随着我国经济的快速发展,消费者不仅要求水产品食品安全、营养全面,也更注重其肌肉品质[1],而养殖水产品的品质与饲料中营养成分密切相关[2-3]。其中,脂肪源可通过影响鱼类生长速度和肌肉沉积速度来影响鱼肉质地,也可通过降解和氧化肌肉中脂质和蛋白质来影响鱼肉的气味和滋味[4]。Regost等[5]研究发现,以鱼油(fish oil,FO)、菜籽油(rapeseed oil,RO)、豆油(soybean oil,SO)为脂肪源时,FO组和SO组大西洋鲑(Salmo salar)肌肉质地显著高于RO组。Fountoulaki等[6]SO、棕榈油、RO全部替代FO后,发现SO组、棕榈油组、RO组金头鲷(Sparus aurata)肌肉的质地、滋味、气味均与FO组无显著差异。但也有研究表明,以高水平SO为脂肪源时,大菱鲆(Psetta maxima)肌肉会产生较强的土腥味[7]。目前,鱼类肌肉品质的研究主要集中于金头鲷、大西洋鲑、大黄鱼(Larimichthys crocea)等海水性鱼类。而淡水性鱼类肌肉品质的研究主要集中于草鱼(Ctenopharyngodon idella)、罗非鱼(Oreochromis niloticus)2个品种上,对于有较高经济价值的青鱼(Mylopharyngodon piceus)肌肉品质的研究相对较少。
青鱼作为我国主要的经济养殖鱼类,也是我国传统四大家鱼中唯一的肉食性鱼类。青鱼生长快、个体大、产量高,在长江流域和珠江流域被广泛养殖,2020年我国青鱼年产量高达69.45万t[8]。青鱼肌肉中蛋白质含量高、脂肪含量低、肉味鲜美,受到了广大消费者的喜爱[9]。目前,不同脂肪源对青鱼的研究主要集中于生长性能、脂质代谢和肝脏抗氧化能力等方面[10],对青鱼肌肉品质方面的研究还未见报道。因此,本研究以FO为对照,分别以富含饱和脂肪酸(SFA)的猪油(lard oil,PL)、富含多不饱和脂肪酸(PUFA)的玉米油(corn oil,CO)和SO以及富含单不饱和脂肪酸(MUFA)的RO和花生油(peanut oil,PO)为脂肪源,探究饲料中不同脂肪源对青鱼生长性能、血清生化指标及肌肉品质(抗氧化指标、质地、气味和滋味)的影响,为优化饲料配方并改善青鱼肌肉品质提供理论依据。
1 材料与方法 1.1 试验设计和试验饲料试验饲料以酪蛋白、明胶为蛋白质源,分别以FO、PL、RO、CO、PO、SO为脂肪源,配制6种等氮等能的试验饲料,试验饲料组成及营养水平见表 1。选取平均体重为(5.25±0.25) g的青鱼540尾,随机分为6组,每组3个重复,每个重复30尾。各组分别投喂以FO(FO组)、PL(PL组)、RO(RO组)、CO(CO组)、PO(PO组)和SO(SO组)为脂肪源的试验饲料。饲料原料粉碎后过60目筛,按表 1比例精准称重,充分混匀。采用双螺杆挤条机制成粒径为2 mm颗粒饲料,置于37 ℃烘箱中风干,最后将饲料成品密封后置于-20 ℃冰箱中保存备用。
|
|
表 1 试验饲料组成及营养水平(风干基础) Table 1 Composition and nutrient levels of experimental diets (air-dry basis) |
|
|
表 2 试验饲料脂肪酸组成(占总脂肪酸的百分比) Table 2 Fatty acid composition of experimental diets (percentage of total fatty acid) |
试验用青鱼购于湖州兴旺水产苗种繁育基地,试验鱼暂养2周后,按照试验设计各组分别投喂不同脂肪源的试验饲料,养殖周期为180 d。养殖过程中水温为26~28 ℃,溶氧浓度>5 mg/L。每天饱食投喂2次,投喂时间分别为08:00和17:00。
1.3 样品采集及分析方法 1.3.1 样品采集养殖试验结束后,禁食24 h,将每组青鱼计数称重。每组随机选取6尾鱼称重后置于-20 ℃冰箱中保存,用于全鱼成分分析。每组随机再取12尾鱼称重后进行尾静脉采血,将血液放入装有肝素的1.5 mL离心管中,在4 ℃条件下,静置8 h,2 500 r/min离心10 min,取上清液分装后放入-80 ℃冰箱中保存,用于血清生化指标的测定。将鱼解剖后取出内脏团、肝脏并称重,以计算脏体比(VSI)、肝体比(HSI)。采集肌肉样品,置于-80 ℃冰箱保存待测。
1.3.2 生长性能的测定增重率(WGR)、特定生长率(SGR)、肥满度(CF)、VSI和HSI计算公式如下:

|

|
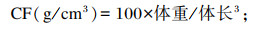
|
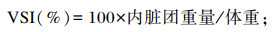
|
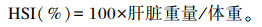
|
饲料、全鱼和肌肉中水分含量的测定参照GB/T 6435—2014方法进行;粗蛋白质含量测定使用Rapid N exceed杜马斯定氮仪(德国Elementar公司),参照GB/T 24318—2009方法进行;粗脂肪含量的测定参照GB 5009.6—2010方法进行;粗灰分含量的测定参照GB/T 6438—2007方法进行。
1.3.4 血清生化指标的测定血清中甘油三酯(triglyceride,TG)、总胆固醇(total cholesterol,TC)、高密度脂蛋白胆固醇(high density lipoprotein cholesterol,HDL-C)、低密度脂蛋白胆固醇(low density lipoprotein cholesterin,LDL-C)含量和碱性磷酸酶(alkaline phosphatase,ALP)活性采用宁波普瑞柏生物技术股份有限公司试剂盒测定,测定方法均参照说明书进行。
1.3.5 肌肉抗氧化指标的测定肌肉中超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽还原酶(glutathione reductase,GR)、谷胱甘肽-S-转移酶(glutathione-S-transferase,GST)活性、总抗氧化能力(total antioxidant capacity,T-AOC)及谷胱甘肽(glutathione,GSH)、丙二醛(malonaldehyde,MDA)含量采用南京建成生物工程研究所试剂盒测定,测定方法均参照说明书进行。
1.3.6 肌肉质地的测定将背侧肌切成1.0 cm×1.0 cm×0.8 cm的肉块,使用TA.XT plus型质构分析仪(英国SMS公司)参照质地剖面分析(texture profile analysis,TPA)法测定肌肉的硬度、韧性、紧实度、坚实度、咀嚼性和弹性,每个样品重复测定3次。
1.3.7 肌肉中蛋白质组成及胶原蛋白合成相关指标、组织蛋白酶含量的测定称取1.00 g肌肉,加入预冷的磷酸缓冲溶液20.00 mL后进行匀浆,将匀浆液置于4 ℃、8 000×g离心机中离心20 min,吸去上清液(水溶性蛋白)于25 mL容量瓶中,用磷酸缓冲溶液定容至25 mL,在4 ℃条件下保存待测。在沉淀中加入20 mL预冷的0.6 mol/L KCl的磷酸缓冲溶液,匀浆后在相同条件下离心20 min,取上清液(盐溶性蛋白)置于25 mL容量瓶中,用0.6 mol/L KCl的磷酸缓冲溶液定容,4 ℃条件下保存待测。肌肉中水溶性蛋白和盐溶性蛋白含量采用考马斯亮蓝法进行测定;胶原蛋白含量测定采用羟脯氨酸法,测得羟脯氨酸含量乘以系数8得到胶原蛋白含量[11];脯氨酸羟化酶(prolyl hydroxylase,PHD)、赖氨酰氧化酶(lysyloxidase,LOX)、胶原吡啶交联(pyridinoline crosslinks,PYD)、组织蛋白酶-B(cathepsin-B,Cath-B)、组织蛋白酶-L(cathepsin-L,Cath-L)含量均采用江苏酶免实业有限公司酶联免疫吸附测定(ELISA)试剂盒测定,测定方法参照说明书进行。
