生理功能和色氨酸及其代谢产物对热应激的可能调节机制进行综述,讨论色氨酸及其代谢产物在热应激条件下与机体免疫的交叉点,为色氨酸在调节畜禽热应激的应用研究提供新的思路。
1 热应激的产生机理及对畜禽的影响 1.1 热应激的产生机理当外界温度高于机体调节上限,畜禽不能通过自身神经内分泌系统维持正常体温,动物便处于热应激状态。在热应激环境中,畜禽机体最初通过增加水摄入量、出汗和呼吸频率,降低心率和采食量等行为和生理反应的启动,将体温维持在正常范围内[5];随后,下丘脑-垂体-肾上腺皮质(HPA)轴和交感神经-肾上腺髓质(SAM)轴的激活,导致循环中的儿茶酚胺和皮质醇含量升高;随着热应激时间的持续,细胞和体液免疫功能遭到损伤,影响消化、生长、生殖和其他代谢过程[6-7]。
1.2 热应激对畜禽生产和免疫的影响动物处于热应激状态,机体难以通过增加饮水、呼吸频率和降低采食量等维持恒定体温时,首先表现为体温升高、生产性能降低[8-9];免疫反应的中枢神经系统(CNS)是一个复杂的网络系统,包括神经内分泌系统和免疫系统,HPA轴和SAM轴调节是免疫调节的2个主要途径。有研究表明,在热应激条件下,HPA轴激活,导致循环中的儿茶酚胺和皮质醇含量升高,其中急性热应激比慢性热应激诱导糖皮质激素水平更高,并引起一系列生化反应,导致脱水,影响能量消耗和蛋白质代谢[6, 10]。热应激会增加活性氧(ROS)的产生,诱导氧化应激,造成肝脏、肠道等器官氧化损伤,破坏肠道黏膜完整性,损害畜禽健康和降低生产性能[11-12]。肠道是机体最大的消化吸收器官,也是重要的防御屏障。在发挥吸收功能的同时,健康肠道的黏膜屏障保护宿主免受具有致病力的毒素和微生物的侵袭。肠道微生物群与肠黏膜免疫密切相关,可同时发挥促炎和抗炎作用。众多研究发现,持续的热应激会导致畜禽肠道的菌群结构和多样性紊乱、有益菌比例降低而有害菌比例增高、黏膜屏障损伤、机体生长性能及免疫力下降[13-15]。
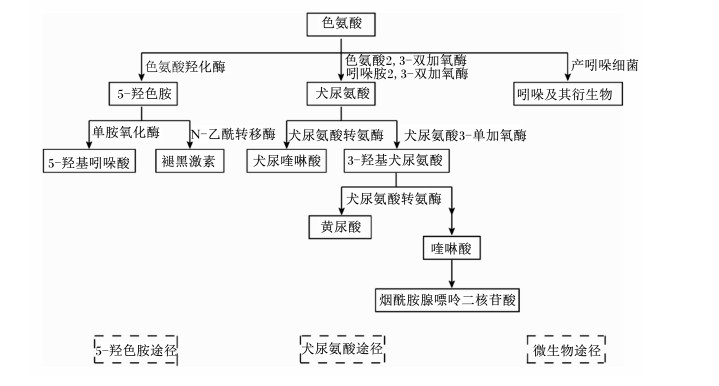
2 色氨酸对热应激的影响 2.1 色氨酸代谢和生理功能色氨酸是构成蛋白质的20种氨基酸之一,是所有动物的必需氨基酸,同时也是生成血清素[如5-羟色胺(5-HT)]、褪黑激素、色胺等一些重要的生物活性分子的前体。色氨酸的代谢途径主要有3种(图 1),其中2种是宿主途径,分别是犬尿氨酸代谢途径和5-HT代谢途径,犬尿氨酸代谢途径限速酶为吲哚胺2, 3-双加氧酶(IDO)和色氨酸2, 3-双加氧酶(TDO)[16],5-HT代谢途径限速酶主要为肠嗜铬细胞中的色氨酸羟化酶1(TPH1)[17];另一种是微生物代谢途径,也就是吲哚途径,其中一些代谢产物可以作为芳香烃受体(AhR)配体。除了色氨酸的直接代谢外,微生物群和微生物代谢物对宿主色氨酸的2种途径也有重要的调节作用[18-20]。研究表明,哺乳动物在感染、应激状态下,大多数色氨酸通过犬尿氨酸途径被分解转化为可能与应激反应相互作用的生物活性物质[21-23]。而急性应激对哺乳动物的大脑色氨酸含量的调节有相反的影响[24],这种脑色氨酸含量增加可能与交感神经激活和血浆儿茶酚胺含量升高有关[25]。陶丝雨等[26]的试验亦表明,肠易激综合征提高结肠TPH1基因表达以及5-HT的含量。那么热应激状态下畜禽机体色氨酸代谢又是怎样的?是否是机体发生急性热应激使大脑色氨酸含量增加,5-HT含量升高,抑制血浆皮质醇含量升高,通过HPA轴缓解应激反应;而随着热应激的持续,色氨酸代谢转向犬尿氨酸代谢途径调控热应激导致的氧化应激和炎症反应,尚不明确,因此其具体代谢途径还需进一步试验研究。
|
图 1 色氨酸主要代谢途径 Fig. 1 Main metabolic pathways of tryptophan[27] |
研究表明,除了作为起始氨基酸参与蛋白质合成外,色氨酸及其代谢产物在调节消化吸收、能量代谢、蛋白质沉积、免疫功能和应激反应等方面有重要作用[28-30]。5-HT是由色氨酸通过色氨酸羟化酶和芳香氨基酸脱羧酶两级酶促反应生成的。在动物体内,血清素主要存在于胃肠道、血小板和中枢神经系统,参与HPA轴调节,促进肠道蠕动[31-32]。另外,5-HT还能通过调节免疫细胞中白细胞介素-6(IL-6)和白细胞介素-10(IL-10)产生影响机体免疫应答[3]。褪黑激素是由动物松果腺分泌,其主要作用是调节昼夜节律,但也被证明影响包括免疫功能、凋亡、增殖和氧化应激在内的多种分子途径[33-34]。犬尿氨酸是犬尿氨酸代谢途径的中间产物,具有抗菌活性,可直接影响肠道菌群的增殖[35],在犬尿氨酸代谢中,犬尿氨酸(KYN)、犬尿喹啉酸(KA)和黄尿酸(XA)等作为AhR的直接配体,以浓度依赖的方式刺激AhR和AhR依赖的基因表达,调节肠道内稳态,AhR自身也在调节IDO1和TDO1表达量方面发挥作用[36]。因此,AhR可能是肠道微生物群、犬尿氨酸代谢途径与免疫应答之间复杂相互作用的重要中介物质。此外,跨膜G蛋白偶联受体(GPCRs)也能感受其代谢中间体,激活信号通路,调节胃肠道稳态和肠道免疫[28]。烟酰胺腺嘌呤二核苷酸(NAD)是生物氢的载体,作为辅酶,在糖酵解、柠檬酸循环、三羧酸循环、糖异生和电子传递等方面发挥重要作用。吲哚及其衍生物是在色氨酸经多种产吲哚细菌产生的色氨酸酶作用下产生的,包括吲哚、吲哚乙醇(IE)、吲哚丙酸(IPA)、吲哚乙酸(IAA)、吲哚醛(IAld)和吲哚丙烯酸(IA)等,这些物质对宿主的生理机能有多种影响。吲哚、IPA和IA通过降低肠通透性影响黏膜内平衡。吲哚还可以诱导肠内分泌细胞释放胰高血糖素样肽1(GLP1),调控食欲、胰岛素分泌和延缓胃排空[37]。
2.2 色氨酸及其代谢产物对热应激的影响饲粮添加色氨酸可缓解畜禽热应激状态。给阉公牛每千克体重静脉滴注38.5 mg色氨酸,可通过增加脑5-HT含量减弱牛在急性热应激环境中直肠温度的升高[38]。在猪研究上,色氨酸亦可提高下丘脑5-HT含量和降低血浆皮质醇含量,通过HPA轴缓解应激反应[39-41]。热应激常伴随严重的氧化应激,饲粮中补充0.15%色氨酸能减轻敌草快注射引起的仔猪肠道损伤、氧化应激和线粒体功能障碍[42]。Zimbelman等[43]研究表明,哺乳期奶牛补充胶囊状的尼克酸会增加热应激状态下的皮肤表面蒸发热损失,降低阴道温度。相似的,王雪莹等[44]在牦牛热应激条件下饲喂烟酸,发现可以通过缓解HPA轴应激反应,调节机体免疫反应,增加血氧供给,提高其对热应激环境的抵抗,提高采食量和营养物质消化率,减少生长性能由于热应激引起的降低,添加烟酸增加烟酰胺腺嘌呤二核苷酸含量可能是其原因之一。褪黑激素是色氨酸代谢产物之一,有研究表明,褪黑激素增加热应激处理后白细胞、单核细胞、粒细胞等免疫细胞数量,清除机体氧自由基,增加超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)等抗氧化酶活性,调节免疫功能,缓解热应激,缓解体重下降程度[45-46]。也有研究表明,饲粮中添加0.75%的高水平色氨酸显著提高仔猪血清二胺氧化酶(DAO)和D-乳酸含量,降低空肠中咬合蛋白(Occludin)和紧密连接蛋白-1(ZO-1)的基因表达,损伤肠道黏膜完整性[47]。因此,热应激条件下各个物种、各个日龄阶段的色氨酸添加量如何仍需试验进一步研究探讨。
3 色氨酸调控畜禽热应激可能的作用机制肠道微生物影响免疫系统响应;反过来,免疫系统亦参与调节肠道菌群的定位和组成。微生物与宿主免疫系统之间的联系是由一系列分子和信号因子介导,继而影响肠道、肝脏、大脑和其他器官。热应激影响肠道菌群组成和多样性,以及肠道菌群与免疫途径相互作用。色氨酸是动物必需的营养物质,其代谢物参与肠道免疫稳态和多种免疫疾病的发生。本文从色氨酸及其代谢物与调节HPA轴,色氨酸及其代谢物与线粒体ROS产生和氧化应激相关信号通路,色氨酸及其代谢产物与肠道黏膜屏障功能3个方面概述色氨酸及其代谢产物与热应激和肠道黏膜屏障功能研究概况,探讨色氨酸及其代谢产物调控畜禽热应激可能的作用机制。
