饲粮纤维是不能被动物自身分泌的消化酶所分解的糖类聚合物,由多种糖单体通过不同的糖苷键链接而成[1]。传统动物营养学除了考虑饲粮中的粗纤维含量,也通过测定中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)、纤维素、半纤维素和木质素的含量来进一步剖分饲粮纤维的组成。然而,由于饲粮纤维在单胃动物前肠无法被消化利用,后肠微生物对饲粮纤维的利用就显得尤为重要。目前研究表明,饲粮纤维的摄入对维持动物肠黏膜屏障完整性、缓解肠道炎症有重要作用[2-3]。同时,一般认为可溶性饲粮纤维(soluble dietary fiber,SDF)比不可溶性饲粮纤维(insoluble dietary fiber,IDF)具有更高的可发酵性[4]。本课题组前期研究表明,小鼠和猪后肠均存在特异性利用SDF和IDF的核心菌群[5-7],后肠微生物可通过改变群落结构实现对不同类型饲粮纤维的有效利用。因此,根据水溶性或可发酵性对饲粮纤维进行分类对探究纤维的作用机理更为合理。肠道主要负责营养物质的消化吸收,同时也是机体最大的免疫器官,肠道健康对维持动物的生长发育极其重要。肠道功能失调不仅会降低营养物质的消化吸收率,还会导致病原微生物、致病性抗原及有毒物质侵入机体,引起诸如肠易激综合征、全身炎症反应综合征、神经性厌食呕吐和微生态失衡等疾病,同时导致机体免疫功能低下、腹泻及生长缓慢等症状[8]。随着单胃动物“后肠营养”概念的提出和相关研究的深入,后肠健康在很大程度上影响动物的养分摄入,由此而言,深入研究饲粮纤维对后肠健康的影响机理意义重大。对生长猪的研究表明,饲粮中添加高水平天然纤维源可明显改善猪的肠道屏障功能,并通过调控模式识别受体相关信号通路影响炎症相关细胞因子的释放,这一效应很可能与纤维类型有关[7]。但值得注意的是,之前的研究是以健康动物为对象,无法证明炎症因子的变化是否处于正常范围。从这个角度而言,肠炎模型动物比健康动物更适用于相关研究。因此,本研究以C57BL/6小鼠为研究对象,通过在其饮水中添加葡聚糖硫酸钠(DSS)建立结肠炎模型,比较分析典型SDF(菊粉)与IDF[微晶纤维素(MCC)]对结肠炎症相关细胞因子表达的影响,为进一步探究SDF与IDF对动物后肠免疫功能的影响机理奠定基础,同时也为动物饲粮中纤维源的选择和科学应用提供参考。
1 材料与方法 1.1 试验设计选取24只体重约为20 g(8周龄)的雄性健康C57BL/6小鼠,预饲3 d后开始正式试验,正式试验分为建模期(7 d)和恢复期(14 d)2个阶段。建模期所有小鼠均饲喂无纤维纯合饲粮,在其饮水中添加3% DSS,保持自由采食和饮水,诱导小鼠产生结肠炎,通过疾病活动指数(DAI)评判模型是否建立成功。建模成功后进入恢复期,按各组体重无差异原则将小鼠分为3组,每组8只,C组(对照组)小鼠仍饲喂无纤维纯合饲粮,试验组小鼠分别饲喂含5%菊粉(I组)或MCC(M组)的饲粮,所有小鼠自由采食和饮水。参照AIN-93标准配制饲粮,各组饲粮组成及营养水平见表 1。
|
|
表 1 各组饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of diets for different groups (air-dry basis) |
试验第1周每天称重,并记录日采食量;第1周结束后每隔3 d称重,每天记录采食量。试验结束后屠宰所有小鼠,采样备用。
1.2 试验材料试验材料包括8周龄雄性C57BL/6小鼠[成都达硕实验动物有限公司,许可证号:SCXK(川)2015-03]、灭菌鼠粮(成都达硕实验动物有限公司)、DSS[0216011080,纯度:99.0%,安倍医疗器械贸易(上海)有限公司]、菊粉[A602227,纯度≥90.0%,生工生物工程(上海)股份有限公司]、MCC[A600279,纯度:99.0%~102.0%,生工生物工程(上海)股份有限公司]、3%双氧水[SDS12-500,斯坦利思生物科技(杭州)有限公司]、Trizol试剂盒[Code No.9108,TaKaRa(北京)公司]、异硫氰酸荧光素(FITC)标记的CD3单克隆抗体[仓鼠抗小鼠免疫球蛋白G1(hamster anti mouse IgG1],CAT: 553061,美国BD Pharmingen公司)、别藻青蛋白(APC)标记的CD4单克隆抗体[大鼠抗小鼠免疫球蛋白G2a(rat anti mouse IgG2a),CAT: 553051,美国BD Pharmingen公司]、荧光素(PE)标记的CD8单克隆抗体[大鼠抗小鼠免疫球蛋白G2a(rat anti mouse IgG2a),CAT: 100708,美国BioLegend公司]、红细胞裂解液(10×,CAT: 349202,美国BD公司)、无水乙醇(CAS: 64-17-5,成都市科隆化学品有限公司)、氯仿(CAS: 67-66-3,成都市科隆化学品有限公司)、逆转录试剂盒[Code No.RR047A,TaKaRa(北京)公司]、全自动生化仪(TBA-40FR,日本Toshiba公司)、荧光定量PCR仪(ABI 7900,美国ABI公司)、流式细胞仪(FACSVerse,美国BD公司)等。
1.3 小鼠结肠炎模型评判方法每天称量小鼠体重。每天记录粪便性状,按肉眼观察到的正常、松散、稀便3个级别进行评分。利用联苯胺法[9]测定隐血情况。根据相关标准[10]对小鼠DAI进行评估。通过DAI判断DSS诱导结肠炎模型是否建立成功。
1.4 血清生化指标测定恢复期试验结束后,所有小鼠经二氧化碳麻醉后进行眼球采血,每只小鼠分别采集2管(各0.5 mL),其中一管进行肝素钠抗凝处理,立即进行T淋巴细胞亚群检测;另一管经室温静置30 min后,4 ℃、12 000×g离心10 min,取血清-20 ℃保存备用。采用全自动生化仪测定血清中总蛋白(TP)、白蛋白(ALB)、免疫球蛋白G(IgG)、C反应蛋白(CRP)、补体3(C3)、免疫球蛋白M(IgM)、非酯化脂肪酸(NEFA)含量。