1.3.8 肌肉气味的测定采用直接顶空吸气法测定肌肉的气味,直接将进样针头插入含样品的顶空瓶中,用PEN3电子鼻(德国Airsense公司)进行测定。测定条件:采样时间为1 s/组,传感器自清洗时间为80 s,传感器归零时间为5 s,样品准备时间为5 s,进样流量为400 mL/min,分析采样时间为80 s。
1.3.9 肌肉滋味的测定样品在室温下解冻,用剪刀将其剪碎,称取20.0 g样品置于烧杯中,添加100 mL纯水,搅拌均匀后倒入搅拌机中搅拌20 s,然后将样品倒入离心管中3 000 r/min离心5 min,取上清液置于TS-5000Z电子舌(日本Insent公司)进行测试。用30 mmol/L KCl溶液与0.3 mmol/L酒石酸溶液配成参比溶液。将传感器置于参比溶液中归零30 s,随后开始进行鲜味测定。测试时间为30 s,测试完毕后用参比溶液清洗3 s,再次进行回味测定,测试时间30 s。每个样品重复4次,取后3次作为测定结果。
1.4 数据统计分析试验数据采用SPSS 25.0统计软件进行单因素方差分析(one-way ANOVA),当差异显著时用Duncan氏法进行多重比较,P < 0.05表示差异显著。数据采用平均值±标准差(mean±SD)表示。
2 结果 2.1 饲料中不同脂肪源对青鱼生长性能的影响如表 3所示,各组之间FBW、WGR、SGR无显著差异(P>0.05)。FO组、RO组和SO组CF和VSI显著高于PL组、CO组和PO组(P<0.05)。FO组和SO组HSI显著高于PL组、RO组、CO组和PO组(P<0.05),CO组HSI最低。
|
|
表 3 饲料中不同脂肪源对青鱼生长性能的影响 Table 3 Effects of dietary different lipid sources on growth performance of black carp |
如表 4所示,PL组、RO组、CO组和PO组全鱼粗蛋白质含量显著高于FO组、SO组(P < 0.05),FO组、PL组、RO组、CO组和PO组肌肉粗蛋白质含量显著高于SO组(P < 0.05)。SO组和PL组全鱼和肌肉粗脂肪含量显著高于FO组、RO组、PO组和CO组(P < 0.05),CO组全鱼和肌肉粗脂肪含量最低。FO组全鱼和肌肉粗灰分含量显著低于其他各组(P < 0.05),PL组和PO组全鱼粗灰分含量显著高于FO组、RO组、CO组和SO组(P < 0.05)。各组之间全鱼和肌肉水分含量无显著差异(P>0.05)。
|
|
表 4 饲料中不同脂肪源对青鱼全鱼和肌肉营养成分的影响 Table 4 Effects of dietary different lipid sources on whole body and muscle nutritional composition of black carp |
如表 5所示,FO组和RO组肌肉SFA含量显著低于PL组、CO组、PO组和SO组(P < 0.05)。FO组和RO组肌肉MUFA含量显著高于PL组、CO组、PO组和SO组(P < 0.05)。RO组肌肉亚麻酸(C18∶3n-3)含量显著高于FO组、PL组、CO组和SO组(P < 0.05)。RO组和SO组肌肉二十碳五烯酸(EPA)+二十二碳六烯酸(DHA)含量显著高于FO组、PL组、CO组和PO组(P < 0.05)。CO组和SO组肌肉PUFA含量显著高FO组、PL组、RO组和PO组(P < 0.05)。
|
|
表 5 饲料中不同脂肪源对青鱼肌肉脂肪酸组成的影响(占总脂肪酸的百分比) Table 5 Effects of dietary different lipid sources on muscle fatty acid composition of black carp (percentage of total fatty acid) |
如表 6所示,FO组、PL组和SO组血清TG含量显著高于RO组、CO组和PO组(P < 0.05)。PO组血清TC含量显著高于其他各组(P < 0.05)。PL组血清HDL-C含量显著低于其他各组(P < 0.05)。FO组和RO组血清ALP活性显著高于PL组、CO组、PO组和SO组(P < 0.05)。各组之间血清LDL-C含量无显著差异(P>0.05)。
|
|
表 6 饲料中不同脂肪源对青鱼血清生化指标的影响 Table 6 Effects of dietary different lipid sources on serum biochemical parameters of black carp |
如表 7所示,PO组肌肉SOD、GR活性显著高于其他各组(P < 0.05),RO组显著高于PL组、CO组、SO组和FO组(P < 0.05)。PO组和SO组肌肉GST活性显著高于FO组、PL组、CO组和RO组(P < 0.05),RO组显著高于FO组、PL组和CO组(P < 0.05),PL组最低。PO组肌肉GSH含量显著高于其他各组(P < 0.05),SO组显著高于FO组、RO组、PL组和CO组(P < 0.05),CO组显著低于其他各组(P < 0.05)。PO组和PL组肌肉T-AOC显著低于其他各组(P < 0.05),CO组显著高于其他各组(P < 0.05)。PO组肌肉MDA含量显著高于其他各组(P < 0.05),SO组、PL组、FO组显著高于RO组和CO组(P < 0.05)。
|
|
表 7 饲料中不同脂肪源对青鱼肌肉抗氧化指标的影响 Table 7 Effects of dietary different lipid sources on muscle antioxidant indexes of black carp |
如表 8所示,FO组、RO组和SO组肌肉硬度显著高于PL组、CO组和PO组(P < 0.05)。FO组、PL组、RO组和SO组肌肉坚实度显著高于CO组和PO组(P < 0.05),CO组最低。FO组、PL组、RO组、PO组和SO组肌肉咀嚼性显著高于CO组(P < 0.05)。FO组、PL组、RO组、PO组和CO组肌肉弹性显著高于SO组(P < 0.05)。各组之间肌肉韧性和紧实度无显著差异(P>0.05)。
|
|
表 8 饲料中不同脂肪源对青鱼肌肉质地的影响 Table 8 Effects of dietary different lipid sources on muscle texture of black carp |
如表 9所示,RO组、CO组、PO组和SO组肌肉水溶性蛋白含量显著低于FO组和PL组(P < 0.05),RO组最低。RO组肌肉盐溶性蛋白含量显著高于其他各组(P < 0.05)。FO组肌肉胶原蛋白和PHD含量显著高于其他各组(P < 0.05)。SO组和CO组肌肉LOX含量显著高于PO组、PL组、FO组和RO组(P < 0.05),RO组显著低于其他各组(P < 0.05)。SO组肌肉PYD含量显著高于其他各组(P < 0.05),RO组显著低于其他各组(P < 0.05)。SO组肌肉Cath-B含量显著高于其他各组(P < 0.05),PL组和FO组显著高于CO组、RO组和PO组(P < 0.05)。PL组、RO组、CO组和SO组肌肉Cath-L含量与FO组无显著差异(P>0.05),而PO组显著低于FO组(P < 0.05)。