3.1 色氨酸与畜禽HPA轴在热应激状态下,HPA轴和SAM轴被激活,维持体内平衡[48]。其中,去甲肾上腺素(NOR)和5-HT参与HPA轴的激活和促肾上腺皮质激素(ACTH)的垂体释放,HPA轴的激活会导致皮质醇分泌增加引起免疫反应,而NOR和5-HT与外周神经免疫激活有关,受白细胞介素-1(IL-1)、IL-6和肿瘤坏死因子-α(TNF-α)诱导[49]。在急性应激期,皮质醇的分泌对免疫系统起到刺激作用,而在慢性应激期,皮质醇的分泌与免疫抑制有关[50-51]。研究表明,灌胃色氨酸促进慢性应激小鼠下丘脑5-HT的合成并增强其抵抗应激能力[52];饲粮中添加色氨酸提高慢性不可预知温和应激肉鸡和小鼠TPH1的表达,提高外周血5-HT的含量,缓解机体应激反应[53]。5-HT通过调节下丘脑促肾上腺皮质激素释放因子(CRF)的释放进而对动物HPA轴发挥重要的调控作用[54-55],并调节行为和神经内分泌对应激的应答[56-57]。此外,应激会导致色氨酸代谢通路改变,而色氨酸代谢通路改变反过来影响机体应激反应,加之5-HT神经传递和HPA轴反应敏感性的个体差异在脊椎动物中是普遍存在的现象[58],因而各种脊椎动物饲粮中最佳色氨酸含量的确定仍有待进一步研究。
3.2 色氨酸与畜禽氧化应激相关信号通路线粒体作为产生细胞内绝大多数活性氧的细胞器,在调节氧化应激的产生和反应中起着关键作用。与细胞核DNA相比,线粒体ROS产生水平高,且对损伤防御能力相对较差,线粒体DNA更容易受到氧化损伤[59]。大量研究表明,畜禽热应激降低畜禽机体抗氧化能力,引发氧化应激[60-62]。热应激是活性氧产生的强诱导剂,一旦由谷胱甘肽(GSH)、GSH-Px、SOD和血红素氧化酶1(HO1)组成的细胞氧化防御系统耗尽,就会导致组织损伤[63]。抗氧化系统的表达主要受红系衍生的核因子2相关因子2(Nrf2)的调控,在生理条件下,Nrf2在细胞质中被Kelch样ECH相关蛋白1(Keap1)抑制。氧化应激时Nrf2从Keap1中解离,导致Nrf2转位到细胞核中与抗氧化反应元件(ARE)结合,诱导抗氧化蛋白的转录,提高应激条件下的细胞存活率[64]。
热应激雏鸡血浆中盐酸和喹啉酸含量升高,而烟酰胺含量降低。在哺乳动物中,色氨酸主要代谢为犬尿氨酸,然后进一步代谢为喹啉酸和烟酰胺,而在鸡研究中,这些代谢过程尚未得到深入研究。此外,从功能的角度来看,犬尿氨酸通过激活芳基烃受体调节免疫系统[36, 65]。喹啉酸通过激活N-甲基-D-天门冬胺酸受体发挥神经毒性作用,并诱导游离自由基的产生和不依赖于受体的氧化应激。然而,这些可能与环境温度的有害影响有关的功能,亦未在鸡中得到证实[65-66]。有研究表明,IA可使LPS刺激下IL-6和白细胞介素-1β(IL-1β)分泌减少,Nrf2-ARE通路激活,AhR激活,黏蛋白2(Muc2)基因表达上调,表明了IA抗氧化和抗炎功能。同时发现,IPA在小鼠巨噬细胞中显示了抗炎作用,而在人类单个核细胞中却没有,这表明IPA诱导的信号通路在小鼠和人类细胞中并不保守[67]。在色氨酸代谢过程中,产生褪黑激素、5-HT和3-羟基犬尿氨酸等几种内源性抗氧化代谢物。Liu等[68]研究表明,饲粮中色氨酸从0.18%增加到1.08%可以提高畜禽机体总抗氧化能力、GSH-Px和过氧化氢酶活性,从而提高机体抗氧化活性,控制ROS的形成。由此,我们推测色氨酸可能在降低机体线粒体ROS产生和调控氧化应激相关信号通路基因表达2个方面调控氧化应激。
3.3 色氨酸与畜禽肠道黏膜屏障功能肠道除了具有消化吸收功能,也是机体重要的免疫器官。健康的肠腔黏膜发挥屏障功能将有害细菌、病毒和其他大分子有害物质与机体内环境分开。肠道黏膜屏障根据结构和功能可划分为:机械屏障、化学屏障、免疫屏障和微生物屏障。
3.3.1 机械屏障细胞间连接的完整性对上皮细胞层的正常功能至关重要,可将肠道内环境与外界隔离开来,形成紧密连接,使细胞紧密结合,控制肠道黏膜通透性。Occludin、闭合蛋白(Claudins)、连接黏附分子(JAMs)和ZO等是细胞间连接重要的结构和功能性蛋白[69]。热应激导致肠道完整性受损和通透性增加,与紧密连接蛋白的表达和分布改变相关[70]。体外和体内研究均表明,吲哚通过增加参与维持上皮细胞结构和功能的基因表达增强细胞间的上皮紧密连接,降低肠道黏膜通透性,改善肠上皮屏障功能[28, 37]。
3.3.2 化学屏障黏蛋白、胆汁、溶菌酶和抗菌肽等是肠上皮细胞的主要化学成分,可以抑制有害细菌的黏附和定植,维持肠黏膜的完整性。热应激损伤肠黏膜绒毛结构,降低碱性磷酸酶活性和抗菌肽含量,减少单位面积内杯状细胞数量,使得黏蛋白分泌水平下降,损伤肠道化学屏障[13, 71-72]。AhR和孕烷X受体(PXR)调节与肠道Muc2基因的表达有关的信号通路,缺乏PXR的小鼠表现出肠道渗漏和小肠中Muc2表达减少[28]。IA作为一种重要的色氨酸微生物源性代谢物,能有效激活小鼠结肠AhR和上调Muc2基因表达,进一步证实了AhR激活和杯状细胞功能之间的联系[67]。此外,吲哚也可以激活AhR信号,进而促进局部白细胞介素-22(IL-22)的产生,并进一步促进抗菌肽的分泌,保护机体免受致病性感染[73]。
3.3.3 免疫屏障免疫屏障主要由肠黏膜相关淋巴组织及分泌型抗体构成,分泌型免疫球蛋白(sIgA)为黏膜免疫的主要抗体,防止有害微生物定植[74]。Toll样受体(TLRs)、核苷酸结合寡聚化结构域(NOD)为广泛表达于各种先天免疫细胞和非免疫细胞上的进化保守的膜受体,作为主要的传感器,可以通过响应细菌、病毒等病原体相关的分子模式来启动先天免疫应答[69]。炎症的启动由促炎细胞因子和抗炎细胞因子之间的不平衡激活。研究表明,热应激状态下过度产生促炎细胞因子,如TNF-α,导致包括肠道在内的器官出血和坏死[75]。AhR是一种胞质配体激活的转录因子,介导外源性代谢,是免疫和炎症的关键调节因子,调节适应性免疫和黏膜屏障功能、肠道稳态的维持和癌变[28, 76-77]。AhR对免疫稳态的促进作用主要有2种机制:首先,AhR介导先天淋巴样细胞的激活,在肠道中产生IL-22,IL-22通过调节微生物组成,调节抗菌肽的释放,影响免疫与菌群间的稳态平衡[78];其次,有证据表明,AhR通过调节上皮内淋巴细胞和先天淋巴样细胞的发育而具有抗炎作用,使得这些细胞在抵御病原微生物渗透和促进肠道内稳态方面发挥重要作用[76]。KYN、KA、XA等几种微生物代谢物作为AhR的配体对宿主免疫至关重要,特别是在保护黏膜免受炎症感染方面[79]。AhR通过与配体结合,直接靶向调控炎症过程中包括IL-6、IL-22、前列腺素G/H合酶2、血管内皮生长因子A、细胞色素P4501A1在内的基因[28]。其中,吲哚可以降低TNF-α介导的核因子-κB(NF-κB)激活,增加抗炎细胞因子IL-10的水平[80]。IPA可显著提高LPS刺激后IL-10的生成,并降低TNF-α的生成[81]。吲哚-3-乙酸(I3A)至少可以通过2种方式直接调节肝细胞和巨噬细胞的炎症反应,I3A作用于巨噬细胞,可减弱促炎细胞因子的释放,诱导肝脏合成游离脂肪酸,进而刺激巨噬细胞;I3A作用于肝细胞,可减弱细胞因子介导的脂肪生成上调[82]。有研究表明,在人树突状细胞中,AhR信号能改变TLRs调节的反应,进一步表明色氨酸分解代谢物对细胞因子产生的影响可能依赖于AhR的激活[83]。此外,有报道称,短链脂肪酸(SCFAs)增强AhR介导的基因表达[84]。在热应激条件下,色氨酸及其代谢与肠道微生物代谢物的关系,色氨酸及其代谢物能否通过AhR影响TLRs、NLRs的表达,进而影响其下游因子在热应激条件下的表达均还需进一步探讨。