1.5 器官指数测定采血后采用颈椎脱臼法处死小鼠,采集肝脏、脾脏、胸腺并称重。按下列公式计算器官指数:
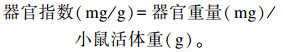
|
将采集的小鼠新鲜静脉血于肝素钠抗凝管中,立即混匀,吸取100 μL抗凝血于流式管,然后加入小鼠的CD3、CD4、CD8单克隆抗体各1头份,涡旋混匀,于4 ℃避光染色30 min,染色后用1 mL红细胞裂解液(1×)破裂红细胞,300×g离心5 min,弃上清,再用500 μL的预冷磷酸盐缓冲液(PBS)重悬细胞,用流式细胞仪进行检测;取新鲜脾脏制备单细胞悬液,获得浓度为1×106~1×107个/mL的单细胞悬液,取细胞悬液100 μL于流式管中,然后加入小鼠的CD3、CD4、CD8单克隆抗体各1头份,涡旋混匀,于4 ℃避光染色30 min,染色后用500 μL的PBS重悬细胞,300×g离心5 min,弃上清,再用500 μL的PBS重悬细胞,使用流式细胞仪进行检测。
1.7 结肠组织炎症相关细胞因子mRNA表达量测定处死小鼠后迅速打开腹腔,用预冷的生理盐水轻轻冲洗肠道内容物后,采集2 cm的结肠中段组织,置于2 mL冻存管中-80 ℃保存备用。采用Trizol试剂盒提取各组小鼠结肠组织总mRNA,1%琼脂糖凝胶电泳检测RNA完整性,采用紫外-可见分光光度计检测RNA浓度,用逆转录试剂盒将RNA反转录为cDNA后使用荧光定量PCR仪进行实时荧光定量PCR(RT-qPCR)分析,检测结肠组织中白细胞介素IL-6(IL-6)、Toll样受体4(TLR4)、核苷酸结合寡聚化结构域蛋白1(NOD1)、髓样分化因子88(MyD88)、肿瘤坏死因子受体相关因子6(TRAF6)、受体互作蛋白激酶2(RIPK2)、白细胞介素-10(IL-10)、核因子-κB(NF-κB)、白细胞介素-1β(IL-1β)的mRNA表达情况。目的基因引物信息如表 2所示。以小鼠3-磷酸甘油醛脱氢酶(GAPDH)作为内参基因,采用2-ΔΔCt法计算结肠组织中各目的基因的mRNA相对表达量。
|
|
表 2 目的基因引物信息 Table 2 Primer information for targeting genes |
采用SPSS 20.0软件的ANOVA模块对所有数据进行单因素方差分析,同时应用最小显著性差异(LSD)法进行多重比较。数据以平均值±标准差表示,以P<0.05为差异显著。
2 结果与分析 2.1 DSS诱导小鼠结肠炎模型的建立建模期间,每天同一时间观察记录小鼠的一般情况。建模前小鼠体重、采食量稳定,反应灵敏且毛发光亮,粪便性状正常。随着试验进行,小鼠出现不同程度的腹泻、便血,摄食减少,拱背,精神萎靡,在第7天肉眼可见便血。
由表 3可知,在建模期第6天,C组和M组小鼠体重显著下降(P<0.05),I组小鼠体重有下降趋势(P=0.051)。由表 4可知,C组和M组小鼠的平均日增重(ADG)在建模期第2、5和6天显著下降(P<0.05),I组小鼠的ADG在建模期第6天显著下降(P<0.05)。由表 5可知,在建模期间各组小鼠的DAI逐渐增加,在建模期第7天时,各组的DAI均显著高于第1天(P<0.05)。
|
|
表 3 建模期各组小鼠体重变化 Table 3 Change of body weight of mice from different groups during modeling period |
|
|
表 4 建模期各组小鼠ADG变化 Table 4 Change of ADG of mice from different groups during modeling period |
|
|
表 5 建模期各组小鼠DAI变化 Table 5 Change of DAI of mice from different groups during modeling period |
由表 6、表 7、表 8可知,恢复期小鼠体重随饲养时间的延长呈上升趋势,但各组小鼠体重和ADG均无显著差异(P>0.05);恢复期第5~7天,与C组相比,M组小鼠平均日采食量(ADFI)显著增加(P<0.05)。
|
|
表 6 恢复期各组小鼠体重变化 Table 6 Change of body weight of mice from different groups during recovery period |
|
|
表 7 恢复期各组小鼠ADG变化 Table 7 Change of ADG of mice from different groups during recovery period |
|
|
表 8 恢复期各组小鼠ADFI变化 Table 8 Change of ADFI of mice from different groups during recovery period |
由表 9可知,各组小鼠肝脏、脾脏和胸腺指数差异均不显著(P>0.05)。
|
|
表 9 各组小鼠恢复期结束时的器官指数 Table 9 Organ indices of mice in different groups at the end of recovery period |
由表 10可知,各组小鼠血清TP、ALB、IgG、C3含量均无显著差异(P>0.05)。与C组相比,M组和I组小鼠血清CRP含量均显著降低(P<0.05),I组小鼠血清NEFA含量显著升高(P<0.05)。与M组相比,I组小鼠血清NEFA含量显著升高(P<0.05),血清IgM含量有降低的趋势(P=0.061)。
|
|
表 10 各组小鼠恢复期结束时的血清生化指标 Table 10 Serum biochemical indexes of mice in different groups at the end of recovery period |