|
|
表 9 饲料中不同脂肪源对青鱼肌肉蛋白质组成及胶原蛋白合成相关指标、组织蛋白酶含量的影响 Table 9 Effects of different dietary lipid sources on muscle protein composition, collagen synthesis related indexes and cathepsin contents of black carp |
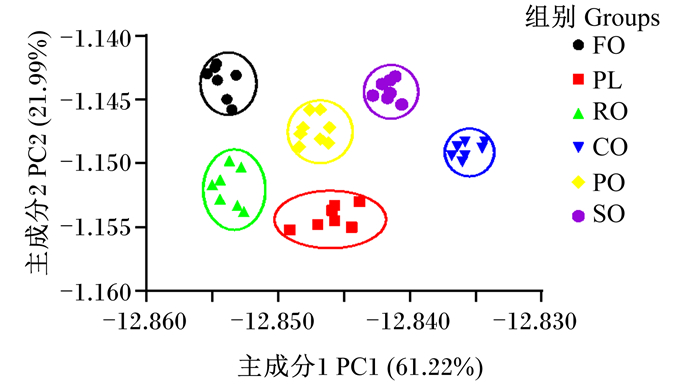
主成分分析(PCA)是使用线性组合将具有强烈针对性的多个变量转换为多个相互无关的综合变量进行分析的方法。图 1为饲料中不同脂肪源对青鱼肌肉气味PCA图,图中显示了2个主要组件轴:主成分1(PC1)和主成分2(PC2),PC1贡献率为61.22%,PC2贡献率21.99%,总贡献率为83.21%,能反映样品的整体气味。饲料中不同脂肪源对青鱼肌肉气味会产生明显差异,RO组肌肉气味与FO组无明显差异,而PL组、PO组、SO组、CO组肌肉气味与FO组有明显差异。
|
图 1 饲料中不同脂肪源对青鱼肌肉气味主成分分析图 Fig. 1 PCA diagram of dietary different lipid sources on muscle odor of black carp |
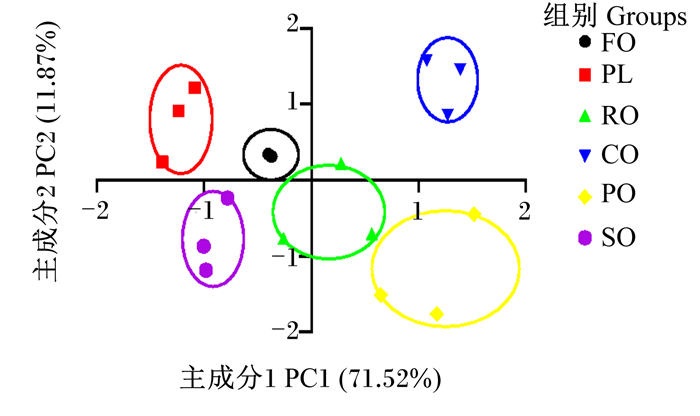
由图 2所示,PC1贡献率为71.52%,PC2贡献率11.87%,总贡献率为83.39%。RO组、SO组肌肉滋味与FO组无明显差异,而PL组、CO组、PO组肌肉滋味与FO组有明显差异;其中,PO组肌肉滋味与FO组差异最为明显。以参比溶液的输出为零点,酸味和咸味的无味点分别为-13和-6,其他指标无味点均为0。如表 10所示,青鱼肌肉酸味、涩味、苦味回味均低于无味点,而咸味、鲜味、甜味、苦味均大于无味点,表明青鱼肌肉中有效味觉指标为咸味、鲜味、甜味、苦味。CO组和PO组肌肉鲜味、咸味显著高于FO组、PL组、RO组和SO组(P < 0.05),且RO组肌肉咸味显著高于FO组、PL组和SO组(P < 0.05)。RO组和CO组肌肉甜味显著高于FO组(P < 0.05),且PL组、PO组和SO组肌肉甜味与FO组无显著差异(P>0.05)。FO组和CO组肌肉苦味显著高于PL组、RO组、PO组和SO组(P < 0.05),SO组最低。
|
图 2 饲料脂肪源对青鱼肌肉滋味主成分分析图 Fig. 2 PCA diagram of dietary different lipid sources on muscle taste of black carp |
|
|
表 10 饲料中不同脂肪源对青鱼肌肉滋味的影响 Table 10 Effects of dietary different lipid sources on muscle taste of black carp |
水产动物对脂肪的利用主要取决于脂肪酸种类及其含量,脂肪源可通过影响水产动物对脂肪酸的消化、吸收和利用,进而对水产动物生长性能产生不同影响[12]。Lin等[13]发现摄食以FO、CO、PO、SO为脂肪源的饲料后,CO组、PO组、SO组石斑鱼(Epinephelus coioides)生长性能与FO组无显著差异。Qiu等[12]也发现摄食以FO、RO、PO、SO为脂肪源的饲料后,PO组和SO组大黄鱼(Larmichthys crocea)生长性能与FO组无显著差异,而RO组大黄鱼的生长性能有所提高,可能是由于RO富含n-3PUFA,从而促进了其生长。本研究中,PL组、RO组、CO组、PO组、SO组WGR、FBW和SGR与FO组无显著差异,但SO组WGR、FBW和SGR均低于其他各组,在石斑鱼[13]上也发现了相似的结果。Li等[14]发现SO中亚油酸含量比FO高,而n-6PUFA含量增加,可能在一定程度上抑制了鱼类的生长。
脂肪源作为重要营养成分,对鱼体组成有重要调控作用。Yu等[15]发现摄食以FO、SO、PO、RO、CO为脂肪源的饲料对杂交鲟(Acipenser baeri Brandt♀×Acipenser schrenckii Brandt ♂)肌肉粗脂肪含量无显著影响。而在本研究中,PL组和SO组全鱼和肌肉粗脂肪含量显著高于FO组、RO组、CO组、PO组。这与上述结果存在一定差异,可能是由于物种和鱼类生存环境不同造成的。本试验结果提示摄食以SO、PL为脂肪源的饲料,可能促进青鱼全鱼和肌肉中脂肪的沉积。这可能是由于PL中PUFA含量低,从而促进了鱼体脂肪沉积。Hosseini等[16]也发现SO中富含n-6PUFA,而饲料中n-6PUFA高也会引起鱼体粗脂肪含量增加。Yu等[15]发现摄食以FO、SO、PO、RO、CO为脂肪源的饲料对杂交鲟肌肉粗蛋白质含量无显著影响。本研究中也发现PL组、RO组、CO组、PO组肌肉粗蛋白质含量与FO组无显著差异,而SO组肌肉粗蛋白质最低。这可能是由于SO促进了鱼体脂肪的沉积,而肝脏脂肪转运能力有限,大量的脂肪无法转运会影响鱼类对于蛋白质的吸收和转运能力,使得鱼体粗蛋白质含量降低[17]。
3.2 饲料中不同脂肪源对青鱼血清生化指标的影响血清TG和TC含量可反映机体对于脂质的吸收能力和脂肪代谢情况,在水产动物中TG和TC含量过高可引起脂肪肝的发生[18]。Yu等[15]发现以FO、RO、CO、SO、PO为脂肪源时对杂交鲟血清TG和TC含量无显著影响。张媛媛等[19]也发现摄食以FO、RO、SO为脂肪源的饲料后,RO组、SO组异育银鲫(Carassius auratus gibelio)血清TG含量与FO组无显著差异。而在本研究中,RO组、CO组和PO组血清TG含量显著低于FO组、PL组和SO组,这与全鱼和肌肉粗脂肪含量结果一致。EPA和DHA可抑制脂类合成,亚麻酸是EPA和DHA合成的前体物质,富含亚麻酸的RO可能对TG合成有抑制作用[20]。而PO组血清TG含量降低可能是因为摄食以PO为脂肪源的饲料增强了鱼体的氧化反应,大部分脂肪酸用来氧化供能,用于合成TG的脂肪酸含量降低,使得血清TG含量降低[21]。脂肪甘油三酯脂肪酶(ATGL)作为TG分解代谢的关键酶,可将TG分解为二酰甘油和脂肪酸。陈炼[10]发现摄食以CO、PO、FO为脂肪源的饲料后,CO组和PO组肝脏中ATGL表达量显著高于FO组,这提示CO和PO可能提高了肝脏中ATGL表达量,加速了TG的分解,引起了TG含量的降低。李婷婷等[22]发现摄食以FO、RO、SO为脂肪源的饲料后,对杂交鲟(Acipenser baerii ♀×Acipenser schrenckii ♂)血清中TC含量无显著影响。Qiu等[12]也发现以FO、RO、SO、PO为脂肪源时对大黄鱼血清TC含量无显著影响。本研究中,PL组、RO组、CO组、SO组血清TC含量与FO组无显著差异,而PO组血清TC含量显著高于其他各组,这表明以PO为脂肪源时可能会促进TC的合成,导致血清TC含量增加[18]。
