2. 湖南农业大学, 湖南省特色水产资源利用工程技术中心, 长沙 410128
2. Hunan Characteristic Aquatic Resources Utilization Engineering Technology Center, Hunan Agricultural University, Changsha 410128, China
近年来,我国克氏原螯虾稻田养殖产业不断扩大,发展势头良好,产业化前景广阔,已成为我国淡水虾类中的主要优势物种,加之其肉味鲜美、风味独特、具有丰富的营养价值,深受广大消费者的喜爱[1-2]。随着人们生活水平的提高,对虾肉的消费需求以由“量”转变到“质”,但针对克氏原螯虾不同生长阶段肉质的相关研究较少,且目前克氏原螯虾养殖在市场需求、加工现状等因素的影响下[3],出现了优良性状逐步丢失、规格差异大、肉质不齐和金属沉积等一系列问题[4]。因此,本试验通过对湖南省南县稻田养殖的不同规格克氏原螯虾的出肉率、肝体比以及肌肉营养成分、质构特性、肌纤维特性和相关肉质基因表达量进行测定,旨在对其营养品质进行科学评估,为今后针对不同生长阶段克氏原螯虾的科学人工养殖和专用配合饲料的开发提供参考依据,促进稻田克氏原螯虾养殖产业的可持续健康发展。
1 材料与方法 1.1 试验设计本试验所用克氏原螯虾为2020年8月采集于湖南省南县稻虾共作田,雌雄比例随机,共采集1 775尾,将鲜活样品带回实验室,逐一称量体长、体重,按体重分为0~10 g(A1组)、10~20 g(A2组)、20~30 g(A3组)和30~50 g(A4组)4组,各组体长和体重参数见表 1。
|
|
表 1 克氏原螯虾规格与分组 Table 1 Procambarus clarkii specification and grouping |
用吸水滤纸擦干克氏原螯虾体表水分,称取虾全重;将试验虾去头去螯,剪去附肢,用镊子将头胸甲和腹部甲壳打开,取肝胰腺及腹部肌肉,再用镊子剥离剩余甲壳上的肌肉,放置滤纸上吸干表面水分,用精确度为0.01 g的电子天平称量并记录。

|
水分含量采用105 ℃烘箱直接干燥法(GB 5009.3—2016)测定,粗蛋白质含量采用凯氏定氮法(GB 5009.3—2016)测定,粗脂肪含量采用索氏抽提法(GB 5009.3—2016)测定,粗灰分含量采用高温灰化法(GB 5009.3—2016)测定,样品为混样;分别委托青岛市科创有限技术公司和北京百得维斯生物技术有限公司检测脂肪酸、氨基酸含量,每组测定3个样品。根据1973年联合国粮农组织(FAO)/世界卫生组织(WHO)建议的每1 g氨基酸评分标准模式和全鸡蛋蛋白质的氨基酸模式,分别计算得出氨基酸评分(AAS)、化学评分(CS)和必需氨基酸指数(EAAI)[5];根据公式计算动脉粥样硬化指数(AI)和血栓形成指数(TI),评估不同规格克氏原螯虾肌肉脂肪酸对人类心血管疾病发生的影响;根据公式计算多烯指数(PI),以反映不同规格克氏原螯虾多烯不饱和脂肪酸的氧化程度。
氨基酸评价模型如下:

|

|
式中:n为比较的氨基酸数;a,b,c,…,h为克氏原螯虾肌肉蛋白质中某种氨基酸含量(mg/g N);A,B,C,…,H为全鸡蛋蛋白质中同种氨基酸含量(mg/g N)。
脂肪酸评价模型如下:
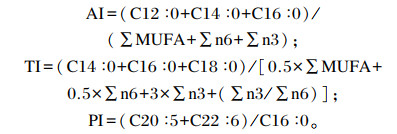
|
式中:MUFA为单不饱和脂肪酸。
1.2.3 肌肉特性分析取完整虾仁,对虾仁的第3腹节中央位置,利用TA.XT.Plus型物性测试仪(英国Stable Micro Systems公司)进行质地多面分析(TPA),测定硬度、咀嚼性、内聚性、胶黏性和弹性等指标。测试参数: 平底柱形探头P/36,测前速度2 mm/s,测试和测后速度均为0.5 mm/s,应变为60%,停留间隔时间5 s。每组测定3个样品,每个样品重复测定3次。取虾背部肌肉,切成3 mm3左右的小块,于4%的多聚甲醛固定液中保存,每组随机包埋3个样品,每个样品选3张切片,苏木素-伊红染色后使用显微镜观察肌纤维组织学结构,用Image-pro plus 6.0软件测量肌纤维直径、根数,每张片子选6个视野,计算得出肌纤维的密度和横截面积,肌纤维密度为测出每个视野内的肌纤维根数和该视野的面积后,换算成每平方毫米的根数。
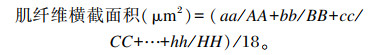