3.3.4 微生物屏障肠道中存在大量与宿主共生的微生物,在调节宿主营养和代谢、刺激肠道成熟、发育、增殖和免疫稳态等方面发挥重要作用[64, 85]。饮食、免疫反应、感染和抗生素的使用都会影响各种宿主肠道菌群结构和多样性。除机体条件改变,环境高温等外界应激刺激亦会引起菌群平衡改变,导致肠道病原体定植[86]和肠道炎症反应[87]。微生物群的作用不仅局限于肠道,还向大脑发出信号,影响中枢神经系统炎症,并参与神经精神疾病[88],Trp-AhR通路是沿微生物-内脏-脑轴传递信号的重要通路之一,在宿主免疫系统、肠道免疫耐受和肠道菌群之间平衡的维持中起着至关重要的作用[81]。微生物代谢产物如SCFAs和多种微生物色氨酸代谢产物可以激活肠道或肝脏中的AhR和AhR靶基因,AhR信号反过来又能影响小肠内的微生物组成,AhR是宿主-微生物通讯的重要调节因子,影响宿主的新陈代谢,调节免疫系统[28]。外源性补充色氨酸可优化益生菌在肠道感染或化学损伤过程中抵抗有害细菌入侵的能力,其中乳酸菌促进AhR依赖的IL-22转录,维持微生物多样性,抑制白色念珠菌定植,保护肠道黏膜免受炎症[81]。KYN具有抗菌活性,可直接影响肠道菌群的增殖[35]。在肠道中,色氨酸被微生物降解为吲哚及其衍生物,产吲哚细菌能抑制非产吲哚细菌的生长和存活[89]。研究发现,吲哚和IE等某些吲哚类化合物对革兰氏阴性肠杆菌有抑菌作用,尤其是沙门氏菌属和志贺氏菌属。此外,研究发现,IAA可抑制乳酸菌的生长和生存,特别是对副干酪乳杆菌[90]。还有一个有趣的发现,饲粮中添加0.42%的色氨酸在肉鸡中具有类似益生元的作用,对致病性和非致病性菌种进行定量分析发现,非致病性菌(双歧杆菌属和肠球菌)数量随着致病性大肠杆菌、梭状芽孢杆菌、肠杆菌和大肠杆菌数量的减少显著增加[91],其背后的确切原因尚不清楚,需要进一步的多学科研究来阐明这个问题。然而,可以推测,产吲哚的细菌可能会抑制不产吲哚的细菌如肠杆菌的生长和存活,特别是沙门氏菌和志贺氏菌属[89];Nowak等[90]也报道了添加到乳酸菌培养物中的IAA具有同样的生长抑制作用,但其背后的作用机制还需进一步探讨。
4 小结色氨酸具有调节生长性能、抗应激和免疫反应的重要功能。越来越多的研究表明,色氨酸及其代谢产物(5-HT、褪黑激素、犬尿氨酸、吲哚及其衍生物等)对肠道微生物组成和功能、宿主免疫系统与中枢神经系统的作用具有深远的影响。相应地,肠道菌群影响宿主对色氨酸的吸收和代谢,并直接或间接地调节随后宿主的生理和代谢。在热应激状态下,饲粮中补充色氨酸可以增加采食量、调节能量代谢、增强肠道黏膜屏障功能、调节畜禽免疫功能和抗应激能力。热应激是一个综合反应,色氨酸是一系列生物活性物质前体,畜禽处于热应激,机体内色氨酸的3条主要代谢途径会如何变化,其与机体免疫的交叉点仍有待研究。热应激导致肠道微生物紊乱,肠道菌群的改变又是如何通过调节色氨酸代谢影响宿主免疫?从细胞到整个机体,热应激状态下色氨酸复杂代谢及其调控热应激作用机理需要进一步的研究,并针对色氨酸代谢物或色氨酸-肠道菌群-免疫相互作用设计可行的可能缓解畜禽热应激的方法。另外,国内外研究关于热应激条件下色氨酸在各个物种、品种、日龄阶段的添加量存在较大差异,目前亟需确定畜禽不同品种、不同日龄色氨酸最佳需要量,来指导和完善抗应激饲粮的综合配制。
| [1] |
MUND M D, RIAZ M, MIRZA M A, et al. Effect of dietary tryptophan supplementation on growth performance, immune response and anti-oxidant status of broiler chickens from 7 to 21 days[J]. Veterinary Medicine and Science, 2020, 6(1): 48-53. DOI:10.1002/vms3.195 |
| [2] |
朱艳芝, 马文锋, 陈晓晨, 等. 色氨酸分解代谢及其在猪饲粮中的应用进展[J]. 动物营养学报, 2020, 32(3): 1019-1024. ZHU Y Z, MA W F, CHEN X C, et al. Tryptophan catabolism and its application in pig diets[J]. Chinese Journal of Animal Nutrition, 2020, 32(3): 1019-1024 (in Chinese). |
| [3] |
王贤泽, 谭碧娥, 丁浩, 等. 色氨酸及其代谢产物的生理功能研究进展[J]. 饲料研究, 2020, 43(5): 130-133. WANG X Z, TAN B E, DING H, et al. Research progresses on physiological function of tryptophan and its metabolites[J]. Feed Research, 2020, 43(5): 130-133 (in Chinese). |
| [4] |
JIANG S Q, GOU Z Y, LIN X J, et al. Effects of dietary tryptophan levels on performance and biochemical variables of plasma and intestinal mucosa in yellow-feathered broiler breeders[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(1): e387-e394. DOI:10.1111/jpn.12757 |
| [5] |
RATNAKARAN A P, SEJIAN V, JOSE V S, et al. Behavioral responses to livestock adaptation to heat stress challenges[J]. Asian Journal of Animal Sciences, 2016, 11(1): 1-13. DOI:10.3923/ajas.2017.1.13 |
| [6] |
KADIM I T, MAHGOUB O, AL-KINDI A, et al. Effects of transportation at high ambient temperatures on physiological responses, carcass and meat quality characteristics of three breeds of Omani goats[J]. Meat Science, 2006, 73(4): 626-634. DOI:10.1016/j.meatsci.2006.03.003 |
| [7] |
BERNABUCCI U, LACETERA N, BAUMGARD L H, et al. Metabolic and hormonal acclimation to heat stress in domesticated ruminants[J]. Animal, 2010, 4(7): 1167-1183. DOI:10.1017/S175173111000090X |
| [8] |
唐湘方.基于蛋白质组与代谢组的肉鸡热应激分子机制研究[D].博士学位论文.北京: 中国农业科学院, 2015. TANG X F.Proteomics and metabolomics analysis on the molecular mechanism of heat stress in broilers[D]. Ph.D.Thesis.Beijing: Chinese Academy of Agricultural Sciences, 2015.(in Chinese) |
| [9] |
蒲启建, 王之盛, 彭全辉, 等. 热应激对不同品种(系)青年肉牛生产性能、营养物质表观消化率及血液生化指标的影响[J]. 动物营养学报, 2017, 29(9): 3120-3131. PU Q J, WANG Z S, PENG Q H, et al. Effects of heat stress on performance, nutrient apparent digestibility and blood biochemical indices of different breeds of young beef cattle[J]. Chinese Journal of Animal Nutrition, 2017, 29(9): 3120-3131 (in Chinese). DOI:10.3969/j.issn.1006-267x.2017.09.013 |
| [10] |