HDL-C可将血液中的TC转入肝脏,从而减少血液中TC含量,降低心血管疾病的发病风险[23]。Gao等[24]发现摄食以FO、RO、CO、SO为脂肪源的饲料后,RO组、CO组和SO组克氏原螯虾(Procambarus clarkia)血清HLD-C含量与FO组无显著差异。李婷婷等[22]也发现RO组、SO组杂交鲟血清HLD-C含量与FO组无显著差异。本研究结果也显示,RO组、CO组、PO组、SO组血清HDL-C含量与FO组无显著差异,而PL组显著低于其他各组。这表明摄食以PL为脂肪源的饲料,可能导致青鱼血清中TC不能充分转运回肝脏进行分解代谢,容易引起部分TC在血管中沉积[23]。ALP在水产动物机体非特异性免疫过程中发挥重要作用,是巨噬细胞溶酶体的标志酶,ALP活性可反映水产动物非特异性免疫能力的强弱[25]。本研究中,FO组和RO组血清ALP活性显著高于PL组、CO组、PO组和SO组,这与异育银鲫(Carassius auratus gibelio)的结果[26]相似。这表明与PL、CO、PO和SO相比,摄食以FO、RO为脂肪源的饲料可能会提高鱼体的非特异性免疫能力[25]。
当机体受到应激时会产生大量活性氧和自由基,这不仅会引起细胞和组织产生氧化损伤,而且当机体受到高强度氧化应激时,会引起肌肉中脂质和蛋白质的降解,产生不良风味物质,最终影响肌肉品质[27]。SOD是清除体内活性氧的第1道防线,可将氧离子(O2-)转化为过氧化氢(H2O2),达到清除O2-的目的。GSH最为重要的非酶类抗氧化物,可与GST共同作用降低机体氧化损伤,而GR可维持细胞内GSH含量[28]。Yu等[15]发现摄食以FO、RO、CO、SO、PO为脂肪源的饲料后,RO组、CO组、SO组和PO组杂交鲟(Acipenser baeri Brandt ♀ ×Acipenser schrenckii Brandt ♂)血清SOD活性与FO组无显著差异。Guo等[29]也发现摄食以FO、RO、PO为脂肪源的饲料后,RO组金鲳鱼(Trachinotus ovatus)血清SOD活性与FO组无显著差异,但PO组显著低于FO组。而本研究中,PO组肌肉SOD、GR、GST活性和GSH含量均显著高于FO组、PL组、RO组、CO组、SO组,这表明青鱼摄食以PO为脂肪源的饲料后,增强了机体氧化代谢,并产生大量活性氧,容易对机体造成一定程度的氧化损伤[28],这与上述杂交鲟和金鲳鱼的结果不一致,可能是由于物种差异引起的。
T-AOC是机体抗氧化能力的总体体现,而MDA是脂质过氧化的代表产物[24]。Guo等[29]发现摄食以FO、RO、PO为脂肪源的饲料后,RO组、FO组金鲳鱼血清T-AOC显著高于PO组,且PO组血清MDA含量显著高于FO组。刘燕等[30]发现摄食以FO、CO为脂肪源的饲料后,CO组津新鲤(Cyprinus carpio)血清T-AOC显著高于FO组。在本研究中,PL组、PO组肌肉T-AOC显著低于FO组、RO组、CO组和SO组,而CO组显著高于其他各组;PO组肌肉MDA含量显著高于其他各组,且RO组、CO组显著低于FO组。这与上述金鲳鱼、津新鲤和鲤鱼研究结果相似。PO组肌肉T-AOC降低、MDA含量升高,可能是由于PO中MUFA含量高,加速了机体氧化代谢,同时产生了相对较多的活性氧,机体通过提高SOD、GR、GST等抗氧化酶活性和GSH含量抵抗氧化造成的胁迫[28]。同时,本研究结果也表明摄食RO、CO为脂肪源的饲料可以提高青鱼肌肉抗氧化能力。这可能是由于CO富含PUFA,使得肌肉抗氧化能力提高[10]。而RO中n-3PUFA可通过核因子相关因子2(Nrf2)途径抑制细胞中H2O2的生成,进而提高了机体的抗氧化能力[31]。抗氧化能力的提高可降低肌肉中脂质氧化产生的异味物质,从而改善肌肉品质[32]。
3.3 饲料中不同脂肪源对青鱼肌肉品质的影响饲料成分是影响肌肉质地的主要因素之一,脂肪源可通过影响鱼类生长速度和健康状况来改变肌肉质地。肌肉的质地与口感直接相关,肌肉的硬度、韧性、紧实度、坚实度、咀嚼性和弹性等指标越高,其品质越好。其中,硬度和弹性是反映淡水鱼肌肉质地的主要指标[33]。Kim等[34]发现用SO全部替代FO后对牙鲆(Paralichthys olivaceus)肌肉硬度、胶黏性、咀嚼性等指标无显著影响。本研究中,RO组肌肉硬度、韧性、紧实度、坚实度、咀嚼性和弹性均与FO组无显著差异,而PL组、CO组、PO组硬度显著低于FO组,SO组肌肉弹性显著低于FO组、PL组、RO组、CO组、PO组。这表明以RO全部替代FO后,对青鱼肌肉质地无显著影响。可能是由于RO促进氧化型肌纤维的合成,肌纤维直径和横截面积减小,使得肌肉质地得到改善[35]。肌肉的弹性与Cath-B的含量相关,Cath-B含量越高,肌肉弹性越小。在本研究中,SO组肌肉Cath-B含量显著高于其他各组,这表明摄食以SO为脂肪源的饲料可能通过提高肌肉中Cath-B含量而降低肌肉的弹性。除此之外,SO组肌肉弹性降低可能与肌内结缔组织肌原纤维的性能及相互作用方式有关[36]。
胶原蛋白作为结缔组织的主要成分,其含量直接影响肌肉硬度。Wei等[37]发现添加适量的羟脯氨酸可以促进大黄鱼肌肉胶原蛋白的合成,进而提高了大黄鱼的肌肉品质。杨航等[38]发现通过提高胶原蛋白合成过程中的关键酶,如PHD、LOX,进而提高草鱼肌肉中胶原蛋白含量,达到改善肌肉品质的目的。但目前,脂肪源对水产动物肌肉中胶原蛋白含量的研究较少。本研究结果显示,FO组肌肉胶原蛋白和PHD含量显著高于其他各组,且RO组最低。同时,RO组肌肉LOX、PYD含量均显著低于FO组。这表明摄食以PL、RO、CO、PO、SO为脂肪源的饲料会降低肌肉中胶原蛋白的沉积,其中RO组最为明显。RO可通过降低肌肉PHD、LOX、PYD含量,使得肌肉中胶原蛋白的合成量减少。而PL组、CO组、PO组和SO组肌肉胶原蛋白含量的降低,除了与胶原蛋白的合成含量有关,还可能与胶原蛋白降解含量有关。一般来说,肌肉中胶原蛋白含量与硬度呈正相关[39],而本研究中肌肉胶原蛋白含量结果与上述肌肉硬度结果不一致。而肌肉质地除了与肌肉中胶原蛋白含量有关,还与肌纤维类型[40]、直径[41]、肌肉中水分和脂肪含量[42]等指标有关,具体原因有待于进一步研究。
不同脂肪源主要是脂肪酸组成和含量不同,肌肉中的脂肪酸组成基本上反映了饲料的脂肪酸组成[43]。鱼肉脂肪酸种类和含量的变化,可通过脂肪酸降解和氧化影响肌肉中挥发性风味化合物的种类和含量,进而影响肌肉气味[44]。本研究结果显示,RO组、PO组肌肉气味与FO组相近,PL组、SO组、CO组肌肉气味与FO组有明显差异。Mu等[43]也发现摄食以FO、RO、SO为脂肪源的饲料后,RO组大黄鱼肌肉气味与FO组相近。这可能是由于FO、RO中富含n-3PUFA,而n-3PUFA产生的挥发性醛是一种可以使鱼肉产生香味的物质[45]。肌肉气味还与脂肪酸种类和不饱和程度有关,一般新鲜鱼肉中的挥发性化合物主要是由不饱和脂肪酸氧化分解产生的羰基和醇类,而PL中富含饱和脂肪酸,氧化后产物不同直接影响了鱼肉的气味[43]。陈炼[10]发现PO中n-6PUFA含量低于CO、SO。n-6系列脂肪酸氧化衍生的挥发性醛类对鱼肉的整体气味会产生负面影响[45],这也导致摄食以PO为脂肪源的饲料,肌肉产生的异味物质含量低于CO组和SO组。水产品肌肉滋味的主要贡献物质包括脂肪酸、氨基酸、核苷酸、矿物质等。Izquierdo等[46]发现以60%RO、SO替代FO后,RO组、SO组金头鲷(Sparus aurata)和鲈鱼肌肉滋味与FO组无显著差异。本研究结果显示,RO组、SO组肌肉滋味与FO组无差异。这可能与肌肉蛋白质经组织蛋白酶降解成多肽,进而形成游离氨基酸的种类和含量有关[47]。PO组肌肉Cath-B和Cath-L含量显著低于FO组,组织蛋白酶含量降低抑制了肌肉中蛋白质降解,使得蛋白质降解后产生的游离氨基酸含量降低,进而影响了PO组肌肉的滋味。CO组、PO组肌肉鲜味显著高于FO组,可能是由于CO组、PO组肌肉中肌苷酸(IMP)与谷氨酸盐协同作用后使得鲜味增强[48-49]。RO组肌肉鲜味与FO组无显著差异,表明以RO为脂肪源时,不会影响青鱼肌肉的鲜味。