|
式中:aa,bb,cc,…,hh为各视野下肌纤维总横切面积;AA,BB,CC,…,HH为各视野下肌纤维根数。
1.2.4 肉质相关基因表达特性分析从各组随机挑选5尾虾,根据相关文献[6-7]和已有小龙虾转录组数据分析结果选择4个与肉质相关的基因进行表达量测定,分别为肌肉生长抑制素(myostatin, MSTN)、原肌球蛋白(tropomyosin, TM)、脂肪酸结合蛋白(fatty acid binding protein, FABP)、脂蛋白脂肪酶(liportein lipase, LPL)基因。按RNA提取试剂盒(美国Omega公司,)说明书提取虾腹部肌肉总RNA,微量检测仪(BioPHotometer,德国Eppendorf公司)和1%琼脂糖凝胶电泳检测总RNA的浓度和纯度,利用Thermo反转录试剂盒将总RNA反转录为cDNA,所有引物由北京擎科生物科技有限公司合成,引物信息见表 2。实时荧光定量PCR反应体系为10 μL,其中包括1 μL cDNA,5 μL SYBR Green Ⅰ荧光染料(Vazyme),0.4 μL 10 μmol/L目的基因的上游引物,0.4 μL 10 μmol/L目的基因的下游引物,3.2 μL ddH2O。反应程序如下:95 ℃ 30 s;95 ℃ 5 s;60 ℃ 40 s;40个循环,所有样品均设置3个重复,以18S rRNA[8]和β-肌动蛋白(β-actin)作为内参基因,运用2-△△Ct法计算相关基因的表达量。
|
|
表 2 用于克氏原螯虾实时荧光定量PCR分析的引物 Table 2 Primers for real time qPCR analysis of Procambarus clarkii |
数据用平均值±标准差(mean±SD)表示,原始数据经Excel 2019初步整理后,采用SPSS 26.0分析软件对数据进行单因素方差分析(one-way ANOVA),若组间差异显著,再采用LSD法进行多重比较,以P < 0.05表示差异显著。
2 结果与分析 2.1 肝体比、出肉率与肌肉营养成分分析 2.1.1 肝体比、出肉率与肌肉常规营养成分含量由表 3可知,不同规格克氏原螯虾的肝体比相近,除了A1与A4组差异显著(P < 0.05)外,其他组间无显著差异(P>0.05);A1组出肉率显著高于其他3组(P < 0.05);肌肉水分、粗蛋白质含量均以A1组为最高,且随着规格增大均呈逐渐降低趋势,A1与A4组差异显著(P < 0.05);肌肉粗脂肪含量随着规格增大呈逐渐升高趋势,A4组最高,A3组次之,且A4和A3组均与A1组差异显著(P < 0.05);肌肉粗灰分含量随着规格增大均呈逐渐降低趋势,A4和A3组差异不显著(P>0.05),但A1组与其他3组差异显著(P < 0.05)。
|
|
表 3 克氏原螯虾肝体比、出肉率与肌肉常规营养成分含量(干物质基础) Table 3 Hepatosomatic index, meat rate and muscle conventional nutritional component contents of Procambarus clarkii (DM basis) |
由表 4可知,在克氏原螯虾腹肌中共检测出20种脂肪酸,包含7种饱和脂肪酸(SFA)和13种不饱和脂肪酸(UFA),其中单不饱和脂肪酸(MUFA)3种、多不饱和脂肪酸(PUFA)10种。饱和脂肪酸含量以A1组最高,A2组最低;单不饱和脂肪酸含量以A2组最高,A4组最低;多不饱和脂肪酸含量以A2组最高,A1组最低。在A1~A4组中,饱和脂肪酸中C18:0含量最高,C22:0含量最低;单不饱和脂肪酸中C18:1n9c含量最高,C20:1含量最低;多不饱和脂肪酸中C20:5n3(EPA)含量最高,C18:3n6含量最低。A2组的AI值和A3组的TI值最小,表明A2和A3组脂肪酸的不饱和度高;A4组的PI值最小,表明A4组的多不饱和脂肪酸降解及氧化程度低。
|
|
表 4 克氏原螯虾肌肉脂肪酸组成与含量(占总脂肪酸的比例) Table 4 Fatty acid composition and contents in muscle of Procambarus clarkii (proportion of total fatty acids) |