GONZALEZ-RIVAS P A, CHAUHAN S S, HA M, et al. Effects of heat stress on animal physiology, metabolism, and meat quality:a review[J]. Meat Science, 2020, 162: 108025. DOI:10.1016/j.meatsci.2019.108025 |
| [11] |
LIU F, COTTRELL J J, FURNESS J B, et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs[J]. Experimental Physiology, 2016, 101(7): 801-810. DOI:10.1113/EP085746 |
| [12] |
ZHANG J F, BAI K W, SU W P, et al. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase Ⅱ detoxifying enzyme systems in broiler chickens[J]. Poultry Science, 2018, 97(4): 1209-1219. DOI:10.3382/ps/pex408 |
| [13] |
李秋粉.益生菌对热应激肉鸡肠道屏障功能的影响[D].硕士学位论文.南昌: 江西农业大学, 2017. LI Q F.Effect of probiotic on intestinal barrier function of heat-stressed broilers[D]. Master's Thesis.Nanchang: Jiangxi Agricultural University, 2017.(in Chinese) |
| [14] |
李永洙, 陈常秀, 金泽林, 等. 热应激环境下育成鸡肠道菌群多样性及黏膜结构的相关性分析[J]. 中国农业大学学报, 2016, 21(1): 71-80. LI Y Z, CHEN C X, JIN Z L, et al. Correlation analysis on adult chicken intestinal flora diversity and mucosal structure under heat stress environment[J]. Journal of China Agricultural University, 2016, 21(1): 71-80 (in Chinese). |
| [15] |
WANG Z, ZHANG D W, YU W J, et al. Probiotic cocktails alleviate heat-induced intestinal barrier dysfunction and oxidative stress in broiler chickens[J]. Animal Husbandry and Feed Science, 2019(Suppl.1): 107-114. |
| [16] |
CERVENKA I, AGUDELO L Z, RUAS J L. Kynurenines:tryptophan's metabolites in exercise, inflammation, and mental health[J]. Science, 2017, 357(6349): eaaf9794. DOI:10.1126/science.aaf9794 |
| [17] |
CÔTÉ F, THÉVENOT E A, FLIGNY C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(23): 13525-13530. DOI:10.1073/pnas.2233056100 |
| [18] |
YANO J M, YU K, DONALDSON G P, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis[J]. Cell, 2015, 161(2): 264-276. DOI:10.1016/j.cell.2015.02.047 |
| [19] |
DESBONNET L, CLARKE G, TRAPLIN A, et al. Gut microbiota depletion from early adolescence in mice:implications for brain and behaviour[J]. Brain, Behavior, and Immunity, 2015, 48: 165-173. DOI:10.1016/j.bbi.2015.04.004 |
| [20] |
REIGSTAD C S, SALMONSON C E, RAINEY Ⅲ J F, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells[J]. The FASEB Journal, 2015, 29(4): 1395-1403. DOI:10.1096/fj.14-259598 |
| [21] |
LE FLOC'H N, OTTEN W, MERLOT E. Tryptophan metabolism, from nutrition to potential therapeutic applications[J]. Amino Acids, 2011, 41(5): 1195-1205. DOI:10.1007/s00726-010-0752-7 |
| [22] |
O'FARRELL K, HARKIN A. Stress-related regulation of the kynurenine pathway:relevance to neuropsychiatric and degenerative disorders[J]. Neuropharmacology, 2015, 112: 307-323. |
| [23] |
O'MAHONY S M, CLARKE G, BORRE Y E, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis[J]. Behavioural Brain Research, 2015, 277: 32-48. DOI:10.1016/j.bbr.2014.07.027 |
| [24] |
DUNN A J. Changes in plasma and brain tryptophan and brain serotonin and 5-hydroxyindoleacetic acid after footshock stress[J]. Life Sciences, 1988, 42(19): 1847-1853. DOI:10.1016/0024-3205(88)90023-9 |
| [25] |
DUNN A J, WELCH J E. Stress-and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activity[J]. Journal of Neurochemistry, 1991, 57(5): 1615-1622. DOI:10.1111/j.1471-4159.1991.tb06359.x |
| [26] |
陶丝雨, 肖丛瑞, 王晶, 等. 缬草醛对肠易激综合征模型大鼠结肠CRF、TPH1 mRNA及5-HT表达的影响[J]. 中国中药杂志, 2017, 42(2): 347-351. TAO S Y, XIAO C R, WANG J, et al. Effects of baldrinal of Valeriana jatamansi on expression of CRF, TPH1 mRNA and 5-HT in rats with irritable bowel syndrome[J]. China Journal of Chinese Materia Medica, 2017, 42(2): 347-351 (in Chinese). |