由表 11可知,各组小鼠血液中各T淋巴细胞亚群的比例无显著差异(P>0.05)。由表 12可知,与C组相比,M组与I组小鼠脾脏中CD3+(P=0.053)和CD4+(P=0.091)T淋巴细胞的比例有升高的趋势。
|
|
表 11 各组小鼠恢复期结束时血液中T淋巴细胞亚群的比例 Table 11 Percentages of T lymphocyte subsets in blood of mice in different groups at the end of recovery period |
|
|
表 12 各组小鼠恢复期结束时脾脏中T淋巴细胞亚群的比例 Table 12 Percentages of T lymphocyte subsets in spleen of mice in different groups at the end of recovery period |
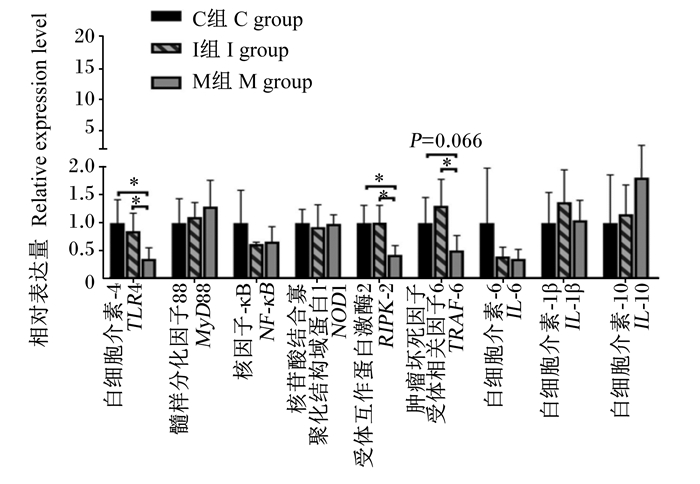
由图 1可知,与C组相比,M组小鼠结肠组织中TLR4、RIPK2的mRNA相对表达量显著下降(P<0.05)。与I组相比,M组小鼠结肠组织中TLR4、RIPK2、TRAF6的mRNA相对表达量显著下降(P<0.05)。
|
“*”表示组间差异显著(P<0.05)。 "*" mean a significant difference between two groups (P < 0.05). 图 1 各组小鼠恢复期结束时结肠组织中炎症相关细胞因子的mRNA相对表达量 Fig. 1 Relative expression levels of mRNA of inflammation-related cytokines in colonic tissue of mice at the end of recovery period |
饲粮纤维作为一种重要的营养素,其对宿主肠道生理和微生态平衡的特殊作用已被证实[12-13]。除了添加浓度以外,饲粮纤维的理化特性也是决定其生理功能的一个重要因素[14-16]。菊粉是典型的SDF,关于菊粉对小鼠生长性能影响的研究目前多集中于代谢疾病模型。研究发现,每天摄入2.5和10.0 g/kg菊芋菊粉均可降低高脂饮食诱导的肥胖小鼠的体重[17],且在高脂、高糖诱导的肥胖小鼠饲粮中添加5%短链菊粉也能有效降低小鼠体重[18]。对于便秘模型小鼠而言,菊粉对小鼠体重无显著影响[19]。但有限的研究表明,在肠道炎症情况下,菊粉对小鼠体重的影响不尽一致。例如,在2.5%DSS诱导的结肠炎小鼠的普通饲粮中添加200 g/kg菊粉对其体重无显著影响[20],而在另外一项DSS诱导的结肠炎小鼠的试验中,添加3%菊粉可显著提高小鼠体重[21]。本研究结果显示,当小鼠发生结肠炎后,饲粮中添加5%菊粉不影响其生长性能。与菊粉相反,MCC在人和模式动物的相关研究中常被作为一种典型的IDF,但其与小鼠生长性能关系的相关研究极为有限。本课题组前期研究表明,在饲粮中短期添加MCC对健康小鼠的终末体重和全期ADG均无显著影响[6]。但本试验结果表明,在小鼠发生结肠炎的情况下,在其饲粮中添加5%MCC可提高小鼠恢复期的阶段采食量,且其作用效果略优于菊粉,暗示MCC可能对结肠炎小鼠的生长性能有潜在的促进作用。
已有研究证实,适宜添加浓度的饲粮纤维对动物的系统免疫机能可能有促进作用[22-23]。胸腺和脾脏分别是机体的中枢和外周免疫器官,而肝脏中存在大量参与免疫应答与免疫反应的细胞,上述器官指数在一定程度上反映了机体免疫功能的强弱[24-25]。本试验结果表明,在DSS诱导小鼠产生结肠炎后,饲粮中添加5%菊粉或MCC对小鼠胸腺、脾脏、肝脏指数均未产生显著影响。本课题组前期对另一种SDF(燕麦β-葡聚糖)与MCC的研究也表明,即使这2种饲粮纤维在健康小鼠饲粮中添加浓度高达20%也不影响其器官指数[6]。除器官指数外,血清生化指标也常被用于衡量机体的免疫功能[26]。CRP是由肝脏合成的急性期蛋白,也是机体炎症反应的一项敏感指标,在正常血清中含量甚微,而在炎症情况下其含量增高。血清CRP含量通常作为评价炎症活动性及其严重程度的有效指标[27]。前人研究发现,菊粉和一些合生元可改善人术后肠道功能[28-29]。同时,高剂量菊粉可降低代谢综合征大鼠血清中的超敏CRP含量[30]。本研究发现,当饲粮中缺乏饲粮纤维时,结肠炎小鼠血清中CRP含量在恢复期下降缓慢。反之,饲粮中添加5%菊粉或MCC则可使结肠炎小鼠恢复期血清CRP含量快速下降,说明这2种类型的饲粮纤维均可有效缓解机体炎症。但是,血清NEFA含量的分析结果暗示菊粉与MCC缓解机体炎症的效果可能存在差异。血清中高含量的NEFA不仅干扰正常糖代谢,还会诱发轻度炎症及胰岛素抵抗[31]。本研究发现,在结肠炎小鼠饲粮中添加5%菊粉可显著提高其血清NEFA含量,且与摄入5%MCC的小鼠相比,其血清IgM含量降低。作为免疫球蛋白,血清IgM含量能在一定程度上反映机体的整体免疫状态[32-33]。就此而言,本试验结果暗示,饲粮中添加5%MCC对结肠炎小鼠免疫功能的改善程度可能大于菊粉。
相关研究表明,机体细胞免疫在肠道炎症发病过程中起重要作用,其中T淋巴细胞是介导细胞免疫应答的主要免疫细胞,根据其细胞表面分化抗原的不同,可以将其分为CD4+细胞和CD8+细胞。CD4+细胞为辅助型T细胞,通过合成与分泌细胞因子,协助其他淋巴细胞发挥免疫功能。CD8+细胞又称细胞毒性T细胞,能直接杀伤或抑制病毒等胞内感染病原体的靶细胞[34]。本研究发现,饲粮中添加5%菊粉或MCC均能提高结肠炎小鼠脾脏中CD3+和CD4+T淋巴细胞的比例,说明菊粉和MCC或可能通过改善细胞免疫功能来缓解小鼠的结肠炎。