由表 5可知,在克氏原螯虾腹肌中共检测出17种氨基酸(不含色氨酸),其中必需氨基酸7种、半必需氨基酸2种、非必需氨基酸8种。A1组的氨基酸、非必需氨基酸和鲜味氨基酸总量均高于其余3组,但差异不显著(P>0.05);A2组的必需氨基酸总量最高,且甲硫氨酸含量最高,显著高于A3组(P < 0.05);除此之外,谷氨酸含量在A1~A4组中皆为最高;A2组必需氨基酸总量/氨基酸总量和必需氨基酸总量/非必需氨基酸总量的比值最大,但与其他3组差异不显著(P>0.05)。
|
|
表 5 克氏原螯虾肌肉氨基酸组成与含量 Table 5 Amino acid composition and contents in muscle of Procambarus clarkia |
由表 6可知,4个组的克氏原螯虾肌肉中赖氨酸的AAS和CS皆为最高。根据AAS,必需氨基酸中第一限制氨基酸为蛋氨酸+半胱氨酸,第二限制氨基酸为苏氨酸;根据CS,必需氨基酸中第一限制氨基酸为缬氨酸,第二限制氨基酸为蛋氨酸+半胱氨酸。根据公式计算出的A1~A4组的EAAI分别为88.22、98.36、87.02、95.86,以A2组的EAAI最高。
|
|
表 6 克氏原螯虾肌肉必需氨基酸评价 Table 6 Evaluation of muscle EAA of Procambarus clarkii |
由表 7可知,克氏原螯虾肌肉硬度、胶黏性、弹性和内聚性随着规格增大而增加,A4组硬度、胶黏性、弹性、咀嚼性和内聚性均为最大,且咀嚼性与其他3组差异显著(P < 0.05),硬度、胶黏性与A1和A2组差异显著(P < 0.05)。
|
|
表 7 克氏原螯虾肌肉质构特性 Table 7 Texture property of Procambarus clarkii muscle |
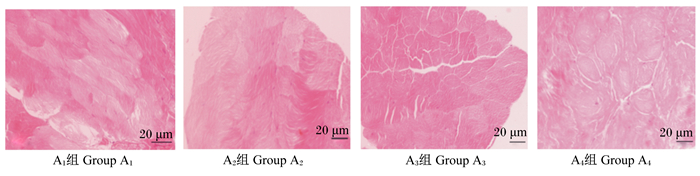
由图 1和表 8可知,A3组的肌纤维直径最大,并显著大于A1和A2组(P < 0.05)。A1组的肌纤维密度显著大于其他3组(P < 0.05),而这3组之间则差异不显著(P>0.05)。A3组肌纤维横截面积最大,显著高于A1和A2组(P < 0.05)。
|
图 1 克氏原螯虾肌肉组织学结构 Fig. 1 Histological structure of Procambarus clarkii muscle (400×) |
|
|
表 8 克氏原螯虾肌纤维特性 Table 8 Muscle fiber characteristics of Procambarus clarkii |
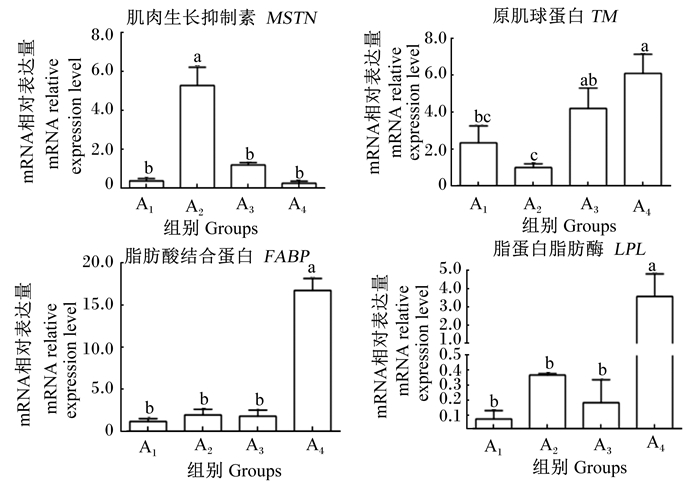
如图 2所示,MSTN基因在A2组的mRNA相对表达量最高,与其他3组差异显著(P < 0.05),其中A4组中最低;TM基因在A4组的mRNA相对表达量最高,A3组次之,A4组显著高于A1和A2组(P < 0.05);FABP和LPL基因在A4组的mRNA相对表达量最高,与其他3组差异显著(P < 0.05),而A1、A2和A3组之间无显著差异(P>0.05)。
|
数据柱形标注相同小写字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P < 0.05)。 Value columns with the same small letters mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P < 0.05). 图 2 肉质相关基因在克氏原螯虾肌肉中表达的qRT-PCR分析 Fig. 2 Expression of meat quality related genes in muscle of Procambarus clarkii analyzed by qRT-PCR |
常规营养成分主要包括粗蛋白质、水分、粗脂肪和粗灰分,其含量对虾类品质评价起着重要作用,但主要通过粗蛋白质和粗脂肪含量来反映。本试验中,A1组克氏原螯虾的出肉率以及肌肉水分、粗蛋白质和粗灰分含量均为最高,而粗脂肪含量显著低于A4组,但高于南美白对虾(2.48%)[9]和斑节对虾(3.85%)[10]。影响虾体营养成分的因素很多,除物种差异外,还有虾的生理状况、虾的大小、捕获季节、饲料种类等,从出肉率和肌肉粗蛋白质含量考虑,小规格克氏原螯虾更具有营养。