| [27] |
LAVELLE A, SOKOL H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease[J]. Nature Reviews Gastroenterology & Hepatology, 2020, 17(4): 223-237. |
| [28] |
GAO J, XU K, LIU H N, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism[J]. Frontiers in Cellular and Infection Microbiology, 2018, 8: 13. DOI:10.3389/fcimb.2018.00013 |
| [29] |
徐敏杰.L-色氨酸和褪黑激素对中华绒螯蟹血糖代谢及免疫性能的影响[D].硕士学位论文.上海: 上海海洋大学, 2018. XU M J.Analysis of the effect of L-tryptophan and melatonin on the hemolymph glucose metabolism and immune performance in the Eriocheir sinensis[D]. Master's Thesis.Shanghai: Shanghai Ocean University, 2018.(in Chinese) |
| [30] |
TEJPAL C S, PAL A K, SAHU N P, et al. Dietary supplementation of L-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings[J]. Aquaculture, 2009, 293(3/4): 272-277. |
| [31] |
BAI M M, LIU H N, XU K, et al. A review of the immunomodulatory role of dietary tryptophan in livestock and poultry[J]. Amino Acids, 2017, 49(1): 67-74. DOI:10.1007/s00726-016-2351-8 |
| [32] |
MARKUS C R. Dietary amino acids and brain serotonin function; implications for stress-related affective changes[J]. Neuromolecular Medicine, 2008, 10(4): 247-258. DOI:10.1007/s12017-008-8039-9 |
| [33] |
GHEORGHE C E, MARTIN J A, MANRIQUEZ F V, et al. Focus on the essentials:tryptophan metabolism and the microbiome-gut-brain axis[J]. Current Opinion in Pharmacology, 2019, 48: 137-145. DOI:10.1016/j.coph.2019.08.004 |
| [34] |
SIMONNEAUX V, RIBELAYGA C. Generation of the melatonin endocrine message in mammals:a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters[J]. Pharmacological Reviews, 2003, 55(2): 325-395. |
| [35] |
NIÑO-CASTRO A, ABDULLAH Z, POPOV A, et al. The IDO1-induced kynurenines play a major role in the antimicrobial effect of human myeloid cells against Listeria monocytogenes[J]. Innate Immunity, 2014, 20(4): 401-411. DOI:10.1177/1753425913496442 |
| [36] |
BESSEDE A, GARGARO M, PALLOTTA M T, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway[J]. Nature, 2014, 511(7508): 184-190. DOI:10.1038/nature13323 |
| [37] |
ROAGER H M, LICHT T R. Microbial tryptophan catabolites in health and disease[J]. Nature Communications, 2018, 9: 3294. DOI:10.1038/s41467-018-05470-4 |
| [38] |
SUTOH M, KASUYA E, YAYOU K I. Effects of intravenous tryptophan infusion on thermoregulation in steers exposed to acute heat stress[J]. Animal Science Journal, 2018, 89(5): 777-783. DOI:10.1111/asj.12988 |
| [39] |
HÖGLUND E, ØVERLI O, WINBERG S. Tryptophan metabolic pathways and brain serotonergic activity:a comparative review[J]. Frontiers in Endocrinology, 2019, 10: 158. DOI:10.3389/fendo.2019.00158 |
| [40] |
ADEOLA O, BALL R O. Hypothalamic neurotransmitter concentrations and meat quality in stressed pigs offered excess dietary tryptophan and tyrosine[J]. Journal of Animal Science, 1992, 70(6): 1888-1894. DOI:10.2527/1992.7061888x |
| [41] |
JAN KOOPMANS S, RUIS M, DEKKER R A, et al. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs[J]. Physiology & Behavior, 2005, 85(4): 469-478. |
| [42] |
LIU J B, ZHANG Y, LI Y, et al. L-tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function[J]. Animal, 2019, 9(5): 266. DOI:10.3390/ani9050266 |
| [43] |
ZIMBELMAN R B, BAUMGARD L H, COLLIER R J. Effects of encapsulated niacin on evaporative heat loss and body temperature in moderately heat-stressed lactating Holstein cows[J]. Journal of Dairy Science, 2010, 93(6): 2387-2394. DOI:10.3168/jds.2009-2557 |
| [44] |