4 结论综上所述,饲料中不同脂肪源对青鱼生长性能、血清生化指标及肌肉品质会产生不同影响。饲料中不同脂肪源对青鱼生长性能未产生负面影响,而摄食以FO和RO为脂肪源的饲料更有利于青鱼健康并且还可改善肌肉质地、气味、滋味。同时。摄食以FO、RO、CO为脂肪源的饲料可提高青鱼肌肉抗氧化能力。与FO相比,摄食以PL、PO、SO为脂肪源的饲料会降低青鱼肌肉抗氧化能力、质地、气味和滋味。
| [1] |
HARRIS W S. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor[J]. Pharmacological Research, 2007, 55(3): 217-223. DOI:10.1016/j.phrs.2007.01.013 |
| [2] |
HU L, YUN B, XUE M, et al. Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus)[J]. Aquaculture, 2013, 372/375: 52-61. DOI:10.1016/j.aquaculture.2012.10.025 |
| [3] |
DUAN Q Y, MAI K S, SHENTU J K, et al. Replacement of dietary fish oil with vegetable oils improves the growth and flesh quality of large yellow croaker (Larmichthys crocea)[J]. Journal of Ocean University of China, 2014, 13(3): 445-452. DOI:10.1007/s11802-014-2188-2 |
| [4] |
LIU H H, HU F, LI P F. Volatile compositions analysis of tilapia (Oreochram niloticus) and fishy odour development in the muscle[J]. Advanced Materials Research, 2014, 1033/1034: 767-776. DOI:10.4028/www.scientific.net/AMR.1033-1034.767 |
| [5] |
REGOST C, JAKOBSEN J V, RØRÅ A M B. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage[J]. Food Research International, 2004, 37(3): 259-271. DOI:10.1016/j.foodres.2003.12.003 |
| [6] |
FOUNTOULAKI E, VASILAKI A, HURTADO R, et al. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures[J]. Aquaculture, 2009, 289(3/4): 317-326. |
| [7] |
REGOST C, ARZEL J, CARDINAL M, et al. Total replacement of fish oil by soybean or linseed oil with a return to fish oil in turbot (Psetta maxima): 2.Flesh quality properties[J]. Aquaculture, 2003, 220(1/4): 737-747. |
| [8] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会编制. 中国渔业统计年鉴-2020[M]. 北京: 中国农业出版社, 2020. Fishery Administration of the Ministry of Agriculture and Rural Areas, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistics yearbook 2020[M]. Beijing: China Agriculture Press, 2020 (in Chinese). |
| [9] |
HUANG H Y, WANG Y X, SHI W Z. Effects of different drying methods on the quality and nonvolatile taste compounds of black carp[J]. Journal of Food Processing and Preservation, 2021, 45(6): e15507. |
| [10] |
陈炼. 不同脂肪源对青鱼鱼种生长, 代谢, 抗氧化反应及非特异性免疫的影响[D]. 硕士学位论文. 宁波: 宁波大学, 2018. CHEN L. Effects of different lipid sources on growth, metabolism, antioxidant response and non-specific immunity of black carp[D]. Master's Thesis. Ningbo: Ningbo University, 2018. (in Chinese) |
| [11] |
SUN W T, HE M, XU X Y, et al. Comparison study of three compounds in Eucommia ulmoides on growth, flesh quality of grass carp (Ctenopharyngodon idella)[J]. Aquaculture Nutrition, 2019, 25(4): 906-916. DOI:10.1111/anu.12909 |
| [12] |
QIU H, JIN M, LI Y, et al. Dietary lipid sources influence fatty acid composition in tissue of large yellow croaker (Larmichthys crocea) by regulating triacylglycerol synthesis and catabolism at the transcriptional level[J]. PLoS One, 2017, 12(1): e0169985. DOI:10.1371/journal.pone.0169985 |
| [13] |
LIN H Z, LIU Y J, HE J G, et al. Alternative vegetable lipid sources in diets for grouper, Epinephelus coioides (Hamilton): effects on growth, and muscle and liver fatty acid composition[J]. Aquaculture Research, 2007, 38(15): 1605-1611. DOI:10.1111/j.1365-2109.2007.01811.x |