根据《中国居民膳食营养素参考摄入量速查手册》,人体理想的膳食脂肪酸构成比例为饱和脂肪酸: 单不饱和脂肪酸: 多不饱和脂肪酸=1:1:1[11],此次测得的克氏原螯虾腹部肌肉中多不饱和脂肪酸含量相对较低。试验结果显示,A1组饱和脂肪酸含量最高,A2组单不饱和脂肪酸和多不饱和脂肪酸含量皆为最高,多不饱和脂肪酸中EPA含量最高。多不饱和脂肪酸不仅具有营养和保健功能,而且可以在一定程度上增加虾的香味和多汁性。本研究结果表明,克氏原螯虾腹部肌肉中含有丰富的脂肪酸,在规格较小的克氏原螯虾(A1和A2组)中尤为突出。根据FAO/WHO模式,必需氨基酸总量/氨基酸总量的比值在40%左右,必需氨基酸/非必需氨基酸的比值在60%以上的为质量较好的蛋白质,本研究结果显示克氏原螯虾的蛋白质营养价值满足FAO/WHO对理想蛋白质源的评价标准。AAS和CS从不同的角度反映了蛋白质构成和利用率的关系,在A1~A4组中AAS和CS最高的均为赖氨酸,超过FAO/WHO模式。肉质食物的鲜美程度主要取决于食物中呈鲜味的谷氨酸和天门冬氨酸以及呈甘味的甘氨酸和丙氨酸含量[12],其中,谷氨酸是鲜味最强的氨基酸,4组克氏原螯虾肌肉中谷氨酸含量丰富,且A1组的谷氨酸含量最高,说明克氏原螯虾肉质鲜美。
3.2 肌肉特性分析出肉率是衡量鱼、虾等水产动物品质和生产性能的重要指标之一,本试验测得同一时期克氏原螯虾腹部肌肉的出肉率随着虾规格增大而逐渐降低,其中规格最小的A1组虾的出肉率显著高于其他规格虾。本次试验测得的稻虾共作模式下的克氏原螯虾的出肉率低于唐黎等[13]测得的湄潭地区稻田养殖的克氏原螯虾(18.40%)和田娟等[14]测得的洞庭湖区野生克氏原螯虾(20.21%),这可能与此次所采虾为秋季虾有关,姚根娣等[15]研究得出秋季大小虾整体的出肉率低于春季和夏季,且容易受生长时期、产地等影响。此外,本次试验测得的克氏原螯虾的出肉率还低于澳洲淡水龙虾(17.97%)[16]和凡纳滨对虾(52.2%)[17],表明克氏原螯虾的出肉率较其他经济虾类偏低。TPA是目前用于评价水产品肉质最广泛的方法之一[18],包括硬度、内聚性、弹性、胶黏性和咀嚼性等[19-20];研究表明,随着动物规格增加,肌纤维会增大增粗,从而硬度增大,且一定范围内的脂肪含量的增加也会引起胶黏性增大[21],本试验中,A4组的肌肉硬度、胶黏性、弹性、咀嚼性和内聚性均最大,且咀嚼性与其他3组差异显著,硬度和胶黏性与规格在20 g以下的A1和A2组差异显著;A3组肌肉咀嚼性小于A1和A2组,可能是由于动物肌纤维密度大,肌肉间填充物多,并且咀嚼性作为一个综合性指标,其结果受到多种因素影响[22]。一般认为,肉的硬度、内聚性和弹性越高,肉质口感越好[23-24],因此,A4组克氏原螯虾在口感上总体更优。
肌肉的基本组成单位是肌纤维[25],肌纤维特性有肌纤维直径、密度、横截面积等。本试验统计分析了4组不同规格克氏原螯虾的肌纤维组织学特性,结果显示,A3组的肌纤维直径最大,且显著大于A1和A2组;A1组的肌纤维密度显著高于其他3组,而后3组之间肌纤维密度差异不显著;A3组肌纤维横截面积最大,显著高于A1和A2组。大多数研究认为,肌纤维直径是衡量肌纤维粗细的指标,肌纤维越细则肉品质越好;肌纤维密度越大则肉质越细腻[26-27],肌纤维的密度与直径呈负相关。本试验中测得A1组克氏原螯虾的肌纤维直径最小,肌纤维密度最大,与其他3组相比肉质更细嫩。
3.3 肉质相关基因表达特性分析MSTN是脊椎动物骨骼肌发生调控中已发现的重要负调控因子[28],MSTN基因缺失突变能促进肌纤维的增生和肥大,增加肌肉的韧性[29]。本试验结果显示A4组中MSTN基因的mRNA相对表达量最低,与A2组差异显著,提示A4组肌肉韧性更强,口感更佳。TM可以通过形成大分子作为肉味增强剂的前体,明显地提高肉味[30],在本试验中TM基因在A3和A4组中的mRNA相对表达量显著高于A1和A2组,提示规格大的虾在肉味上要更优;FABP和LPL都参与脂质的分解代谢过程[31-32],FABP家族能够促进细胞摄取脂肪酸,防止脂肪酸在细胞内的堆积。本试验中,FABP基因在A4组中的mRNA相对表达量最高,显著高于其他3组,而其他3组间无显著差异。