王雪莹, 王之盛, 薛白, 等. 烟酸对热应激牦牛生长性能、营养物质表观消化率和血液指标的影响[J]. 动物营养学报, 2020, 32(5): 2228-2240. WANG X Y, WANG Z S, XUE B, et al. Effects of niacin on growth performance, nutrient apparent digestibility and blood indexes of yak under heat-stress[J]. Chinese Journal of Animal Nutrition, 2020, 32(5): 2228-2240 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.05.032 |
| [45] |
尹华祺.褪黑素对热应激小鼠抗氧化性能和免疫功能影响的研究[D].硕士学位论文.成都: 四川农业大学, 2009. YIN H Q.Reseach in melatonin's effection on antioxidaion and immunologic function of rats under heat-stress[D]. Master's Thesis.Chengdu: Sichuan Agricultural University, 2009.(in Chinese) |
| [46] |
HYDER I, SEJIAN V, BHATTA R, et al. Biological role of melatonin during summer season related heat stress in livestock[J]. Biological Rhythm Research, 2017, 48(2): 297-314. DOI:10.1080/09291016.2016.1262999 |
| [47] |
TOSSOU M C B, LIU H N, BAI M M, et al. Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig[J]. BioMed Research International, 2016, 2016: 2912418. |
| [48] |
SEJIAN V, BHATTA R, GAUGHAN J B, et al. Review:adaptation of animals to heat stress[J]. Animal, 2018, 12(Suppl.2): S431-S444. |
| [49] |
CALEFI A S, FONSECA J G A, NUNES C A D Q, et al. Heat stress modulates brain monoamines and their metabolites production in broiler chickens co-infected with clostridium perfringens type a and Eimeria spp.[J]. Veterinary Sciences, 2019, 6(1): 4. DOI:10.3390/vetsci6010004 |
| [50] |
BAGATH M, KRISHNAN G, DEVARAJ C, et al. The impact of heat stress on the immune system in dairy cattle:a review[J]. Research in Veterinary Science, 2019, 126: 94-102. DOI:10.1016/j.rvsc.2019.08.011 |
| [51] |
罗勇.海马在高温高湿应激致大鼠HPA轴功能紊乱中的作用及其药物干预研究[D].博士学位论文.上海: 中国人民解放军海军军医大学, 2019. LUO Y.Effects of hippocampus on HPA axis dysfunction in rats under high temperature and high humidity stress combined condition and its drug intervention[D]. Ph.D.Thesis.Shanghai: The Second Military Medical University, 2019.(in Chinese) |
| [52] |
李萌萌, 崔晓, 朱凌峰, 等. 色氨酸对慢性应激小鼠行为的影响[J]. 食品与生物技术学报, 2013, 32(8): 838-843. LI M M, CUI X, ZHU L F, et al. Effect of tryptophan on behavior of chronically stressed mice[J]. Journal of Food Science and Biotechnology, 2013, 32(8): 838-843 (in Chinese). |
| [53] |
岳云双.色氨酸调节慢性不可预知应激动物肠道屏障及免疫功能的机制研究[D].博士学位论文.北京: 中国农业大学, 2017. YUE Y S.Mechanism of tryptophan on intestinal epithelial barrier and immune functions in chronic unpredictable stress-exposed animals[D]. Ph.D.Thesis.Beijing: China Agricultural University, 2017.(in Chinese) |
| [54] |
DINAN T G. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function[J]. Life Sciences, 1996, 58(20): 1683-1694. DOI:10.1016/0024-3205(96)00066-5 |
| [55] |
WINBERG S, NILSSON A, HYLLAND P, et al. Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish[J]. Neuroscience Letters, 1997, 230(2): 113-116. DOI:10.1016/S0304-3940(97)00488-6 |
| [56] |
DE KLOET E R, JOËLS M, HOLSBOER F. Stress and the brain:from adaptation to disease[J]. Nature Reviews Neuroscience, 2005, 6(6): 463-475. DOI:10.1038/nrn1683 |
| [57] |
MOLTESEN M, LAURSEN D C, THÖRNQVIST P O, et al. Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss)[J]. The Journal of Experimental Biology, 2016, 219(24): 3907-3914. DOI:10.1242/jeb.139857 |
| [58] |
ØVERLI Ø, SØRENSEN C, PULMAN K G T, et al. Evolutionary background for stress-coping styles:relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates[J]. Neuroscience & Biobehavioral Reviews, 2007, 31(3): 396-412. |
| [59] |