值得注意的是,前人研究表明,饲粮纤维对机体免疫功能的影响可能主要集中在肠道[35-39]。摄入纤维能降低DSS诱导的结肠炎小鼠肠道促炎因子的表达量、上调抗炎因子的表达量,并通过改变肠道微生物的组成来调节肠系膜淋巴结中的Treg细胞数量,从而缓解结肠炎[40-41]。研究表明,饲粮添加菊粉和MCC能显著提高生长猪的结肠杯状细胞数量,从而促进其肠道免疫功能[42],然而在肥胖模型小鼠中,MCC并不影响肠形态和炎症因子水平[43]。本课题组前期的研究结果显示,SDF与IDF含量不同的天然纤维对生长猪结肠免疫功能的改善与模式识别受体信号通路的调控有关[7],他人研究也有类似结果[44]。TLR4和核苷酸结合寡聚化结构域样受体(NLR)都属于模式识别受体,相关信号通路对调节机体的先天免疫反应有重要作用[45-48]。TLR4和TRAF6是TLR4信号通路的关键基因。TLR4能激发一系列信号通路,促进炎症的发生及加重[49-50]。NOD1和RIPK2是NOD信号通路的关键基因。TLR4和NLR与配体(如病原微生物)结合后,可激活通过下游信号分子,最终导致NF-κB的激活,从而启动炎症相关细胞因子表达[51]。IL-10是由免疫细胞分泌的白细胞介素,IL-6是由单核巨噬细胞产生的多向性的促炎细胞因子,其在溃烂性结肠炎中表达上升[52-53]。IL-10有抗炎和抗过敏作用,能抑制促炎细胞因子的释放和抗原递呈[54]。因此,本试验进一步测定了小鼠结肠组织中上述通路关键基因的表达情况。结果发现,与自然恢复的结肠炎小鼠相比,当饲粮中含有5%MCC时,小鼠结肠组织中TLR4、TRAF6、RIPK2的mRNA相对表达量与对照组和菊粉组相比均有不同程度的下降,暗示MCC主要通过调控TLR4和NLR相关信号通路,降低促炎细胞因子的表达量和提高抗炎细胞因子的表达量来改善炎症小鼠的结肠免疫功能,并且其效果可能优于菊粉。由此可见,虽然SDF和IDF这2种类型饲粮纤维的添加均有助于增强炎症小鼠结肠的免疫功能,但二者缓解小鼠结肠炎症的途径很可能存在差异。由于微生物是模式识别受体的主要配体,故推测上述2种类型饲粮纤维促进小鼠结肠免疫功能的机制应与结肠菌群的调节有关,但具体机制还需进一步研究论证。
4 结论① 饲粮中添加5%菊粉或MCC对DSS诱导的结肠炎小鼠恢复期生长性能均无显著影响。
② 饲粮中添加5%菊粉或MCC对结肠炎小鼠恢复期胸腺、脾脏和肝脏指数均无显著影响。
③ 饲粮中添加5%菊粉或MCC均可促使结肠炎小鼠血清CRP含量快速下降,加速炎症缓解,且MCC的作用较菊粉更为显著。
④ 饲粮中添加5%菊粉或MCC均可提高结肠炎小鼠脾脏中CD3+和CD4+T淋巴细胞的比例,提高细胞免疫功能,从而缓解结肠炎;此外,MCC主要通过下调促炎细胞因子和上调抗炎细胞因子的表达改善结肠炎小鼠肠道免疫功能,且效果优于菊粉,可能与TLR4和NLR相关信号通路的调控有关。
| [1] |
吕东海. 日粮纤维对单胃动物消化生理的影响[J]. 饲料工业, 2002, 23(6): 12-14. LV D H. Effect of dietary fiber on digestivity physilogy for monogastric animal[J]. Feed Industry, 2002, 23(6): 12-14 (in Chinese). DOI:10.3969/j.issn.1001-991X.2002.06.005 |
| [2] |
冯江鑫, 陈代文, 余冰, 等. 饲粮纤维对猪肠道屏障功能的影响[J]. 动物营养学报, 2018, 30(10): 3828-3835. FENG J X, CHEN D W, YU B, et al. Effect of dietary fiber on intestinal barrier function in pigs[J]. Chinese Journal of Animal Nutrition, 2018, 30(10): 3828-3835 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.10.005 |
| [3] |
KIM Y, HWANG S W, KIM S, et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota[J]. Gut Microbes, 2020, 11(4): 944-961. DOI:10.1080/19490976.2020.1730149 |
| [4] |
HOWLETT J F, BETTERIDGE V A, CHAMP M, et al. The definition of dietary fiber-discussions at the ninth vahouny fiber symposium: building scientific agreement[J]. Food & Nutrition Research, 2010, 54: 54. |
| [5] |
LUO Y H, ZHANG L, LI H, et al. Different types of dietary fibers trigger specific alterations in composition and predicted functions of colonic bacterial communities in BALB/c mice[J]. Frontiers in Microbiology, 2017, 8: 966. DOI:10.3389/fmicb.2017.00966 |
| [6] |
张玲, 陈代文, 余冰, 等. 饲粮中短期内单独及混合添加高水平燕麦β-葡聚糖和微晶纤维素对小鼠生长性能、器官指数和粪便细菌群落结构的影响[J]. 动物营养学报, 2017, 29(7): 2407-2415. ZHANG L, CHEN D W, YU B, et al. Short-term adding high-level oat β-glucan, microcrystalline cellulose and their mixture in diets affects growth performance organ indexes and fecal bacterial community structure of mice[J]. Chinese Journal of Animal Nutrition, 2017, 29(7): 2407-2415 (in Chinese). DOI:10.3969/j.issn.1006-267x.2017.07.024 |