有研究报道,FABP与碳链较短的脂肪酸如棕榈酸、油酸亲和力较高,上述脂肪酸皆在A1组含量最高,且A1组中总脂肪酸含量最高,这与FABP基因表达规律不符,可能是由于总脂肪酸含量在不同规格克氏原螯虾中差异不大,且可能只有在线粒体氧化受阻或采用了难以代谢的脂肪酸类似物的情况下才会有FABP含量的提升[33],脂肪酸含量高不一定会提升FABP基因的mRNA相对表达量。LPL是脂质分解代谢过程中关键的限速酶,与肌肉中水分含量呈负相关,与肌内脂肪含量呈正相关[34-35],本试验所得结果与此相符。本试验结果显示,A4组中LPL基因的mRNA相对表达量显著高于其他3组,且水分含量随着克氏原螯虾规格增大而减少,而A4组粗脂肪含量显著高于其他3组。此外,FABP和LPL这类脂肪沉积相关基因可以调控肌内脂肪含量[36],肌内脂肪含量与肉的多汁性和风味呈正相关[37-38],与肉的吞咽咀嚼次数和酸涩度呈负相关关系[39],提示A4组在多汁性和口感风味上更优。
4 结论综上所述,稻虾共作模式下的克氏原螯虾具有丰富的营养价值,富含油酸等多种人体必需脂肪酸,且氨基酸组成合理,因含有较多的呈味氨基酸和不饱和脂肪酸,使克氏原螯虾具有良好的风味和一定的保健作用;此外,从出肉率以及肌肉粗蛋白质、脂肪酸总量和氨基酸总量考虑,规格小的0~10 g克氏原螯虾在营养价值上较高;但从肌肉质构特性和4个肉质相关基因表达结果考虑,规格大的30~50 g克氏原螯虾在风味口感上更优。
| [1] |
YU L M, SONG C, ZHANG C, et al. Occurrence of sulfonamides in fish in the lower reaches of Yangtze river, China and estimated daily intake for understanding human dietary exposure[J]. Aquaculture, 2018, 495: 538-544. DOI:10.1016/j.aquaculture.2018.06.033 |
| [2] |
黄春红, 梁洲勇, 陈蕴, 等. 不同饲料对小龙虾日摄食率、消化率、生长及肌肉品质的影响[J]. 动物营养学报, 2020, 32(5): 2361-2368. HUANG C H, LIANG Z Y, CHEN Y, et al. Effects of different feeds on daily feeding rate, digestibility, growth and muscle quality of crayfish (Procambarus clarkii)[J]. Chinese Journal of Animal Nutrition, 2020, 32(5): 2361-2368 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.05.045 |
| [3] |
WANG H, SHI W J, WANG L, et al. Genetic determination of processing traits in the red swamp crayfish, Procambarus clarkii (Girard)[J]. Aquaculture, 2020, 529: 735602. DOI:10.1016/j.aquaculture.2020.735602 |
| [4] |
ANANDKUMAR A, LI J, PRABAKARAN K, et al. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans[J]. Journal of Food Composition and Analysis, 2020, 88: 103449. DOI:10.1016/j.jfca.2020.103449 |
| [5] |
World Health Organization/Food and Agriculture Organization. Energy and protein requirements report of a joint FAO/WHO ad hoc expert committee. WHO technical report series, No. 522 Geneva: WHO, 1973.