| [14] |
LI Y, LIANG X, ZHANG Y, et al. Effects of different dietary soybean oil levels on growth, lipid deposition, tissues fatty acid composition and hepatic lipid metabolism related gene expressions in blunt snout bream (Megalobrama amblycephala) juvenile[J]. Aquaculture, 2016, 451: 16-23. DOI:10.1016/j.aquaculture.2015.08.028 |
| [15] |
YU H H, XING W, LI T L, et al. Effects of alternative dietary lipid sources on growth performance, health status and fillet fatty acid composition of hybrid sturgeon (Acipenser baeri Brandt ♀×Acipenser schrenckii Brandt)[J]. Aquaculture Nutrition, 2020, 26(5): 1419-1430. DOI:10.1111/anu.13086 |
| [16] |
HOSSEINI S V, KENARI A A, REGENSTEIN J M, et al. Effects of alternative dietary lipid sources on growth performance and fatty acid composition of Beluga sturgeon, Huso huso, juveniles[J]. Journal of the World Aquaculture Society, 2010, 41(4): 471-489. DOI:10.1111/j.1749-7345.2010.00389.x |
| [17] |
VAN AN L, HONG T T T, OGLE B, et al. Utilization of ensiled sweet potato (Ipomoea batatas (L.) Lam.) leaves as a protein supplement in diets for growing pigs[J]. Tropical Animal Health and Production, 2005, 37(1): 77-88. DOI:10.1023/B:TROP.0000047937.41355.4d |
| [18] |
WU F, TIAN J, YU L J, et al. Effects of dietary rapeseed meal levels on growth performance, biochemical indices and flesh quality of juvenile genetically improved farmed tilapia[J]. Aquaculture Reports, 2021, 20: 100679. DOI:10.1016/j.aqrep.2021.100679 |
| [19] |
张媛媛, 刘波, 戈贤平, 等. 不同脂肪源对异育银鲫生长性能、机体成分、血清生化指标、体组织脂肪酸组成及脂质代谢的影响[J]. 水产学报, 2012, 36(7): 1111-1118. ZHANG Y Y, LIU B, GE X P, et al. Effect of dietary oil sources on growth performance, body composition, the serum biochemical indices, fatty acids composition and lipid metabolism of Carassius auratus gibelio[J]. Journal of Fisheries of China, 2012, 36(7): 1111-1118 (in Chinese). |
| [20] |
SHEARER G C, SAVINOVA O V, HARRIS W S. Fish oil-how does it reduce plasma triglycerides?[J]. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 2012, 1821(5): 843-851. DOI:10.1016/j.bbalip.2011.10.011 |
| [21] |
吴美焕, 安文强, 董晓慧, 等. 饲料脂肪源对珍珠龙胆石斑鱼生长性能、血清生化指标及肝脏脂肪酸组成、脂肪代谢相关指标的影响[J]. 动物营养学报, 2020, 32(3): 1315-1326. WU M H, AN W Q, DONG X H, et al. Effects of dietary lipid sources on growth performance, serum biochemical indexes and liver fatty acids composition, lipid metabolism related indexes of Epinephelus lanceolatus×Epinephelus fuscoguttatus[J]. Chinese Journal of Animal Nutrition, 2020, 32(3): 1315-1326 (in Chinese). |
| [22] |
李婷婷, 褚志鹏, 李创举, 等. 饲料中不同脂肪源对杂交鲟幼鱼生长性能、体成分、养分表观消化率、肝脏脂肪代谢酶活性和血清生化指标的影响[J]. 动物营养学报, 2021, 33(6): 3447-3460. LI T T, CHU Z P, LI C J, et al. Effects of different lipid sources in diet on growth performance, body composition, nutrient apparent digestibilities, liver lipid metabolism enzymes activities and serum biochemical parameters of juvenile hybrid sturgeon[J]. Chinese Journal of Animal Nutrition, 2021, 33(6): 3447-3460 (in Chinese). |
| [23] |
PHILLIPS M C. Molecular mechanisms of cellular cholesterol efflux[J]. Journal of Biological Chemistry, 2014, 289(35): 24020-24029. DOI:10.1074/jbc.R114.583658 |
| [24] |