| [7] |
赵瑶. 日粮纤维源对生长猪生产性能和肠道健康的影响机制及肠道微生物的媒介作用[D]. 硕士学位论文. 雅安: 四川农业大学, 2018. ZHAO Y. The influence of different dietary fiber son the production performance and intestinal health of growing pigs and the role of intestinal microflora as a medium[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2018. (in Chinese) |
| [8] |
FASANO A, SHEA-DONOHUE T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases[J]. Nature Clinical Practice Gastroenterology & Hepatology, 2005, 2: 416-422. |
| [9] |
叶应妩, 王毓三. 全国临床检验操作规程[M]. 2版. 南京: 东南大学出版社, 1997: 143. YE J W, WANG Y S. National guide to clinical laboratory proceduers[M]. 2nd ed. Nanjing: Southeast University Press, 1997: 143 (in Chinese). |
| [10] |
COOPER H. Clinicopathologic study of dextran sulfate sodium experimental murine colitis[J]. Laboratory Investigation, 1993, 69(2): 238-249. |
| [11] |
PU J N, CHEN D W, TIAN G, et al. Protective effects of benzoic acid, Bacillus coagulans, and oregano oil on intestinal injury caused by enterotoxigenic Escherichia coli in weaned piglets[J]. BioMed Research International, 2018, 2018: 1829632. |
| [12] |
HOLSCHER H D. Dietary fiber and prebiotics and the gastrointestinal microbiota[J]. Gut Microbes, 2017, 8(2): 172-184. DOI:10.1080/19490976.2017.1290756 |
| [13] |
SIMPSON H L, CAMPBELL B J. Review article: dietary fibre-microbiota interactions[J]. Alimentary Pharmacology & Therapeutics, 2015, 42(2): 158-179. |
| [14] |
CHEN T T, CHEN D W, TIAN G, et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model[J]. BioMed Research International, 2019, 2019: 7809171. |
| [15] |
SATOH H, URUSHIDANI T. Soluble dietary fiber can protect the gastrointestinal mucosa against nonsteroidal anti-Inflammatory drugs in mice[J]. Digestive Diseases and Sciences, 2016, 61: 1903-1914. DOI:10.1007/s10620-016-4086-5 |
| [16] |
SHOAIB M, SHEHZAD A, OMAR M, et al. Inulin: properties, health benefits and food applications[J]. Carbohydrate Polymers, 2016, 147: 444-454. DOI:10.1016/j.carbpol.2016.04.020 |
| [17] |
于秋红, 李苑禾, 宗尧, 等. 菊芋源菊粉对肥胖小鼠缓解作用的研究[J]. 天然产物研究与开发, 2017, 29(1): 141-146, 151. YU Q H, LI Y H, ZONG Y, et al. Mitigation effect of Jerusalem artichoke inulin on obese mice[J]. Natural Product Research and Development, 2017, 29(1): 141-146, 151 (in Chinese). |
| [18] |