|
| [6] |
LEAL-GUTIÉRREZ J D, ELZO M A, CARR C, et al. RNA-seq analysis identifies cytoskeletal structural genes and pathways for meat quality in beef[J]. PLoS One, 2020, 15(11): e0240895. DOI:10.1371/journal.pone.0240895 |
| [7] |
ZHUO R Q, ZHOU T T, YANG S P, et al. Characterization of a molt-related myostatin gene (FmMstn) from the banana shrimp Fenneropenaeus merguiensis[J]. General and Comparative Endocrinology, 2017, 248: 55-68. DOI:10.1016/j.ygcen.2017.03.010 |
| [8] |
DAI Y J, WANG Y Q, ZHANG Y H, et al. The role of ficolin-like protein (PcFLP1) in the antibacterial immunity of red swamp crayfish (Procambarus clarkii)[J]. Molecular Immunology, 2017, 81: 26-34. DOI:10.1016/j.molimm.2016.11.017 |
| [9] |
KIM E J, PARK M A, SEO H C, et al. Changes in the growth and body composition of juvenile white shrimp Litopenaeus vannamei fed diets containing fish meal and soybean meal as protein sources[J]. Korean Journal of Fisheries and Aquatic Sciences, 2012, 45(6): 659-665. DOI:10.5657/KFAS.2012.0659 |
| [10] |
VASAGAM K P K, RAMESH S, BALASUBRAMANIAN T. Dietary value of different vegetable oil in black tiger shrimp Penaeus monodon in the presence and absence of soy lecithin supplementation: effect on growth, nutrient digestibility and body composition[J]. Aquaculture, 2005, 250(1/2): 317-327. |
| [11] |
中国营养学会. 中国居民膳食营养素参考摄入量(2013版)[M]. 北京: 科学出版社, 2014. Chinese Nutrition Society. Reference intake of dietary nutrients for Chinese residents (2013 version)[M]. Beijing: Science Press, 2014 (in Chinese). |
| [12] |
刘峰, 吕小康, 刘阳阳, 等. 饥饿对大黄鱼幼鱼肌肉中氨基酸和脂肪酸组成的影响[J]. 渔业科学进展, 2018, 39(5): 58-65. LIU F, LV X K, LIU Y Y, et al. Effect of starvation on amino acids and fatty acids of juvenile Larimichthys crocea[J]. Progress in Fishery Sciences, 2018, 39(5): 58-65 (in Chinese). |
| [13] |
唐黎, 杨家军, 林艳红, 等. 贵州稻田养殖克氏原螯虾肌肉营养成分分析[J]. 河北渔业, 2018(9): 15-19, 45. TANG L, YANG J J, LIN Y H, et al. Analysis on flesh nutrient component of freshwater crayfish Procambarus clarkii farmed in ricefield in Guizhou province[J]. Hebei Fisheries, 2018(9): 15-19, 45 (in Chinese). DOI:10.3969/j.issn.1004-6755.2018.09.003 |
| [14] |
田娟, 许巧情, 田罗, 等. 洞庭湖克氏原螯虾肌肉成分分析及品质特性分析[J]. 水生生物学报, 2017, 41(4): 870-877. TIAN J, XU Q Q, TIAN L, et al. The muscle composition analysis and flesh quality of Procambarus clarkii in the Dongting lake[J]. Acta Hydrobiologica Sinica, 2017, 41(4): 870-877 (in Chinese). |
| [15] |
姚根娣, 孙振中, 郭履骥, 等. 克氏原螯虾(Cambarus clarkii)含肉率和营养成分分析[J]. 水产科技情报, 1993(4): 177-179. YAO G D, SUN Z Z, GUO L J, et al. Analysis of meat content and nutritional components of Cambarus clarkii[J]. Fisheries Science & Technology Information, 1993(4): 177-179 (in Chinese). |
| [16] |
王广军, 孙悦, 郁二蒙, 等. 澳洲淡水龙虾与克氏原螯虾肌肉营养成分分析与品质评价[J]. 动物营养学报, 2019, 31(9): 4339-4348. WANG G J, SUN Y, YU E M, et al. Analysis and quality evaluation of nutrient components in muscle of Cherax quadricarinatus and Procambarus clarkii[J]. Chinese Journal of Animal Nutrition, 2019, 31(9): 4339-4348 (in Chinese). |
| [17] |