GAO F, LIU J, WANG A M, et al. Dietary lipid sources modulate the intestinal transport of fatty acids in the red swamp crayfish Procambarus clarkii[J]. Aquaculture, 2020, 521: 735091. DOI:10.1016/j.aquaculture.2020.735091 |
| [25] |
ZHU M, WU S J. The growth performance and nonspecific immunity of loach Paramisgurnus dabryanus as affected by dietary β-1, 3-glucan[J]. Fish & Shellfish Immunology, 2018, 83: 368-372. |
| [26] |
陈家林, 韩冬, 朱晓鸣, 等. 不同脂肪源对异育银鲫的生长、体组成和肌肉脂肪酸的影响[J]. 水生生物学报, 2011, 35(6): 988-997. CHEN J L, HAN D, ZHU X M, et al. Dietary lipid sources for gibel carp Carassius auratus gibelio: growth performance, tissue composition and muscle fatty acid profiles[J]. Acta Hydrobiologica Sinica, 2011, 35(6): 988-997 (in Chinese). |
| [27] |
宋益贞. 不同脂肪源对罗非鱼生长特性和肌肉品质的影响[D]. 硕士学位论文. 无锡: 江南大学, 2012. SONG Y Z. Effects of different lipid sources on growth and muscle qualities of tilapia[D]. Master's Thesis. Wuxi: Jiangnan University, 2012. (in Chinese) |
| [28] |
YUAN X, ZHOU Y, LIANG X F, et al. Effect of dietary glutathione supplementation on the biological value of rapeseed meal to juvenile grass carp, Ctenopharyngodon idellus[J]. Aquaculture Nutrition, 2015, 21(1): 73-84. DOI:10.1111/anu.12142 |
| [29] |
GUO H J, CHEN C Y, YAN X, et al. Effects of different dietary oil sources on growth performance, antioxidant capacity and lipid deposition of juvenile golden pompano Trachinotus ovatus[J]. Aquaculture, 2021, 530: 735923. DOI:10.1016/j.aquaculture.2020.735923 |
| [30] |
刘燕, 程镇燕, 曲木, 等. 不同脂肪源饲料对津新鲤生长、消化及生理生化指标的影响[J]. 饲料研究, 2016(11): 38-46, 51. LIU Y, CHENG Z Y, QU M, et al. Effects of different lipid source diets on growth, digestion and physiological and biochemical indexes of Jinxin carp[J]. Feed Research, 2016(11): 38-46, 51 (in Chinese). |
| [31] |
KUSUNOKI C, YANG L, YOSHIZAKI T, et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes[J]. Biochemical and Biophysical Research Communications, 2013, 430(1): 225-230. DOI:10.1016/j.bbrc.2012.10.115 |
| [32] |
胡宇超, 王园, 孟子琪, 等. 发酵麸皮多糖对肉羊肉品质、肌肉氨基酸组成及肌肉抗氧化酶和肌纤维类型相关基因表达的影响[J]. 动物营养学报, 2020, 32(2): 932-940. HU Y C, WANG Y, MENG Z Q, et al. Effects of fermented wheat bran polysaccharides on meat quality, muscle amino acid composition and expression of antioxidant enzymes and muscle fiber type-related genes in muscle of mutton sheep[J]. Chinese Journal of Animal Nutrition, 2020, 32(2): 932-940 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.02.049 |
| [33] |
胡芬, 李小定, 熊善柏, 等. 5种淡水鱼肉的质构特性及与营养成分的相关性分析[J]. 食品科学, 2011, 32(11): 69-73. HU F, LI X D, XIONG S B, et al. Texture properties of freshwater fish and their correlation with nutritional components[J]. Food Science, 2011, 32(11): 69-73 (in Chinese). |
| [34] |
KIM D K, KIM K D, SEO J Y, et al. Effects of dietary lipid source and level on growth performance, blood parameters and flesh quality of sub-adult olive flounder (Paralichthys olivaceus)[J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(6): 869-879. DOI:10.5713/ajas.2011.11470 |
| [35] |
RYU Y C, KIM B C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle[J]. Meat Science, 2005, 71(2): 351-357. DOI:10.1016/j.meatsci.2005.04.015 |
| [36] |
常海军. 肌内结缔组织与肉嫩度关系研究[J]. 肉类研究, 2009(11): 94-97. CHANG H J. Research on relationship between intramuscular connective tissue and meat tenderness[J]. Meat Research, 2009(11): 94-97. DOI:10.3969/j.issn.1001-8123.2009.11.024 |