王晨. 朝浩鹏, 谢宇端. 菊粉对高脂高糖饮食诱导肥胖小鼠的减肥作用与其调节肠道菌群的关系分析[J]. 安徽医药, 2020, 24(4): 666-670. WANG C. Anti-obesity effect of inulin on obesity mice induced by high-fat and high-glucose diet and its relationship with regulating intestinal floras[J]. Anhui Medical and Pharmaceutical Journal, 2020, 24(4): 666-670. (in Chinese) |
| [19] |
徐小琴, 杨立琛, 邓桂龙, 等. 菊粉与谷物粉润肠通便作用的初步研究[J]. 食品研究与开发, 2018, 39(12): 183-186. XU X Q, YANG L C, DENG G L, et al. Preliminary study on action of inulin and cereal powder in relaxing bowel[J]. Food Research and Developent, 2018, 39(12): 183-186 (in Chinese). DOI:10.3969/j.issn.1005-6521.2018.12.035 |
| [20] |
MILES J P, ZOU J, KUMAR M V, et al. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis[J]. Inflammatory Bowel Diseases, 2017, 23(7): 1133-1143. DOI:10.1097/MIB.0000000000001155 |
| [21] |
LIU Z, LIU F, WANG W, et al. Study of the alleviation effects of a combination of Lactobacillus rhamnosus and inulin on mice with colitis[J]. Food & Function, 2020, 11(5): 3823-3837. |
| [22] |
WELLINGTON M O, HTOO J K, VAN KESSEL A G, et al. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs[J]. Journal of Animal Science, 2018, 96(12): 5222-5232. |
| [23] |
TROMPETTE A, GOLLWITZER E S, PATTARONI C, et al. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism[J]. Immunity, 2018, 48(5): 992-1005. DOI:10.1016/j.immuni.2018.04.022 |
| [24] |
RACANELLI V, REHERMANN B. The liver as an immunological organ[J]. Hepatology, 2006, 43(2): S54-S62. |
| [25] |
KUBES P, JENNE C. Immune responses in the liver[J]. Annual Review of Immunology, 2018, 36: 247-277. DOI:10.1146/annurev-immunol-051116-052415 |
| [26] |
刘娓娓, 顾文君, 沈锡中. 血清生化指标对慢性乙型肝炎肝纤维化及炎症程度的诊断价值[J]. 中国临床医学, 2005, 12(1): 65-66. LIU W W, GU W J, SHEN X Z. Diagnostic value of serum biochemical markers for hepatic fibrosis and inflammation in patients with chronic hepatitis B[J]. Clinical Medical Journal of China, 2005, 12(1): 65-66 (in Chinese). DOI:10.3969/j.issn.1008-6358.2005.01.025 |
| [27] |
BLACK S, KUSHNER I, SAMOLS D. C-reactive protein[J]. The Journal of Biological Chemistry, 2004, 279(47): 48487-48490. DOI:10.1074/jbc.R400025200 |
| [28] |
单新辉. 菊粉低聚糖降脂机制研究[D]. 硕士学位论文. 大连: 大连医科大学, 2016. SHAN X H. Study on the molecular mechanism of Jiangzhi inulooligosaccharide[D]. Master's Thesis. Dalian: Dalian Medical University, 2016. (in Chinese) |
| [29] |
赵明利. 合生元对胃肠手术后肠黏膜屏障功能保护作用的基础与临床研究[D]. 博士学位论文. 北京: 北京协和医学院, 2009. ZHAO M L. Protective effect of synbiotics on intestinal barrier function after gastrointestinal surgery[D]. Ph. D. Thesis. Beijing: Peking Union Medical College, 2009. (in Chinese) |