李晓, 王颖, 李红艳, 等. 凡纳滨对虾虾头与肌肉营养成分分析与评价[J]. 水产科学, 2018, 37(1): 66-72. LI X, WANG Y, LI H Y, et al. Analysis and assessment of nutrient composition in head and muscle of pacific white leg shrimp Litopenaeus vannamei[J]. Fisheries Science, 2018, 37(1): 66-72 (in Chinese). |
| [18] |
万金娟, 陈友明, 邵俊杰, 等. 盱眙地区不同养殖模式下克氏原螯虾肌肉品质的比较分析[J]. 动物营养学报, 2020, 32(2): 965-972. WANG J J, CHEN Y M, SHAO J, et al. Comparative analysis on muscle quality of red swamp crayfish (Procambarus clarkia) cultured under different aquaculture modes in Xuyi region[J]. Chinese Journal of Animal Nutrition, 2020, 32(2): 965-972 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.02.053 |
| [19] |
LIN W L, ZENG Q X, ZHU Z W, et al. Relation between protein characteristics and TPA texture characteristics of crisp grass carp (Ctenopharyngodon idellus C. ET V) and grass carp (Ctenopharyngodon idellus)[J]. Journal of Texture Studies, 2012, 43(1): 1-11. DOI:10.1111/j.1745-4603.2011.00311.x |
| [20] |
CASAS C, MARTINEZ O, GUILLEN M D, et al. Textural properties of raw Atlantic salmon (Salmo salar) at three points along the fillet, determined by different methods[J]. Food Control, 2006, 17(7): 511-515. DOI:10.1016/j.foodcont.2005.02.013 |
| [21] |
柳明, 朱晓鸣, 雷武, 等. 不同饲料营养对池塘养殖长吻生长性能和鱼肉品质的影响[J]. 水生生物学报, 2010, 34(3): 598-610. LIU M, ZHU X M, LEI W, et al. Effect of diet formulation on the growth performance and quality of tank reared Chinese longsnout catfish[J]. Acta Hydrobiologica Sinica, 2010, 34(3): 598-610 (in Chinese). |
| [22] |
YANG Y B, BIAN J P, MA X Y, et al. Comparative study on muscle fiber characteristics and muscle nutritional quality between two groups of Yellow river carps[J]. Agricultural Science & Technology, 2017, 18(8): 1432-1436, 1483. |
| [23] |
梁洁, 庞敏, 李林静, 等. 稻田与清水养殖模式下小龙虾肉的理化特性比较[J]. 湖南农业科学, 2018(5): 79-81. LIANG J, PANG M, LI L J, et al. Comparison of physicochemical properties of Procambarus clarkii breeded in paddy field and clean water aquaculture model[J]. Hunan Agricultural Sciences, 2018(5): 79-81 (in Chinese). |
| [24] |
田万强, 李林强, 梅楚刚, 等. 肌肉组织质构特性差异比较及肌纤维分析[C]//第十八届中国科协年会. 西安: 中国科学技术学会, 陕西省人民政府, 2016: 8. TIAN W Q, LI L Q, MEI C G, et al. Comparison of differences of texture properties of tissue and analysis of muscle fibers[C]//The 18th Annual Meeting of China Association for Science and Technology. Xi'an: China Association for Science and Technology, Shaanxi Provincial People's Government, 2016: 8. (in Chinese) |
| [25] |
斯琴其木格. 乌珠穆沁羊生长过程中MRFs基因特性和肌纤维形态学变化分析[D]. 博士学位论文. 呼和浩特: 内蒙古农业大学, 2017. SI QIN Q M G. Analysis of MRFs gene characteristics and morphological changes of muscle fibers in Wuzhumuqin sheep during postnatal development[D]. Ph. D. Thesis. Hohhot: Inner Monggolia Agriculture University, 2017. (in Chinese) |
| [26] |
侯粲, 杨曙明, 周宇, 等. 不同营养水平对虹鳟肌肉组织学的影响[J]. 安徽农业科学, 2013, 41(12): 5347-5348, 5353. HOU C, YANG S M, ZHOU Y, et al. Effect of nutrition with different levels on histology of rainbow trout (Oncorhynchus mykiss)[J]. Jonurnal of Anhui Agricultural Science, 2013, 41(12): 5347-5348, 5353 (in Chinese). DOI:10.3969/j.issn.0517-6611.2013.12.064 |