| [37] |
WEI Z H, MA J, PAN X Y, et al. Dietary hydroxyproline improves the growth and muscle quality of large yellow croaker Larimichthys crocea[J]. Aquaculture, 2016, 464: 497-504. DOI:10.1016/j.aquaculture.2016.07.015 |
| [38] |
杨航, 徐禛, 谭素梅, 等. 杜仲皮、叶对草鱼生长、肌肉品质及胶原蛋白相关基因表达的影响[J]. 动物营养学报, 2020, 32(12): 5827-5838. YANG H, XU Z, TAN S M, et al. Influences of dietary Eucommia bark and leaf on growth, muscle quality and collagen related genes expression in grass carp (Ctenopharyngodon idellus)[J]. Chinese Journal of Animal Nutrition, 2020, 32(12): 5827-5838 (in Chinese). |
| [39] |
MORENO H M, MONTERO M P, GÓMEZ-GUILLÉN M C, et al. Collagen characteristics of farmed Atlantic salmon with firm and soft fillet texture[J]. Food Chemistry, 2012, 134(2): 678-685. DOI:10.1016/j.foodchem.2012.02.160 |
| [40] |
CHANG K C, DA COSTA N, BLACKLEY R, et al. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs[J]. Meat Science, 2003, 64(1): 93-103. DOI:10.1016/S0309-1740(02)00208-5 |
| [41] |
JOHNSTON I A, MANTHRI S, ALDERSON R, et al. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar L.)[J]. Journal of Experimental Biology, 2003, 206(8): 1337-1351. DOI:10.1242/jeb.00262 |
| [42] |
JOHNSEN C A, HAGEN Ø, BENDIKSEN E Å. Long-term effects of high-energy, low-fishmeal feeds on growth and flesh characteristics of Atlantic salmon (Salmo salar L.)[J]. Aquaculture, 2011, 312(1/2/3/4): 109-116. |
| [43] |
MU H, LI J, PAN X Y, et al. Alterations in fatty acid composition and volatile compounds in muscle of large yellow croaker Larimichthys crocea fed different dietary lipid sources[J]. Aquaculture Reports, 2021, 20: 100688. DOI:10.1016/j.aqrep.2021.100688 |
| [44] |
MOREIRA N, SOARES S, VALENTE L M P, et al. Effect of two experimental diets (protein and lipid vegetable oil blends) on the volatile profile of Senegalese sole (Solea senegalensis Kaup, 1858) muscle[J]. Food Chemistry, 2014, 153: 327-333. DOI:10.1016/j.foodchem.2013.12.071 |
| [45] |
TURCHINI G M, MORETTI V M, MENTASTI T, et al. Effects of dietary lipid source on fillet chemical composition, lavour volatile compounds and sensory characteristics in the freshwater fish tench (Tinca tinca L.)[J]. Food Chemistry, 2007, 102(4): 1144-1155. DOI:10.1016/j.foodchem.2006.07.003 |
| [46] |
IZQUIERDO M S, OBACH A, ARANTZAMENDI L, et al. Dietary lipid sources for seabream and seabass: growth performance, tissue composition and flesh quality[J]. Aquaculture Nutrition, 2003, 9(6): 397-407. DOI:10.1046/j.1365-2095.2003.00270.x |
| [47] |
SENTANDREU M, TOLDRÁ F. Dipeptidyl peptidase activities along the processing of Serrano dry-cured ham[J]. European Food Research and Technology, 2001, 213(2): 83-87. DOI:10.1007/s002170100355 |
| [48] |
MARUJI Y, SHIMIZU M, MURATA M, et al. Multiple taste functions of the umami substances in muscle extracts of yellowtail and bastard halibut[J]. Fisheries Science, 2010, 76(3): 521-528. DOI:10.1007/s12562-010-0231-9 |
| [49] |
MCCABE C, ROLLS E T. Umami: a delicious flavor formed by convergence of taste and olfactory pathways in the human brain[J]. European Journal of Neuroscience, 2007, 25(6): 1855-1864. DOI:10.1111/j.1460-9568.2007.05445.x |