| [30] |
李耀华. 甘肃地产菊粉对代谢综合征大鼠的影响及机制研究[D]. 硕士学位论文. 兰州: 甘肃中医药大学, 2017. LI Y H. The effect and mechanism of Gansu native inulin on rats with metabolic syndrome[D]. Master's Thesis. Lanzhou: Gansu University of Chinese Medicine, 2017. (in Chinese) |
| [31] |
CAMPOS G, FERNÁNDEZ V, FERNÁNDEZ E, et al. Association of free fatty acids with the insulin-resistant state but not with central obesity in individuals from Venezuela[J]. Investigacion Clinica, 2010, 51(1): 115-126. |
| [32] |
LIU J, WANG Y, XIONG E M, et al. Role of the IgM Fc receptor in immunity and tolerance[J]. Frontiers in Immunology, 2019, 10: 529. DOI:10.3389/fimmu.2019.00529 |
| [33] |
OUCHIDA R, MORI H, HASE K, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(40): E2699-E2706. DOI:10.1073/pnas.1210706109 |
| [34] |
何维. 医学免疫学[M]. 北京: 人民卫生出版社, 2005. HE W. Medical immunology[M]. Beijing: People's Medical Publishing House, 2005 (in Chinese). |
| [35] |
DESAI M S, SEEKATZ A M, KOROPATKIN N M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility[J]. Cell, 2016, 167(5): 1339-1353. DOI:10.1016/j.cell.2016.10.043 |
| [36] |
MACIA L, TAN J, VIEIRA A T, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome[J]. Nature Communications, 2015, 6: 6734. DOI:10.1038/ncomms7734 |
| [37] |
JHA R, FOUHSE J M, TIWARI U, 等. 膳食纤维与单胃动物肠道健康[J]. 张洪丽, 译. 中国饲料添加剂, 2019(9): 25-32. JHA R, FOUHSE J M, TIWARI U, et al. Dielary fiber and intestinal health of monogastric animals[J]. ZHANG H L, translation. China Feed Additive, 2019(9): 25-32. (in Chinese) |
| [38] |
张艳红, 高洁. 膳食纤维与肠道健康[J]. 健康与营养, 2016(增刊1): 54-57. ZHANG Y H, GAO J. Dietary fiber and intestinal health[J]. Health and Nutrition, 2016(Suppl.1): 54-57 (in Chinese). |
| [39] |
赵奕敏, 杨凌君, 叶玲, 等. 饲粮纤维对畜禽肠道调控研究进展[J]. 中国饲料, 2019(11): 11-15. ZHAO Y M, YANG L J, YE L, et al. Research progress on dietary fiber regulating and controlling the intestines of livestock and poultry[J]. China Feed, 2019(11): 11-15 (in Chinese). |
| [40] |
HARTOG A, BELLE F N, BASTIAANS J, et al. A potential role for regulatory T-cells in the amelioration of DSS induced colitis by dietary non-digestible polysaccharides[J]. The Journal of Nutritional Biochemistry, 2015, 26(3): 227-233. DOI:10.1016/j.jnutbio.2014.10.011 |
| [41] |
PRAENGAM K, SAHASAKUL Y, KUPRADINUN P, et al. Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice[J]. Food & Function, 2017, 8(12): 4630-4643. |
| [42] |
吴维达, 解竞静, 张宏福. 不同日粮纤维对生长猪养分消化率和肠道结构形态的影响[J]. 畜牧与兽医, 2016, 48(6): 57-60. WU W D, XIE J J, ZHANG H F. Effects of the different dietary fiber on nutrients digestibility and intestinal morphology[J]. Animal Husbandry & Veterinary Medicine, 2016, 48(6): 57-60 (in Chinese). |