CASTRO-PORTUGUEZ R, SUTPHIN G L. Kynurenine pathway, NAD+ synthesis, and mitochondrial function:targeting tryptophan metabolism to promote longevity and healthspan[J]. Experimental Gerontology, 2020, 132: 110841. DOI:10.1016/j.exger.2020.110841 |
| [60] |
崔艳军.热应激和氧化应激对肥育猪骨骼肌代谢的影响及硫辛酸的调控作用[D].博士学位论文.北京: 中国农业科学院, 2016. CUI Y J.Effects of heat stress and oxidative stress on metabolism of skeletal muscle and protection of lipoic acid in finishing pigs[D]. Ph.D.Thesis.Beijing: Chinese Academy of Agricultural Sciences, 2016.(in Chinese) |
| [61] |
钟光, 邵丹, 胡艳, 等. 持续热应激对黄羽肉鸡生长性能、器官指数、血清生化指标和抗氧化功能的影响[J]. 动物营养学报, 2018, 30(11): 4425-4432. ZHONG G, SHAO D, HU Y, et al. Effects of persistent heat stress on growth performance, organ indices, serum eiochemical indices and antioxidant function of yellow-feathered broilers[J]. Chinese Journal of Animal Nutrition, 2018, 30(11): 4425-4432 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.11.019 |
| [62] |
刘秀楠, 郭亮, 郝慧, 等. 急性热应激对肉仔鸡抗氧化能力的影响[J]. 今日畜牧兽医, 2018, 34(9): 4-5. LIU X N, GUO L, HAO H, et al. Effect of acute heat stress on antioxidant capacity of broilers[J]. Today Animay Husbandry and Veterinary Medicine, 2018, 34(9): 4-5 (in Chinese). DOI:10.3969/j.issn.1673-4092.2018.09.003 |
| [63] |
AKBARIAN A, MICHIELS J, DEGROOTE J, et al. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals[J]. Journal of Animal Science and Biotechnology, 2016, 7: 37. DOI:10.1186/s40104-016-0097-5 |
| [64] |
LIAN P Q, BRABER S, GARSSEN J, et al. Beyond heat stress:intestinal integrity disruption and mechanism-based intervention strategies[J]. Nutrients, 2020, 12(3): 734. DOI:10.3390/nu12030734 |
| [65] |
FUJIGAKI H, YAMAMOTO Y, SAITO K. L-tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases:focus on cell type differences[J]. Neuropharmacology, 2017, 112: 264-274. DOI:10.1016/j.neuropharm.2016.01.011 |
| [66] |
TOMONAGA S, OKUYAMA H, TACHIBANA T, et al. Effects of high ambient temperature on plasma metabolomic profiles in chicks[J]. Animal Science Journal, 2018, 89(2): 448-455. DOI:10.1111/asj.12951 |
| [67] |
WLODARSKA M, LUO C W, KOLDE R, et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation[J]. Cell Host & Microbe, 2017, 22(1): 25-37. |
| [68] |
LIU Y, YUAN J M, ZHANG L S, et al. Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density[J]. Poultry Science, 2015, 94(8): 1894-1901. DOI:10.3382/ps/pev155 |
| [69] |
HE J N, MA L X, QIU J L, et al. Effects of compound organic acid calcium on growth performance, hepatic antioxidation and intestinal barrier of male broilers under heat stress[J]. Asian-Australasian Journal of Animal Sciences, 2019, 33(7): 1156-1166. |
| [70] |
PEARCE S C, MANI V, BODDICKER R L, et al. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs[J]. Journal of Animal Science, 2012, 90(Suppl.4): 257-259. |
| [71] |
YI H B, HU W Y, CHEN S, et al. Cathelicidin-WA improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic Escherichia coli O157 : H7 infection[J]. The Journal of Immunology, 2017, 198(4): 1696-1705. DOI:10.4049/jimmunol.1601221 |
| [72] |
YI H B, XIONG Y X, WU Q W, et al. Effects of dietary supplementation with L-arginine on the intestinal barrier function in finishing pigs with heat stress[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(4): 1134-1143. DOI:10.1111/jpn.13277 |
| [73] |
LEVY M, BLACHER E, ELINAV E. Microbiome, metabolites and host immunity[J]. Current Opinion in Microbiology, 2017, 35: 8-15. DOI:10.1016/j.mib.2016.10.003 |
| [74] |