| [27] |
SCHIAFFINO S, REGGIANI C. Fiber types in mammalian skeletal muscles[J]. Physiological Reviews, 2011, 91(4): 1447-1531. |
| [28] |
SOUSA-JUNIOR L P B, MEIRA A N, AZEVEDO H C, et al. Variants in myostatin and MyoD family genes are associated with meat quality traits in Santa Inês sheep[J]. Animal Biotechnology, 2020. DOI:10.1080/10495398.2020.1781651 |
| [29] |
FIEMS L O. Double muscling in cattle: genes, husbandry, carcasses and meat[J]. Animals, 2012, 2(3): 472-506. DOI:10.3390/ani2030472 |
| [30] |
WOOD J D, ENSER M, FISHER A V, et al. Fat deposition, fatty acid composition and meat quality: a review[J]. Meat Science, 2008, 78(4): 343-358. DOI:10.1016/j.meatsci.2007.07.019 |
| [31] |
JOO S T, KIM J D, HWANG Y H, et al. Control of fresh meat quality through manipulation of muscle fiber characteristics[J]. Meat Science, 2013, 95(4): 828-836. DOI:10.1016/j.meatsci.2013.04.044 |
| [32] |
YIN B Z, FANG J C, ZHANG J S, et al. Correlations between single nucleotide polymorphisms in FABP4 and meat quality and lipid metabolism gene expression in Yanbian yellow cattle[J]. PLoS One, 2020, 15(6): e0234328. DOI:10.1371/journal.pone.0234328 |
| [33] |
HOCQUETTE J F, GONDRET F, BAÉZA E, et al. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers[J]. Animal, 2010, 4(2): 303-319. |
| [34] |
KAMATH S D, SCHEIBLHOFER S, JOHNSON C M, et al. Effect of structural stability on endolysosomal degradation and T-cell reactivity of major shrimp allergen tropomyosin[J]. Allergy, 2020, 75(11): 2909-2919. DOI:10.1111/all.14410 |
| [35] |
NAKAMURA F, TAKAHASHI K. Paratropomyosin: a new myofibrillar protein that modifies the actin-myosin interaction in postrigor skeletal muscle[J]. The Japanese Biochemical Society, 2008, 97(4): 1043-1051. |
| [36] |
KURODA M, HARADA T. Fractionation and characterization of the macromolecular meaty flavor enhancer from beef meat extract[J]. Journal of Food Science, 2004, 69(7): 542-548. |
| [37] |
王鑫. Myostatin基因编辑牛F1代肉质分析[D]. 硕士学位论文. 呼和浩特: 内蒙古大学, 2019. WANG X. Meat quality analysis of the crossbred cattle with myostatin gene editing[D]. Master's Thesis. Hohhot: Inner Mongolia University, 2019. (in Chinese) |
| [38] |
郭勇, 张雄, 史开志, 等. 猪脂肪沉积相关酶基因表达与胴体及肉质性状的相关性研究[J]. 中国畜牧杂志, 2021, 57(3): 91-96. GUO Y, ZHANG X, SHI K Z, et al. Correlation analysis between the expression of porcine fat deposition-related enzyme genes and carcass and meat quality traits[J]. Chinese Journal of Animal Science, 2021, 57(3): 91-96 (in Chinese). |
| [39] |
刘嘉欣, 张木子, 黎明, 等. 氨氮胁迫下饥饿与复投喂对黄颡鱼肝脏中脂质代谢相关酶活性及相关基因表达的影响[J]. 动物营养学报, 2021, 33(1): 436-447. LIU J X, ZHANG M Z, LI M, et al. Effects of starvation and re-feeding on enzyme activities and gene expression involved in liver lipid metabolism of yellow catfish (Pelteobagrus fulvidraco) under ammonia nitrogen stress[J]. Chinese Journal of Animal Nutrition, 2021, 33(1): 436-447 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.01.044 |