| [43] |
李娟娟. 金纳米颗粒和纳米微晶纤维素对小鼠肠道和血液指标的影响[D]. 博士学位论文. 北京: 中国地质大学: 北京, 2019. LI J J. Effects of gold nanoparticles and nanocrystalline cellulose on intestinal and blood indices in mice[D]. Ph. D. Thesis. Beijing: China University of Geosciences, 2019. (in Chinese) |
| [44] |
YANG L, LIN Q, HAN L, et al. Soy hull dietary fiber alleviates inflammation in BALB/C mice by modulating the gut microbiota and suppressing the TLR-4/NF-κB signaling pathway[J]. Food & Function, 2020, 11(7): 5965-5975. |
| [45] |
HU X L, YU Y N, CHIN Y E, et al. The role of acetylation in TLR4-mediated innate immune responses[J]. Immunology and Cell Biology, 2013, 91(10): 611-614. DOI:10.1038/icb.2013.56 |
| [46] |
LIU C H, LIU H, GE B. Innate immunity in tuberculosis: host defense vs pathogen evasion[J]. Cellular & Molecular Immunology, 2017, 14(12): 963-975. |
| [47] |
MUKHERJEE S, HUDA S, SINHA B S. Toll-like receptor polymorphism in host immune response to infectious diseases: a review[J]. Scandinavian Journal of Immunology, 2019, 90(1): e12771. |
| [48] |
TARTEY S, TAKEUCHI O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells[J]. International Reviews of Immunology, 2017, 36(2): 57-73. DOI:10.1080/08830185.2016.1261318 |
| [49] |
PŁÓCIENNIKOWSKA A, HROMADA-JUDYCKA A, BORZECKA K, et al. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling[J]. Cellular and Molecular Life Sciences, 2015, 72(3): 557-581. |
| [50] |
ZHANG W, ZHUANG N, LIU X, et al. The metabolic regulator Lamtor5 suppresses inflammatory signaling via regulating mTOR-mediated TLR4 degradation[J]. Cellular & Molecular Immunology, 2020, 17(10): 1063-1076. |
| [51] |
FUKATA M, VAMADEVAN A S, ABREU M T. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders[J]. Seminars in Immunology, 2009, 21(4): 242-253. |
| [52] |
李琪佳, 徐敏, 宫恩聪. 细胞因子与溃疡性结肠炎[J]. 临床内科杂志, 1997, 4(6): 297-298. LI Q J, XU M, GONG E C. Cytokines and ulcerative colitis[J]. Chinese Journal of Coal Industry Medicine, 1997, 4(6): 297-298 (in Chinese). |
| [53] |
丁伟群, 林庚金, 徐三荣, 等. 溃疡性结肠炎发病中白介素水平的变化[J]. 复旦学报(医学科学版), 2001, 28(4): 330-333. DING W Q, LIN G J, XU S R, et al. Changes of interleukin level in ulcerative colitis patients[J]. Journal of Fudan University (Medical Science), 2001, 28(4): 330-333 (in Chinese). |
| [54] |
MOORE K W, DE WAAL MALEFYT R, COFFMAN R L, et al. Interleukin-10 and the interleukin-10 receptor[J]. Annual Review of Immunology, 2001, 19: 683-765. |