熊云霞, 王丽, 易宏波, 等. 热应激对猪禽肠道健康的影响及其机制研究进展[J]. 中国畜牧杂志, 2020, 56(2): 17-22. XIONG Y X, WANG L, YI H B, et al. Research progress on effects of heat stress on intestinal health of poultry and pigs and its mechanism[J]. Chinese Journal of Animal Science, 2020, 56(2): 17-22 (in Chinese). |
| [75] |
李思勉, 程康, 闫恩法, 等. 虎杖苷对热应激大鼠空肠消化吸收、抗氧化和屏障功能的影响[J]. 中国兽医科学, 2020, 50(1): 118-127. LI S M, CHENG K, YAN E F, et al. Effects of polydatin on jejunal digestion, absorption, antioxidant and barrier function in rats with heat stress[J]. Chinese Veterinary Science, 2020, 50(1): 118-127 (in Chinese). |
| [76] |
HUBBARD T D, MURRAY I A, PERDEW G H. Indole and tryptophan metabolism:endogenous and dietary routes to ah receptor activation[J]. Drug Metabolism and Disposition, 2015, 43(10): 1522-1535. DOI:10.1124/dmd.115.064246 |
| [77] |
KORECKA A, DONA A, LAHIRI S, et al. Bidirectional communication between the Aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism[J]. NPJ Biofilms and Microbiomes, 2016, 2: 16014. DOI:10.1038/npjbiofilms.2016.14 |
| [78] |
ZELANTE T, IANNITTI R G, CUNHA C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22[J]. Immunity, 2013, 39(2): 372-385. DOI:10.1016/j.immuni.2013.08.003 |
| [79] |
ROOKS M G, GARRETT W S. Gut microbiota, metabolites and host immunity[J]. Nature Reviews Immunology, 2016, 16(6): 341-352. DOI:10.1038/nri.2016.42 |
| [80] |
BANSAL T, ALANIZ R C, WOOD T K, et al. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(1): 228-233. DOI:10.1073/pnas.0906112107 |
| [81] |
SUN M G, MA N, HE T, et al. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR)[J]. Critical Reviews in Food Science and Nutrition, 2020, 60(10): 1760-1768. DOI:10.1080/10408398.2019.1598334 |
| [82] |
KRISHNAN S, DING Y F, SAEDI N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages[J]. Cell Reports, 2018, 23(4): 1099-1111. DOI:10.1016/j.celrep.2018.03.109 |
| [83] |
KADO S, CHANG W L W, CHI A N, et al. Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells[J]. Archive Für Toxikologie, 2017, 91(5): 2209-2221. |
| [84] |
JIN U H, CHENG Y T, PARK H, et al. Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and caco-2 human colon cancer cells[J]. Scientific Reports, 2017, 7: 10163. DOI:10.1038/s41598-017-10824-x |
| [85] |
JOHNSON-HENRY K C, ABRAHAMSSON T R, WU R Y, et al. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis[J]. Advances in Nutrition, 2016, 7(5): 928-937. DOI:10.3945/an.116.012237 |
| [86] |
FARAG M R, ALAGAWANY M. Physiological alterations of poultry to the high environmental temperature[J]. Journal of Thermal Biology, 2018, 76: 101-106. DOI:10.1016/j.jtherbio.2018.07.012 |
| [87] |
RIBET D, COSSART P. How bacterial pathogens colonize their hosts and invade deeper tissues[J]. Microbes and Infection, 2015, 17(3): 173-183. DOI:10.1016/j.micinf.2015.01.004 |
| [88] |
MA N, HE T, JOHNSTON L J, et al. Host-microbiome interactions:the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling[J]. Gut Microbes, 2020, 11(5): 1203-1219. DOI:10.1080/19490976.2020.1758008 |
| [89] |
SMITH E A, MACFARLANE G T. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine[J]. Microbial Ecology, 1997, 33(3): 180-188. DOI:10.1007/s002489900020 |
| [90] |
NOWAK A, LIBUDZISZ Z. Influence of phenol, p-cresol and indole on growth and survival of intestinal lactic acid bacteria[J]. Anaerobe, 2006, 12(2): 80-84. DOI:10.1016/j.anaerobe.2005.10.003 |
| [91] |
BELLO A U, IDRUS Z, MENG G Y, et al. Gut microbiota and transportation stress response affected by tryptophan supplementation in broiler chickens[J]. Italian Journal of Animal Science, 2017, 17(1): 107-113. |




